Abstract
We conducted an international phase 2 trial to evaluate 2 dose levels of ofatumumab, a human CD20 mAb, combined with fludarabine and cyclophosphamide (O-FC) as frontline therapy for chronic lymphocytic leukemia (CLL). Patients with active CLL were randomized to ofatumumab 500 mg (n = 31) or 1000 mg (n = 30) day 1, with fludarabine 25 mg/m2 and cyclophosphamide 250 mg/m2 days 2-4, course 1; days 1-3, courses 2-6; every 4 weeks for 6 courses. The first ofatumumab dose was 300 mg for both cohorts. The median age was 56 years; 13% of patients had a 17p deletion; 64% had β2-microglobulin > 3.5 mg/L. Based on the 1996 National Cancer Institute Working Group (NCI-WG) guidelines, the complete response (CR) rate as assessed by an independent review committee was 32% for the 500-mg and 50% for the 1000-mg cohort; the overall response (OR) rate was 77% and 73%, respectively. Based on univariable regression analyses, β2-microglobulin and the number of O-FC courses were significantly correlated (P < .05) with CR and OR rates and progression-free survival (PFS). The most frequent Common Terminology Criteria (CTC) grade 3-4 investigator-reported adverse events were neutropenia (48%), thrombocytopenia (15%), anemia (13%), and infection (8%). O-FC is active and safe in treatment-naive patients with CLL, including high-risk patients. This trial was registered at www.clinicaltrials.gov as NCT00410163.
Introduction
Chronic lymphocytic leukemia (CLL) remains the most common form of adult leukemia in Western countries, with approximately 14 990 new cases and 4390 deaths in 2010 in the United States.1 The clinical course for CLL is highly variable, ranging from patients who never require therapy to patients who require immediate therapy, rapidly develop refractory disease, and succumb to their disease; up to two-thirds of all patients will eventually require treatment.2,3 Strategies for initial treatment have evolved over the last decade. Historically, treatment was palliative, with complete response (CR) rates < 10% for patients treated frontline with alkylating agents such as chlorambucil monotherapy.4 With the advent of purine analogs such as fludarabine, both response rate and progression-free survival (PFS) have improved compared with chlorambucil.5 Subsequently, 3 large, randomized phase 3 frontline studies reported superior PFS and response rates with combined fludarabine and cyclophosphamide (FC) compared with fludarabine monotherapy.6-8 The introduction of the CD20 mAb rituximab represented a significant advance, especially in combination with fludarabine-based regimens.
The Cancer and Leukemia Group B 9712 phase 2 trial reported a 47% CR rate and 90% overall response (OR) rate with concurrent fludarabine and rituximab.9,10 The phase 2 frontline study of rituximab combined with FC (FCR) from the M.D. Anderson Cancer Center reported a 72% CR rate, 95% OR rate, and median time to progression of 80 months.11,12 The combination of FC with mitoxantrone and rituximab (FCM-R) has been evaluated in phase 2 frontline studies and also showed high response rates: 82%-83% CR rates and 93%-96% OR rates.13,14 In one of the studies, the authors concluded that outcomes with the FCM-R regimen did not appear to differ from those of patients treated with FCR.14 More recently, the randomized phase 3 CLL8 study of the German CLL Study Group reported significantly longer median PFS (52 vs 33 months; P < .001) associated with superior response rates for FCR versus FC (OR rate 95% vs 88% and CR rate 44% vs 22%, respectively; P < .001).15 This represented one of the first randomized studies of CLL showing superior overall survival (OS) for a frontline treatment: the 3-year OS rate was 87% with FCR compared with 83% with FC; P = .012. Therefore, chemoimmunotherapy with a CD20 mAb is the current standard of care for fit patients with CLL who can tolerate myelosuppression; alkylating agents such as chlorambucil remain an option for older patients with comorbidities.16
Ofatumumab (Arzerra; GlaxoSmithKline and Genmab A/S) is a human CD20 mAb that binds to a unique, membrane-proximal epitope composed of both the large and small loops of CD20 that is distinct from the epitope recognized by rituximab.17,18 Ofatumumab showed more rapid and effective in vitro complement–dependent cytotoxicity compared with rituximab, including in primary CLL cells with low expression of CD20.17-19 A phase 1/2 open-label trial of once-weekly ofatumumab for 4 weeks in patients with relapsed/refractory CLL reported an OR rate of 50% for the highest-dose cohort (dose 1, 500 mg; doses 2-4, 2000 mg; n = 26).20 In a subsequent international pivotal trial, ofatumumab monotherapy was administered weekly for 8 weeks, followed by 4 monthly infusions (dose 1, 300 mg; doses 2-12, 2000 mg) to patients with fludarabine- and alemtuzumab-refractory CLL and fludarabine-refractory CLL with bulky (> 5 cm) lymph nodes; the OR rate was 58% and 47%, respectively.21 The estimated median PFS was approximately 6 months and OS was 14-15 months. Given this significant single-agent activity, we investigated the efficacy and safety of 2 dose levels of ofatumumab combined with FC (O-FC) in previously untreated patients with CLL. The primary end point of this study was to estimate CR rates for each dose cohort.
Methods
Patients
The health authorities and local independent ethics committees or institutional review boards of all participating institutions approved the protocol, amendments, and consent forms. The study, initiated on January 9, 2007, is registered at www.clinicaltrials.gov as NCT00410163 and was conducted in accordance with the Declaration of Helsinki and Guidelines for Good Clinical Practice.
All patients provided informed consent. Eligible adult patients (≥ 18 years of age) had active, treatment-naive CLL (CD5+/CD20+/CD23+), circulating lymphocytes > 5 × 109/L, and an indication for treatment according to the 1996 National Cancer Institute-Working Group (NCI-WG) guidelines.22 Exclusion criteria included known Richter transformation, central nervous system involvement, infection requiring systemic treatment, clinically significant cardiac disease, an uncontrolled medical condition, history of cerebrovascular disease, HIV-1 infection, positive hepatitis B serology (unless consistent with vaccination), or Eastern Cooperative Oncology Group (ECOG) performance status > 2.
Study design and treatment
Baseline assessments included a physical examination, evaluation for constitutional symptoms, computed tomography scan, blood sampling for hematology and biochemistry, and bone marrow examination. Analyses of laboratory samples, including bone marrow biopsies and smears, were performed by a central laboratory (Bio-Analytical Research Corporation). Assessments of prognostic factors, including genomic abnormalities by fluorescence in situ hybridization, immunoglobulin heavy chain variable gene (IGHV) mutational status, and CD38 positivity, were also performed by the central laboratory. Disease status was assessed by physical examination, presence of constitutional symptoms, and blood counts every 4 weeks until week 24, and every 3 months after the last course until disease progression or month 24. Bone marrow examination was performed after course 3 and at 3 months after the end of treatment to confirm CR. After month 24, patients were followed every 6 months to monitor B cells until the cell count recovered to the normal range or to at least to the baseline level, up to month 60 or until the start of a new CLL treatment.
This was a randomized, 2-dose, parallel cohort, open-label phase 2 trial (Figure 1). Patients were randomized 1:1 to receive ofatumumab 500 mg or 1000 mg (day 1), combined with intravenous fludarabine 25 mg/m2 and cyclophosphamide 250 mg/m2 (days 2-4 for course 1, days 1-3 for subsequent courses) every 4 weeks for a total of 6 courses. The first dose of ofatumumab was 300 mg for both cohorts. Before all ofatumumab infusions, patients received acetaminophen 1000 mg and cetirizine 10 mg or equivalents. Glucocorticoid equivalent to 100 mg of prednisolone was also administered before the first and second infusion of ofatumumab; if no grade 3 or 4 adverse events were observed with infusion 2, the glucocorticoid dose could be reduced for subsequent infusions at the discretion of the investigator. A dose reduction for FC, but not ofatumumab, was recommended for toxicity. Criteria for dose reduction to fludarabine 20 mg/m2 and cyclophosphamide 200 mg/m2 were toxicities of grade 3 or higher. For continued occurrence of cytopenias of at least grade 3, doses could be further reduced to fludarabine 15 mg/m2 and cyclophosphamide 150 mg/m2. Patients were withdrawn from treatment for toxicities of grade 3 or higher lasting more than 2 weeks in the setting of dose reduction. Allopurinol for tumor lysis syndrome prophylaxis, anti-infective prophylaxis (eg, for herpesvirus infections or Pneumocystis jiroveci pneumonia), and growth factor support were permitted at the discretion of the investigator.
CONSORT (Consolidated Standards Of Reporting Trials) diagram. Phase 2 study of O-FC in previously untreated patients with CLL.
CONSORT (Consolidated Standards Of Reporting Trials) diagram. Phase 2 study of O-FC in previously untreated patients with CLL.
Efficacy evaluations
The primary end point was CR rate based on the 1996 NCI-WG criteria, which was measured during the period from start of treatment until 3 months after the last infusion. Secondary efficacy end points included duration of response (time from initial response to disease progression or death), PFS (time from randomization to disease progression or death), time-to-next CLL therapy (time from randomization to administration of CLL therapy other than the study drug), and correlation of outcomes with prognostic factors. OR and OS (time from randomization to death) were also assessed. Responses and disease progression were determined by an independent review committee. Computed tomography scans were performed 3 months after the end of treatment to confirm CR, but imaging data were not included in the response assessment presented in this paper.
Safety evaluations
Investigators graded adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 and assigned attribution. Blood samples were taken at screening and at each ofatumumab infusion visit for blood chemistries and counts performed by central laboratories (Bio-Analytical Research Corporation). Laboratory abnormalities were graded according to CTCAE Version 3.0. Blood samples were also drawn at screening, after course 3, and at 6 and 18 months after the last ofatumumab infusion for analysis of human anti–human IgG antibodies.
Statistical analysis
The primary objective of the study was to estimate the CR rate and the corresponding 2-sided 95% confidence interval (CI) for each dose cohort. With an expected CR rate of 70%, a sample size of 28 in each cohort was needed to observe a 17% difference from the response rate to the lower limit of the 95% CI. The study was not powered to detect differences in end points between dose cohorts. Analyses were performed on the full analysis set, including all patients who were exposed to study drug. PFS and OS were determined using Kaplan-Meier estimates. Adverse events and clinical safety end points were reported using descriptive statistics.
Results
Patients and treatment
Sixty-one patients were enrolled from 17 centers in 5 countries and randomized to the ofatumumab 500-mg (n = 31) or 1000-mg (n = 30) dose cohorts; the median (range) patient age for both groups was 56 years (range 38-73 and 38-72, respectively; Table 1). The 17p deletion was found in 13% of all patients and the median serum β-2 microglobulin (β-2M) was 4 mg/L. Overall, 64% of patients completed all 6 courses of O-FC therapy and 79% completed 4 or more courses (Table 1). The most common reason for premature treatment discontinuation was prolonged cytopenia, occurring in 38% of patients receiving 3 or fewer courses of O-FC (3 patients with grade 4 neutropenia, 1 patient with grade 3 thrombocytopenia, and 1 patient with grade 3 leukopenia) and in 56% of patients receiving 4-5 courses of O-FC (3 patients with grade 3 or 4 neutropenia and 2 patients with grade 2 thrombocytopenia; supplemental Table A1 [available on the Blood Web site; see the Supplemental Materials link at the top of the online article]).
Pretreatment patient characteristics
| . | Ofatumumab 500 mg (n = 31) . | Ofatumumab 1000 mg (n = 30) . |
|---|---|---|
| Characteristic, median (range) | ||
| Age, y | 56 (38-73) | 56 (38-72) |
| β-2M, mg/L | 4 (1.8-11.5) | 4 (2.1-10.7) |
| Lactate dehydrogenase, IU/L | 216 (125-566) | 234 (147-1144) |
| Thymidine kinase, IU/L | 23 (5-171) | 32 (5-201) |
| Lymphocytes, ×109/L | 93 (4-302) | 77 (8-307) |
| Neutrophils, ×109/L | 5 (1.4-13.9) | 4.4 (0.9-13.4) |
| Hemoglobin, g/dL | 12 (8.0-14.9) | 12.3 (6.6-15.8) |
| Platelets, ×109/L | 168 (58-351) | 135 (50-318) |
| Characteristic, no. of patients (%) | ||
| Rai stage III-IV | 12 (39) | 16 (53) |
| Binet stage C | 8 (26) | 12 (40) |
| β-2M > 3.5 mg/L | 19 (61) | 20 (67) |
| ECOG performance status 1-2 | 15 (48) | 12 (40) |
| Palpable lymph nodes > 5 cm | 5 (16) | 5 (17) |
| CD38-positive (≥ 30%) | 8 (26) | 4 (13) |
| Unmutated IGHV* | 16 (52) | 9 (30) |
| FISH genomic abnormality† | ||
| 17p del | 2 (6) | 6 (20) |
| 11q del | 7 (23) | 3 (10) |
| Trisomy 12 | 4 (13) | 5 (17) |
| No abnormality | 5 (16) | 2 (7) |
| 13q del (sole) | 12 (39) | 13 (43) |
| O-FC courses received, no. | ||
| 6 | 22 (71) | 17 (57) |
| 4-5 | 2 (6) | 7‡ (23) |
| 1-3 | 7 (23) | 6 (20) |
| . | Ofatumumab 500 mg (n = 31) . | Ofatumumab 1000 mg (n = 30) . |
|---|---|---|
| Characteristic, median (range) | ||
| Age, y | 56 (38-73) | 56 (38-72) |
| β-2M, mg/L | 4 (1.8-11.5) | 4 (2.1-10.7) |
| Lactate dehydrogenase, IU/L | 216 (125-566) | 234 (147-1144) |
| Thymidine kinase, IU/L | 23 (5-171) | 32 (5-201) |
| Lymphocytes, ×109/L | 93 (4-302) | 77 (8-307) |
| Neutrophils, ×109/L | 5 (1.4-13.9) | 4.4 (0.9-13.4) |
| Hemoglobin, g/dL | 12 (8.0-14.9) | 12.3 (6.6-15.8) |
| Platelets, ×109/L | 168 (58-351) | 135 (50-318) |
| Characteristic, no. of patients (%) | ||
| Rai stage III-IV | 12 (39) | 16 (53) |
| Binet stage C | 8 (26) | 12 (40) |
| β-2M > 3.5 mg/L | 19 (61) | 20 (67) |
| ECOG performance status 1-2 | 15 (48) | 12 (40) |
| Palpable lymph nodes > 5 cm | 5 (16) | 5 (17) |
| CD38-positive (≥ 30%) | 8 (26) | 4 (13) |
| Unmutated IGHV* | 16 (52) | 9 (30) |
| FISH genomic abnormality† | ||
| 17p del | 2 (6) | 6 (20) |
| 11q del | 7 (23) | 3 (10) |
| Trisomy 12 | 4 (13) | 5 (17) |
| No abnormality | 5 (16) | 2 (7) |
| 13q del (sole) | 12 (39) | 13 (43) |
| O-FC courses received, no. | ||
| 6 | 22 (71) | 17 (57) |
| 4-5 | 2 (6) | 7‡ (23) |
| 1-3 | 7 (23) | 6 (20) |
FISH indicates fluorescence in situ hybridization.
Defined as ≥ 98% homology to closest germline sequence.
Based on Dohner hierarchy.24
Two patients in the 1000-mg cohort received all 6 infusions of ofatumumab but no fludarabine and cyclophosphamide during treatment course 6.
Efficacy
The CR rate (95% CI) by independent review committee evaluation was 32% (17%-51%) in the ofatumumab 500-mg cohort and 50% (31%-69%) in the 1000-mg cohort. The OR rate (95% CI) was 77% (59%-90%) and 73% (54%-88%), respectively, with one nodular partial response in each cohort. Differences in response rates were not statistically significant, although the study was not designed to detect differences in end points between dose cohorts. Compared with studies of chemoimmunotherapy in frontline CLL,12,15 a higher proportion of patients in this study had baseline β-2M levels of > 3.5 mg/L (61% in the 500-mg cohort and 67% in the 1000-mg cohort) and 17p deletion (6% in the 500-mg cohort and 20% in the 1000-mg cohort), indicating that this was a higher-risk group of frontline patients.
Principal investigator and sponsor review of data for the 19 patients who achieved partial response revealed that 8 of these patients had persistent cytopenias (n = 5 in the 500-mg cohort and n = 3 in the 1000-mg cohort) with no evidence of residual disease. These patients fulfilled all criteria for CR with recovery of blood counts during the follow-up period (month 6 or beyond). In addition, 3 patients had no evidence of residual CLL but did not have a confirmatory bone marrow evaluation. Of the 8 patients who achieved PR with evidence of residual disease, 5 had disease in the marrow with > 30% lymphocytes, 2 had splenomegaly, and 1 had an elevated lymphocyte count.
Five patients (n = 3 in the 500-mg cohort and n = 2 in the 1000-mg cohort) had stable disease, 4 of whom received only 2 courses of treatment; 3 patients had persistent disease in the lymph nodes, and 1 patient had persistent disease in the blood. For the patient with stable disease who received 6 courses, persistent hepatomegaly was observed. Among the 7 patients (n = 2 in the 500-mg cohort and n = 5 in the 1000-mg cohort) who had progressive disease, 3 (all in the 1000-mg cohort) had both 17p deletion and elevated (> 4 mg/L) β-2M and 2 patients had elevated β-2M. The predominant site for disease progression was the lymph nodes in 6 patients and the spleen in 1 patient. Three patients (n = 2 in the 500-mg cohort and n = 1 in the 1000-mg cohort) who received only 1 course of O-FC were not evaluable for response and were considered treatment failures.
The CR and OR rates were 41% and 75%, respectively, for the dose cohorts combined. Response rates by patient subgroups are shown in Table 2. Serum β-2M levels of at least 4 mg/L and 17p deletion were associated with lower CR rates. Both the CR and OR rates were lower for patients who received 3 courses of treatment or fewer. Exploratory univariable regression analyses were performed for all characteristics (as continuous variables for noncategorical items) listed in Table 2. Results showed a higher likelihood for CR associated with lower pretreatment β-2M and thymidine kinase (Table 3). A higher likelihood for response was associated with lower β-2M and a smaller sum product of lymph node diameters by physical examination at screening. Finally, longer PFS was associated with lower β-2M level, lower serum lactate dehydrogenase, and ECOG performance status of 0 at screening. Completion of 6 O-FC courses was associated with higher CR and OR rates and longer PFS (Table 3). The median duration of response, time-to-next treatment, PFS (Figure 2A), and OS (Figure 2B) could not be estimated with a median follow-up of 8 months.
Responses to O-FC by pretreatment characteristics and number of treatment courses received
| Characteristic . | n* . | CR rate, % . | OR rate, % . |
|---|---|---|---|
| All patients | 61 | 41 | 75 |
| Sex | |||
| Male | 43 | 35 | 70 |
| Female | 18 | 56 | 89 |
| Age, y | |||
| < 65 | 50 | 44 | 76 |
| ≥ 65 | 11 | 27 | 73 |
| ECOG performance status | |||
| 0 | 34 | 47 | 82 |
| 1-2 | 27 | 33 | 67 |
| Palpable lymph nodes | |||
| ≤ 5 cm | 51 | 39 | 78 |
| > 5 cm | 10 | 50 | 60 |
| Rai stage | |||
| 0-II | 33 | 36 | 73 |
| III-IV | 28 | 46 | 79 |
| Binet stage | |||
| A | 8 | 38 | 75 |
| B | 33 | 42 | 79 |
| C | 20 | 40 | 70 |
| β-2M† | |||
| < 4 mg/L | 32 | 53 | 84 |
| ≥ 4 mg/L | 28 | 29 | 68 |
| Lactate dehydrogenase† | |||
| < 228 IU/L | 30 | 40 | 77 |
| ≥ 228 IU/L | 31 | 42 | 74 |
| Thymidine kinase† | |||
| < 29 IU/L | 29 | 45 | 76 |
| ≥ 29 IU/L | 30 | 40 | 80 |
| ALC† | |||
| < 90 × 109/L | 31 | 45 | 81 |
| ≥ 90 × 109/L | 30 | 37 | 70 |
| CD38 expression (%) | |||
| < 30 | 48 | 44 | 77 |
| ≥ 30 | 12 | 33 | 67 |
| IGHV mutation status | |||
| Mutated | 28 | 46 | 75 |
| Unmutated‡ | 25 | 36 | 84 |
| FISH genomic abnormalitym§ | |||
| 17p del | 8 | 13 | 63 |
| 11q del | 10 | 40 | 70 |
| Trisomy 12q | 8 | 63 | 63 |
| No abnormality | 7 | 71 | 100 |
| 13q del (sole) | 25 | 32 | 80 |
| O-FC courses received, no. | |||
| 6 | 39 | 56‖ | 92‖ |
| 4-5 | 9 | 33 | 78 |
| 1-3 | 13 | 0 | 23 |
| Characteristic . | n* . | CR rate, % . | OR rate, % . |
|---|---|---|---|
| All patients | 61 | 41 | 75 |
| Sex | |||
| Male | 43 | 35 | 70 |
| Female | 18 | 56 | 89 |
| Age, y | |||
| < 65 | 50 | 44 | 76 |
| ≥ 65 | 11 | 27 | 73 |
| ECOG performance status | |||
| 0 | 34 | 47 | 82 |
| 1-2 | 27 | 33 | 67 |
| Palpable lymph nodes | |||
| ≤ 5 cm | 51 | 39 | 78 |
| > 5 cm | 10 | 50 | 60 |
| Rai stage | |||
| 0-II | 33 | 36 | 73 |
| III-IV | 28 | 46 | 79 |
| Binet stage | |||
| A | 8 | 38 | 75 |
| B | 33 | 42 | 79 |
| C | 20 | 40 | 70 |
| β-2M† | |||
| < 4 mg/L | 32 | 53 | 84 |
| ≥ 4 mg/L | 28 | 29 | 68 |
| Lactate dehydrogenase† | |||
| < 228 IU/L | 30 | 40 | 77 |
| ≥ 228 IU/L | 31 | 42 | 74 |
| Thymidine kinase† | |||
| < 29 IU/L | 29 | 45 | 76 |
| ≥ 29 IU/L | 30 | 40 | 80 |
| ALC† | |||
| < 90 × 109/L | 31 | 45 | 81 |
| ≥ 90 × 109/L | 30 | 37 | 70 |
| CD38 expression (%) | |||
| < 30 | 48 | 44 | 77 |
| ≥ 30 | 12 | 33 | 67 |
| IGHV mutation status | |||
| Mutated | 28 | 46 | 75 |
| Unmutated‡ | 25 | 36 | 84 |
| FISH genomic abnormalitym§ | |||
| 17p del | 8 | 13 | 63 |
| 11q del | 10 | 40 | 70 |
| Trisomy 12q | 8 | 63 | 63 |
| No abnormality | 7 | 71 | 100 |
| 13q del (sole) | 25 | 32 | 80 |
| O-FC courses received, no. | |||
| 6 | 39 | 56‖ | 92‖ |
| 4-5 | 9 | 33 | 78 |
| 1-3 | 13 | 0 | 23 |
ALC indicates absolute lymphocyte count; and FISH, fluorescence in situ hybridization.
Patient numbers do not add up to N = 61 for some of the categories due to missing assessments.
Cutoff values were based on median levels at baseline.
Defined as ≥ 98% homology to closest germline sequence.
Based on Dohner hierarchy.24
P < .05, Fisher's exact test.
Univariable logistic and Cox regression analyses for clinical outcomes
| Outcome . | n . | Odds or hazard ratio . | P . |
|---|---|---|---|
| CR | |||
| β-2M*† | 60 | < 1.000 | .022 |
| Thymidine kinase*† | 59 | 0.983 | .044 |
| Completion of 6 O-FC courses | 61 | 7.027 | .002 |
| OR | |||
| β-2M*† | 60 | < 1.000 | .010 |
| βSPD of lymph nodes by physical exam*† | 61 | 0.983 | .034 |
| Completion of 6 O-FC courses | 61 | 11.556 | < .001 |
| PFS | |||
| β-2M*† | 60 | > 1.000 | .007 |
| Lactate dehydrogenase*† | 61 | 1.005 | .009 |
| ECOG performance status 0† | 61 | 0.139 | .013 |
| Completion of 6 O-FC courses | 61 | 0.138 | .004 |
| Outcome . | n . | Odds or hazard ratio . | P . |
|---|---|---|---|
| CR | |||
| β-2M*† | 60 | < 1.000 | .022 |
| Thymidine kinase*† | 59 | 0.983 | .044 |
| Completion of 6 O-FC courses | 61 | 7.027 | .002 |
| OR | |||
| β-2M*† | 60 | < 1.000 | .010 |
| βSPD of lymph nodes by physical exam*† | 61 | 0.983 | .034 |
| Completion of 6 O-FC courses | 61 | 11.556 | < .001 |
| PFS | |||
| β-2M*† | 60 | > 1.000 | .007 |
| Lactate dehydrogenase*† | 61 | 1.005 | .009 |
| ECOG performance status 0† | 61 | 0.139 | .013 |
| Completion of 6 O-FC courses | 61 | 0.138 | .004 |
SPD indicates sum of product of diameters.
Evaluated as continuous variables.
At baseline.
PFS and OS in patients treated with O-FC. (A) PFS in patients (N = 61) treated with O-FC. PFS was defined as the time from randomization until disease progression or death. Median follow-up time was 8 months. (B) OS in patients (N = 61) treated with O-FC. OS was defined as the time from randomization until death. Median follow-up time was 8 months.
PFS and OS in patients treated with O-FC. (A) PFS in patients (N = 61) treated with O-FC. PFS was defined as the time from randomization until disease progression or death. Median follow-up time was 8 months. (B) OS in patients (N = 61) treated with O-FC. OS was defined as the time from randomization until death. Median follow-up time was 8 months.
Safety
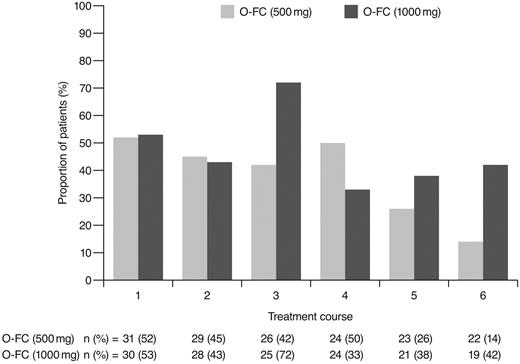
Ofatumumab infusion–related reactions were common and primarily occurred with the first and second courses and decreased with subsequent infusions; all were grade 1 or 2 (Figure 3). The most commonly reported (> 15% of all patients) adverse events (all grades) from the start of treatment until 30 days after the last dose of treatment were neutropenia (48%), infection (38%), nausea (41%), thrombocytopenia (26%), rash (25%), vomiting (23%), fever (21%), headache (18%), and fatigue (18%). The most common grade 3 or 4 reported adverse events are shown in Table 4. Hematologic toxicity by laboratory evaluation (using CTCAE version 3.0 grading) showed that 87% of patients in each dose cohort had at least one event of grade 3 or 4 neutropenia during treatment. Based on laboratory values, 16% of patients in the 500-mg cohort and 27% in the 1000-mg cohort had grade 3 or 4 anemia, and 10% and 27%, respectively, had grade 3 or 4 thrombocytopenia. A possible explanation for the incidence of laboratory-assessed cytopenia with O-FC was the presence of pretreatment baseline cytopenia in 29% of patients in the 500-mg cohort and in 33% of patients in the 1000-mg cohort (data not shown). A total of 26% of patients had baseline grade 1 anemia, 10% had baseline grade 1 thrombocytopenia, and 7% had baseline grade 1 neutropenia as assessed using laboratory values and CTCAE version 3.0 grading.
Ofatumumab infusion–related reactions over the course of treatment. All patients received ofatumumab 300 mg for course 1. All infusion reactions on the day of ofatumumab infusion were CTC grade 1 or 2 events.
Ofatumumab infusion–related reactions over the course of treatment. All patients received ofatumumab 300 mg for course 1. All infusion reactions on the day of ofatumumab infusion were CTC grade 1 or 2 events.
Most common investigator-reported* grade 3-4 adverse events with O-FC
| Adverse event, no. of patients (%) . | Ofatumumab 500 mg (n = 31) . | Ofatumumab 1000 mg (n = 30) . |
|---|---|---|
| Neutropenia | 11 (35) | 18 (60) |
| Thrombocytopenia | 2 (6) | 7 (23) |
| Anemia | 2 (6) | 6 (20) |
| Febrile neutropenia | 3 (10) | 3 (10) |
| Infections | 1 (3) | 4 (13) |
| Sepsis | 0 (0) | 2 (7) |
| Herpes virus | 1 (3) | 0 (0) |
| Respiratory | 0 (0) | 1 (3) |
| Unspecified | 0 (0) | 1 (3) |
| Adverse event, no. of patients (%) . | Ofatumumab 500 mg (n = 31) . | Ofatumumab 1000 mg (n = 30) . |
|---|---|---|
| Neutropenia | 11 (35) | 18 (60) |
| Thrombocytopenia | 2 (6) | 7 (23) |
| Anemia | 2 (6) | 6 (20) |
| Febrile neutropenia | 3 (10) | 3 (10) |
| Infections | 1 (3) | 4 (13) |
| Sepsis | 0 (0) | 2 (7) |
| Herpes virus | 1 (3) | 0 (0) |
| Respiratory | 0 (0) | 1 (3) |
| Unspecified | 0 (0) | 1 (3) |
Reported in ≥ 10% of patients in either dose cohort from start of treatment until 30 days following completion of treatment; grading by CTCAE Version 3.0.
Fludarabine and cyclophosphamide dose reductions or withholding occurred in 14 patients, primarily due to cytopenias (n = 12); 6 patients withdrew from treatment despite dose reductions. In the 500-mg cohort, 9 patients withdrew from treatment for the following reasons: cytopenias (n = 3), autoimmune hemolytic anemia requiring treatment (n = 2), myocardial infarction (n = 1), nonresponse (n = 2), or patient request (n = 1). In the 1000-mg cohort, 13 patients withdrew for the following reasons: cytopenias (n = 7), autoimmune hemolytic anemia requiring treatment (n = 1), nonresponse (n = 1), chest discomfort (n = 1), patient request (n = 1), death (n = 1), or investigator's decision (n = 1; Table 5). Age (< 65 years vs ≥ 65 years) was not significantly correlated with the ability to receive all 6 cycles of O-FC when considering patients who developed grade 3 or 4 neutropenia by laboratory evaluation within 30 days of the last infusion (74% vs 45%: P = .081).
Summary of treatment withdrawals and number of O-FC courses completed
| Number of courses received and reason for treatment withdrawal . | No. of patients . | ||
|---|---|---|---|
| Ofatumumab 500 mg (n = 31) . | Ofatumumab 1000 mg (n = 30) . | Total (N = 61) . | |
| Did not receive 6 courses of O-FC | 9 | 13 | 22 |
| Received 3 or fewer courses of O-FC due to: | |||
| Cytopenias | 2 | 3 | 5 |
| AIHA | 1 | 1 | |
| Nonresponse | 2 | 1 | 3 |
| Patient request | 1 | 1 | |
| Myocardial infarction | 1 | 1 | |
| Chest discomfort | 1 | 1 | |
| Death | 1 | 1 | |
| Received 4-5 courses of O-FC due to: | |||
| Cytopenias | 1 | 4* | 5* |
| AIHA | 1 | 1 | 2 |
| Investigator decision | 1 | 1 | |
| Patient request | 1 | 1 | |
| Number of courses received and reason for treatment withdrawal . | No. of patients . | ||
|---|---|---|---|
| Ofatumumab 500 mg (n = 31) . | Ofatumumab 1000 mg (n = 30) . | Total (N = 61) . | |
| Did not receive 6 courses of O-FC | 9 | 13 | 22 |
| Received 3 or fewer courses of O-FC due to: | |||
| Cytopenias | 2 | 3 | 5 |
| AIHA | 1 | 1 | |
| Nonresponse | 2 | 1 | 3 |
| Patient request | 1 | 1 | |
| Myocardial infarction | 1 | 1 | |
| Chest discomfort | 1 | 1 | |
| Death | 1 | 1 | |
| Received 4-5 courses of O-FC due to: | |||
| Cytopenias | 1 | 4* | 5* |
| AIHA | 1 | 1 | 2 |
| Investigator decision | 1 | 1 | |
| Patient request | 1 | 1 | |
AIHA indicates autoimmune hemolytic anemia.
Two patients in the 1000-mg cohort received all 6 infusions of ofatumumab but no fludarabine and cyclophosphamide during treatment course 6 due to cytopenias.
Two patients died during the study. One patient (500-mg cohort) died due to febrile neutropenia during the follow-up period 50 days after the sixth ofatumumab infusion. One patient (1000-mg cohort) died due to dyspnea (etiology unknown) during the treatment period 19 days after the second ofatumumab infusion. A third patient (1000-mg cohort) died after withdrawal from the study 186 days after the second ofatumumab infusion; this death was related to neutropenia after alternative therapy for progressive disease.
Discussion
The results from the present study demonstrate that O-FC is an active frontline chemoimmunotherapy regimen for patients with CLL. Both dose cohorts showed significant activity with O-FC. Although the trial was not powered to compare outcomes and the CR rate was not statistically different between the cohorts, there was a trend for a higher CR rate with the ofatumumab 1000-mg (50%) cohort compared with the 500-mg (32%) cohort. In addition, principal investigator and sponsor review of the data determined that 5 patients (16%) in the 500-mg cohort and 3 (10%) in the 1000-mg cohort could be considered a CR with incomplete bone marrow recovery at response assessment according to the 2008 NCI-WG guidelines.23 The flat 1000-mg dose is similar to the rituximab dose used in the FCR regimen for courses 2 to 6, with a lower dose of ofatumumab administered for course 1 (300 mg vs 375 mg/m2 with rituximab); the 50% CR rate with O-FC is comparable to that reported with the phase 3 CLL8 frontline FCR study (44% CR).15
The OR rate observed with O-FC in this study (73% and 77%) was lower than that reported with other frontline chemoimmunotherapy regimens.9-12,15 The lower OR rate in our study may have been related to the higher-risk profile of this patient population and to response being evaluated by an independent review committee. Elevated β-2M identified patients at increased risk for lower response and shorter PFS with frontline chemoimmunotherapy.12,15 The median β-2M of 4 mg/L and the proportion of patients with β-2M greater than 3.5 mg/L in our study (64%) were higher than those for patients evaluated in frontline FCR or FCM-R trials.12-15 Results from univariable regression analyses in our study showed that the increased β-2M level was significantly correlated with lower CR and OR rates and shorter PFS. Furthermore, deletion of chromosome 17p is a high-risk feature associated with poor outcomes across treatments, including the FCR and FCM-R regimens.12,13,15 In our study, 17p deletion was detected in 13% of patients, including 20% of patients in the 1000-mg cohort, which was higher than the expected 4%-8% incidence of 17p deletion reported in previously untreated patients.6,8,12,13,15,24 The small patient numbers in our study limited our ability to identify pretreatment factors associated with responses, and the current short follow-up time limits time-to-event analyses.
As expected, ofatumumab infusion–related reactions were mainly observed with the first and second infusions; all were grade 1 or 2 events. No apparent differences were observed in the frequency or severity of these reactions between dose cohorts. The overall incidence of grade 3 or 4 adverse events (70%) appeared comparable to that reported for frontline FCR (77%).15 Investigator-reported myelosuppression was the most frequent grade 3 or 4 adverse event with O-FC, with grade 3 or 4 neutropenia reported in 48% of all patients. Frontline FC can be associated with substantial myelosuppression, with grade 3 or 4 neutropenia reported in 21%-69% of patients.6,8,15 In the phase 3 CLL8 trial, the incidence of investigator-reported grade 3 or 4 neutropenia adverse events was significantly higher in the FCR arm compared with the FC arm (34% vs 21%; P < .0001),15 and was comparable to that observed in this trial. The mechanism for increased neutropenia with the addition of CD20 mAb is unknown and requires further investigation. Based on available laboratory assessments, grade 3 or 4 neutropenia occurred in 87% of patients in each cohort, suggesting that the incidence of neutropenia was likely independent of the ofatumumab dose level (Figure 4). Although the incidence of grade 3 or 4 anemia and thrombocytopenia appeared higher in the 1000-mg cohort, the differences were not statistically significant. These grade 3 or 4 events primarily occurred during the first treatment course and were uncommon in subsequent courses (Figures 5 and 6). Grade 3 or 4 infections were observed in 8% of patients (13% of patients in the 1000-mg cohort), and these infection rates appeared favorable relative to the 26% incidence reported with the FCR regimen.15 Moreover, despite the 87% incidence of grade 3 or 4 neutropenia based on laboratory assessments, infections were manageable, with only 2 patients experiencing sepsis.
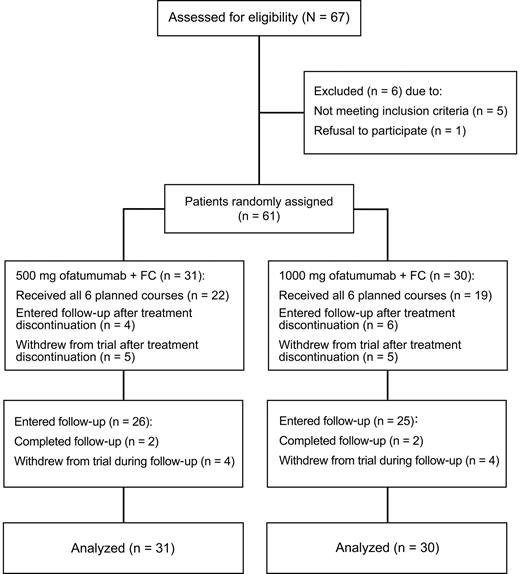
Incidence of laboratory-assessed CTC grade 3 or 4 neutropenia by treatment course. Laboratory values were assigned to treatment course based on date of laboratory measurement in relation to ofatumumab infusion.
Incidence of laboratory-assessed CTC grade 3 or 4 neutropenia by treatment course. Laboratory values were assigned to treatment course based on date of laboratory measurement in relation to ofatumumab infusion.
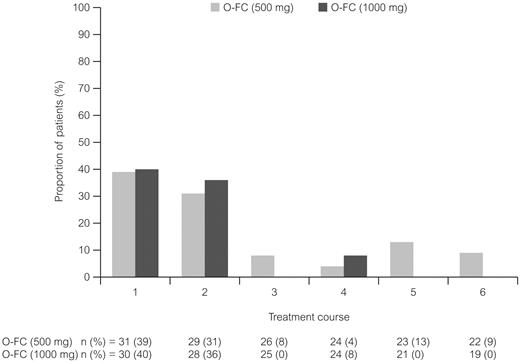
Incidence of laboratory-assessed CTC grade 3 or 4 anemia by treatment course. Laboratory values were assigned to treatment course based on date of laboratory measurement in relation to ofatumumab infusion.
Incidence of laboratory-assessed CTC grade 3 or 4 anemia by treatment course. Laboratory values were assigned to treatment course based on date of laboratory measurement in relation to ofatumumab infusion.
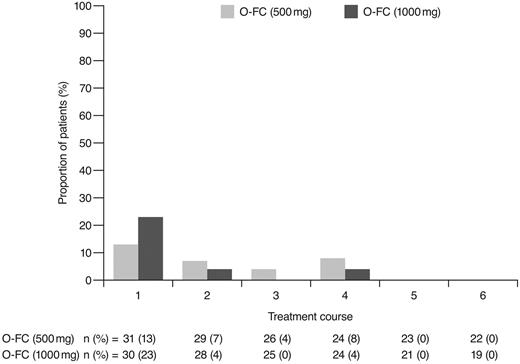
Incidence of laboratory-assessed CTC grade 3 or 4 thrombocytopenia by treatment course. Laboratory values were assigned to treatment course based on date of laboratory measurement in relation to ofatumumab infusion.
Incidence of laboratory-assessed CTC grade 3 or 4 thrombocytopenia by treatment course. Laboratory values were assigned to treatment course based on date of laboratory measurement in relation to ofatumumab infusion.
Although one-third of our patients discontinued treatment, this was not unexpected given that 26% of patients were unable to complete all 6 courses of FCR in the phase 2 and 3 studies.11,12,15 Cytopenias leading to treatment withdrawal were less frequent among patients who received 3 or fewer courses of O-FC compared with those who received 4-5 courses (Table 5). In addition, the overall incidence of cytopenias leading to treatment withdrawal in our study (16%) was similar to that reported with FCR (13%).11,12
The high CR rate (up to 50%) despite the high-risk disease features seen in our patient population suggests that the O-FC regimen may provide another chemoimmunotherapy option for previously untreated patients with CLL who can tolerate potential myelosuppression. Adverse events were manageable, with no unexpected toxicities, and the safety profile of O-FC appeared to be consistent with other CD20 mAb–based chemoimmunotherapy regimens. Analyses of secondary end points are ongoing, including pharmacokinetic analyses. Further follow-up continues on this study to collect time-to-event end points, which will also be important in evaluating outcomes with O-FC treatment in the context of currently available frontline regimens. The 1000-mg dose of ofatumumab is being assessed in additional studies of combination regimens in patients with CLL.
The online version of this article contains a data supplement.
Data from this study were presented in part at the American Society of Hematology Annual Meeting, December 5-8, 2009, New Orleans, LA; the American Society of Clinical Oncology Annual Meeting, June 4-8, 2010, Chicago, IL; and the Congress of the European Hematology Association, June 10-13, 2010, Barcelona, Spain.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients and the following investigators in the Hx-CD20-407 study for their participation: from the Czech Republic, T. Kozák and M. Trněný; from Germany, U. Dührsen and N. Schmitz; from the United Kingdom, G. Follows and P. Hillmen; and from the United States, S. Gregory and L. C. Pinter-Brown. We also thank the Independent Data Monitoring Committee: H. Hagberg, P. Johnson, and B. Nilsson.
Editorial support in the form of data review and compilation, development and revisions of the outline, and subsequent manuscript drafts under the direction of the first author, incorporation of author comments, and development of tables and figures was provided by Maoko Naganuma (GlaxoSmithKline, Collegeville, PA). Editorial support in the form of development of outline and manuscript first draft, collation of author comments, copyediting, referencing, and graphic services was provided in part by Samantha Taylor, PhD, Medicus International New York, and was funded by GlaxoSmithKline. W.G.W. is a Leukemia & Lymphoma Society Clinical Scholar.
This study was cosponsored by Genmab A/S and GlaxoSmithKline.
Authorship
Contribution: W.G.W. conceived and designed the study; J.D., F.J.H.-I., G.H., T.J.K., J.M., S.P., L.S., S.S., and W.G.W. provided study materials or patients; G.C., J.D., I.G., R.G., T.J.K., J.M., C.A.R., and W.G.W. collected and assembled data; C.-N.C., G.C., I.G., M.G., F.J.H.-I., T.N., S.P., C.A.R., S.S., and W.G.W. analyzed and interpreted data; G.C., I.G., M.G., G.H., T.J.K., T.N., S.P., C.A.R., and W.G.W. prepared the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: G.C., C.-N.C, I.G., and M.G. are employees of GlaxoSmithKline; G.C., I.G., and M.G. are GlaxoSmithKline stockholders; T.N. and C.A.R. are former employees of Genmab; C.A.R. is a Genmab stockholder; F.J.H.-I. was a consultant for Amgen; J.M. has consulted or advised for GlaxoSmithKline; J.D. has received honoraria from GlaxoSmithKline; L.S. has consulted or advised for GlaxoSmithKline and received honoraria from Genzyme, Bayer-Schering Pharma, and Roche; S.S. has consulted or advised for GlaxoSmithKline and Genmab, received honoraria from GlaxoSmithKline and Genmab, received research funding from GlaxoSmithKline and Genmab; S.P. has consulted or advised for GlaxoSmithKline and received honoraria from GlaxoSmithKline; and W.G.W. has received honoraria and research funding from GlaxoSmithKline. The remaining authors declare no competing financial interests.
A complete list of the members of the 407 Study Investigators appears in the online supplemental Appendix.
Correspondence: William G. Wierda, University of Texas, M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: wwierda@mdanderson.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal