Abstract
Lymphoid hyperplasia of gastric mucosa associated with Helicobacter pylori (HP) infection represents a preneoplastic condition of the mucosa associated lymphoid tissue (MALT), which may evolve to a B-cell lymphoma. While it is well established that the initial neoplastic proliferation of B cells is antigen-driven and dependent on the helper activity of HP-specific T cells, it needs to be elucidated which cytokine or soluble factor(s) promote B-cell activation and lymphomagenesis. Herein, we originally report that gastric MALT lymphoma express high levels of a proliferation inducing ligand (APRIL), a novel cytokine crucial in sustaining B-cell proliferation. By immunohistochemistry, we demonstrate that APRIL is produced almost exclusively by gastric lymphoma-infiltrating macrophages located in close proximity to neoplastic B cells. We also show that macrophages produce APRIL on direct stimulation with both HP and HP-specific T cells. Collectively, our results represent the first evidence for an involvement of APRIL in gastric MALT lymphoma development in HP-infected patients.
Introduction
Helicobacter pylori (HP) colonizes the human gastric mucosa and triggers a strong local inflammatory response.1 Chronic inflammation because of the persistence of HP infection can give rise to organized lymphoid tissue in the gastric mucosa, the so-called mucosa-associated lymphoid tissue (MALT) that, in a small subset of individuals, can ultimately progress to low-grade gastric B-cell lymphoma of MALT type.2 The current model of MALT lymphoma genesis assumes that one or more neoplastic clones, displaying characteristics of marginal zone B cells, originate from the organized MALT, colonize and replace the original follicles and eventually destroy the gastric glands to form lympho-epithelial lesions.3 Although MALT lymphoma usually grows slowly and has a low propensity to spread, a small percentage of cases undergo high-grade transformation. It is generally accepted that, in early stages of gastric lymphoma development, neoplastic growth is both antigen-driven and dependent of the helper activity of T cells specific for HP.2,4 The fact that the eradication of the bacterium leads to regression of the lymphoma, especially in its early stages, is consistent with such a postulate.5,6 However, less is known about the role of the host immune response in MALT lymphoma pathogenesis.
A proliferation inducing ligand (APRIL) is one of the most recently cloned members of the tumor necrosis factor (TNF) family7-10 expressed by a variety of immune cells including neutrophils, monocytes, macrophages, dendritic cells, and T cells, but also epithelial cells and tumor cells. By binding to its BCMA and TACI receptors, APRIL promotes survival and proliferation of B cells as well as their differentiation to plasma cells. As recently reported, APRIL-transgenic mice develops lymphoid malignancies originating from peritoneal B-1 B cells.11 In addition, not only APRIL is expressed in a wide array of B-cell malignancies, including Hodgkin lymphomas,12 but high levels of APRIL in the serum of these patients correlate with poor prognosis.13 Consistently, in vitro experiments have clearly shown that APRIL promotes cell survival and proliferation of neoplastic B-cells (reviewed by Planelles et al13 and Roosnek et al14 ). Interestingly, APRIL expression in human non-Hodgkin B-cell lymphomas is found in neoplastic cells as well as in associated inflammatory cells, predominantly neutrophils.15
Herein, we report that gastric MALT lymphomas are particularly enriched in APRIL-expressing macrophages. We also show that, in vitro, HP triggers an abundant release of APRIL by human monocyte-derived macrophages and that HP-specific T cells contribute to create an APRIL-enriched environment in MALT lymphoma. Collectively, our results provide the first evidence that APRIL may be involved in MALT lymphoma development in HP-infected patients.
Methods
Tissues
Tissues were obtained from the archive of the Department of Pathology (Spedali Civili di Brescia, Brescia, Italy, San Raffaele Hospital, Milan, Italy and Padova Hospital). Samples included normal gastric mucosa (5 cases), HP-associated gastric lymphoid infiltrates Wotherspoon-Isaacson (WI)6 grades 1 (5 cases), 3 (3 cases), 4 (2 cases), and 5 (9 cases, resection specimens), gastric diffuse large B-cell lymphomas (5 cases) and HP-negative gastritis (6 cases). In the latter group HP was assessed by histology (modified Giemsa staining) and confirmed by clinical history, rapid urease testing, and/or ELISA (Hp-specific IgG antibodies; Gastropanel, Biohit). A second group of samples consisted of matched endoscopic biopsies (pre- and posttreatment) from 6 patients with gastric MALT lymphoma who underwent clinical and histologic remission after HP-eradication therapy. WI-5 cases lack AP12/MALT1 translocation as proved by FISH analysis (MALT1 FISH deoxyribonucleic acid Probe; Split Signal; Dako Cytomation) and the large majority of them (7 of 9; 78%) show IgH clonal rearrangements. For the IgH clonality assay, genomic deoxyribonucleic acid was extracted from tumor tissue sections using the QIAamp Tissue Kit (QIAGEN). PCR was performed using genomic deoxyribonucleic acid (200 ng) with primers targeting the variable region FR2 or FR3 and joining regions of the IgH gene. The amplified products were visualized in 10% polyacrylamide gel.
Immunohistochemistry
Four micron formalin-fixed paraffin embedded tissue sections were stained with anti-APRIL (Ms IgG1, Clone Aprily-2, Alexis Biochemicals) and anti-HP TMDU (Ms, Clone W4-1; provided by Prof Eishi, Department of Human Pathology, Graduate School of Medical Science, Tokyo Medical and Dental University, Tokyo, Japan).16 On appropriate antigen retrieval (water bath at 98°C for 40 minutes in ethylenediaminetetraacetic acid buffer pH 8.0), reactivity was revealed using NovoLink Polymer horseradish peroxidase linked (Novocastra Laboratories) followed by Diaminobenzidine (DAB). Characterization of APRIL positive cells was performed by double immunohistochemistry. After completing the first immune reaction, the second was realized using primary antibodies to the following antigens: CD11c (Clone 5D11; Novocastra Laboratories Ltd); CD68 (Clone KP-1; Dako); CD20 (Clone L26; Dako); CD163 (Clone 10D6; Thermo Scientific); CD1a (Clone 010; Dako); CD15 (Clone MMA; Thermo Scientific) and visualized using Mach 4-AP (Biocare Medical), followed by Ferangi Blue (Biocare Medical) as chromogen. Quantification of APRIL-expressing cells was performed on at least 5 HPF (high power field) on sections double stained for APRIL and CD11c. Immunostained sections were photographed using the DP-70 Olympus digital camera mounted on the Olympus BX60 microscope, and the digital pictures (each corresponding to 0.036 mm2) were used for cell count. Values were expressed as the mean ± SD.
Bacteria and culture conditions
HP strain 342 was maintained in 5% CO2 at 37°C on Columbia agar plates supplemented with 5% horse blood. Colonies were inoculated into brain heart infusion broth containing 5% fetal bovine serum and were cultured for 2 days in rotary shaking at 180 revolutions per minute at 37°C under microaerophilic conditions.
Macrophage differentiation
Monocytes (5 × 105) isolated form buffy-coat and seeded in 24-well plates were cultured in RPMI 20% FBS in the presence of macrophage colony stimulating factor (M-CSF; Immunologic Science; 100 ng/mL) for a 6-day differentiation to M-CSF–differentiated human macrophages (MDM). MDM were cultured for 1 day in culture medium without M-CSF, before being exposed to bacteria (5 × 105 colony-forming unit/mL).
Real-time PCR analysis
Total RNA was isolated from MDM using TRIzol solution (Invitrogen) according to the manufacturer's instructions. RNA was reverse-transcribed and amplified with the following primers: for GAPDH, 5′-AGCAACAGGGTGGTGGAC-3′ and 5′-GTGTGGTGGGGGACTGAG-3′; for APRIL, 5′-AAGGGTATCCCTGGCAGAGT-3′ and 5′-GCAGGACAGAGTGCTGCTT-3′. After the amplification, data analysis was performed using the second derivative method algorithm. For each sample, the amount of messenger RNA (mRNA) of APRIL was expressed as the n-fold of the normalized amount of mRNA in untreated cells (1 arbitrary unit = APRIL mRNA concentration/GAPDH mRNA concentration [both in fmoles/μL]).
Generation of gastric T-cell clones and assay for T-cell clone helper function for APRIL production
On approval of the local Ethical Committee (Department of Internal Medicine, University of Firenze), 5 HP-positive patients with gastric low-grade MALT lymphoma and 5 patients with uncomplicated HP-positive chronic gastritis gave their informed consent and were enrolled in the study. None of the patients had taken antibiotics or gastric proton pump inhibitors within 2 months before the study. Biopsy specimens were obtained during endoscopy from the gastric mucosa for (1) histology and HP detection, (2) rapid urease test, and (3) culture of infiltrating lymphocytes. Diagnosis of HP infection was based on positive urease test, histologic detection of HP, and positive 13C-urea breath test. Biopsy specimens of gastric mucosa were cultured for 7 days in complete medium supplemented with IL-2 (50 U/mL) to preferentially expand in vivo–activated T cells. Mucosal specimens were then disrupted, and single T-cell blasts were cloned by limiting dilution (0.3 cells/well) in round-bottom microwell plates containing 105 irradiated PBMCs as feeder cells, phytohaemagglutinin (0.5% vol/vol; Life Technologies) and IL-2 (20 U/mL). Cell surface marker analysis of T-cell clones was performed on a BD FACSCalibur, according to the CellQuest Version 5.1 software, using anti-CD3, anti-CD4, and anti-CD8 mAb purchased from BD. T-cell clones were then screened in triplicate cultures for responsiveness to medium or HP lysate (10 μg/mL) by measurement of [3H]thymidine deoxyribose uptake after 60 hours in the presence of autologous PBMCs (5 × 104) as antigen presenting cells. Mitogenic index (ratio of mean counts per minute of stimulated to unstimulated cultures) > 5 were considered positive. T-cell blasts of HP-specific clones (8 × 105/mL) were cocultured for 16 hours with autologous macrophages (4 × 105/mL) in the presence of medium or HP antigen (10 μg/mL).
APRIL protein detection in cells and in culture supernatant
For the evaluation of the intracellular APRIL content, cells were collected and lysed in 20mM tris(hydroxymethyl)aminomethane, 150mM NaCl 0.25% NP40 at 4°C. Five micrograms of proteins were loaded on 4%-12% SDS-PAGE and transferred on nitrocellulose. APRIL was revealed using a specific mAb (Alexis Biochemical). The amount of extracellular APRIL was quantified by a commercial ELISA (Invitrogen), according to the manufacturer's instructions. For the evaluation of the intracellular APRIL content, MDM exposed to HP were spread by cytospin on slides and stained for APRIL as indicated in “Immunohistochemistry.”
Statistical analysis
Data are reported as the mean ± SD. The Student t test and 1-way ANOVA were used for statistical analysis of the differences between experimental groups. P values ≤ .05 were considered significant.
Results
HP-associated gastritis and gastric MALT lymphoma are enriched in APRIL-containing tumor-associated macrophages
We initially evaluated by immunohistochemistry whether APRIL might be expressed in gastric MALT lymphomas. To this end, 9 cases of HP-associated WI-5 cases lacking the API2/MALT1 translocation, as detected by FISH analysis (not shown), were used. Compared with normal gastric mucosa, in which APRIL reactivity is scanty (Figure 1A), we found numerous cells reacting to anti-APRIL in all WI-5 cases (Figure 1B). The large proportion of APRIL+ cells showed a macrophage-like morphology and were admixed with CD20+ neoplastic B cells (Figure 1B inset) and to surrounding plasma cells (not shown). By double immunohistochemistry of 5 representative cases, APRIL+ cells were negative for lymphoid markers CD20 and CD3 (Figure 1B inset and data not shown) but strongly reacting to CD11c, CD68 and CD163 but not to CD1a, thus confirming their macrophage identity (Figure 1D-F and data not shown). In addition to macrophages, a very small fraction of CD15+ polymorphonuclear cells also reacted to APRIL (not shown), while, in all cases, no reactivity for APRIL was observed in the large majority of neoplastic cells (Figure 1B-F). A further characterization of APRIL reactivity revealed numerous APRIL+ macrophages also in HP-associated WI grade 1 to 4 (Figure 1C). Of note, the number of APRIL-producing macrophages was significantly higher in WI lesions compared with normal gastric mucosa and HP-negative gastritis, being the mean number of CD11c+ APRIL+ cells, respectively 22.2 [WI-1], 22.6 [WI-3/4], and 28.5 [WI-5] vs 7.2 [normal mucosa] and 9.2 [HP-negative gastritis] (P < .01); on the contrary, differences among WI groups were not significant (P = .07). Remarkably, in matched biopsies obtained from 6 MALT lymphoma patients, the number of CD11c+APRIL+ macrophages was dramatically reduced (19.6 vs 4.1; P < .01) in WI-5 cases, which underwent remission after HP-eradication treatment (Figure 1C). Notably, local production of APRIL was not exclusive of MALT lymphomas, because numerous CD11c+APRIL+ cells were observed also in cases of HP-negative gastric diffuse large B-cell lymphomas (not shown).
APRIL production by tissue macrophages. Sections are from normal gastric mucosa (A), WI grade 5 (B,D-F) and grade 1 (G-L). Compared with normal gastric mucosa where APRIL reactivity is observed in rare large cells of the lamina propria (A), numerous APRIL+ cells are found (B-C) surrounding CD20+ neoplastic cells in MALT lymphoma (panel B inset) [red symbol in panel C indicates significance vs normal and G/HP- (#P < .01) or vs mWI-5/PT (##P < .01)]. Double staining demonstrates that APRIL+ cells coexpress CD11c (D), CD68 (E), and CD163 (F). No APRIL reactivity is observed in neoplastic B cells (B,D-F). In WI grade 1, the anti-HP antibody recognizes bacilli in the gastric mucous layer (panel G inset); HP reactivity is also observed in the cytoplasm of numerous cells in the gastric lamina propria (H). Double stain demonstrates that HP-containing cells are represented by macrophages coexpressing CD11c (I), CD163 (J), and CD68 (K) and some of them produce APRIL (L). For immunohistochemistry, sections are counterstained with Meyer haematoxylin and secondary antibodies revealed with DAB (brown, anti-APRIL in panels A,BD,E,F,L; anti-HP in panels G-K), and Ferangi blue (blue, anti-CD11c in panels D,I; anti-CD68 in panels E,K; anti-CD163 in panels F,J; APRIL in panel L). Scale bar: 100 μm (panels A,B,G) and 20 μm (panels D-F,I-L). Legend in panel C: G/HP- indicates HP-negative gastritis; mWI-5, matched WI-5; and mWI-5/PT, matched WI-5 posteradication treatment.
APRIL production by tissue macrophages. Sections are from normal gastric mucosa (A), WI grade 5 (B,D-F) and grade 1 (G-L). Compared with normal gastric mucosa where APRIL reactivity is observed in rare large cells of the lamina propria (A), numerous APRIL+ cells are found (B-C) surrounding CD20+ neoplastic cells in MALT lymphoma (panel B inset) [red symbol in panel C indicates significance vs normal and G/HP- (#P < .01) or vs mWI-5/PT (##P < .01)]. Double staining demonstrates that APRIL+ cells coexpress CD11c (D), CD68 (E), and CD163 (F). No APRIL reactivity is observed in neoplastic B cells (B,D-F). In WI grade 1, the anti-HP antibody recognizes bacilli in the gastric mucous layer (panel G inset); HP reactivity is also observed in the cytoplasm of numerous cells in the gastric lamina propria (H). Double stain demonstrates that HP-containing cells are represented by macrophages coexpressing CD11c (I), CD163 (J), and CD68 (K) and some of them produce APRIL (L). For immunohistochemistry, sections are counterstained with Meyer haematoxylin and secondary antibodies revealed with DAB (brown, anti-APRIL in panels A,BD,E,F,L; anti-HP in panels G-K), and Ferangi blue (blue, anti-CD11c in panels D,I; anti-CD68 in panels E,K; anti-CD163 in panels F,J; APRIL in panel L). Scale bar: 100 μm (panels A,B,G) and 20 μm (panels D-F,I-L). Legend in panel C: G/HP- indicates HP-negative gastritis; mWI-5, matched WI-5; and mWI-5/PT, matched WI-5 posteradication treatment.
Together, these results strongly support the notion that macrophages represent a relevant source of APRIL available from the early phases of MALT lymphoma progression.
HP-infected macrophages produce APRIL
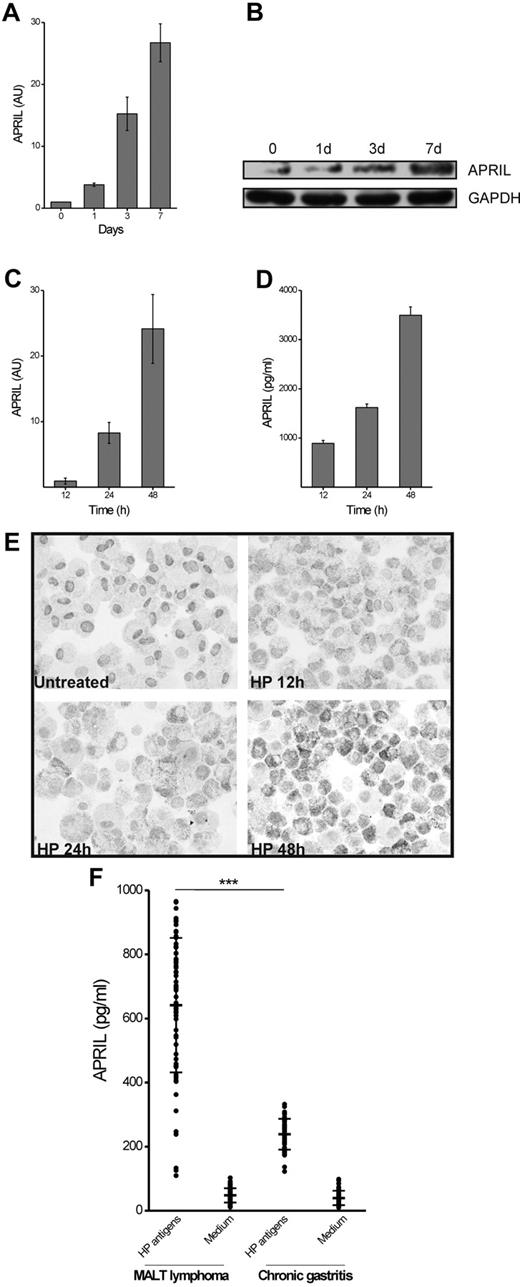
It was recently reported that cells containing HP-derived products can be found in the lamina propria of patients with gastric cancer and chronic gastritis,16 confirming early and more recent studies proving that mucosal invasion by HP might occur in HP-dependent pathologies.17-20 Using the same antibody (recognizing an HP-specific form of LPS),16 we were able to confirm this finding on cases of WI grade 1 densely colonized by HP. In particular, in addition to its classic localization in the gastric surface epithelium or in the spaces between mucous-secreting cells, we detected anti-HP reactivity within the cytoplasm of macrophages localized in multiple foci in the gastric lamina propria (Figures 1G-H). Remarkably, HP-containing macrophages were positive for CD11c, CD163 and CD68 (Figure 1I-K), as well as intensely stained by the anti-APRIL antibody (Figure 1L). In parallel with these tissue sample evaluations, we subsequently addressed whether HP could trigger, directly or indirectly, APRIL production by macrophages in vitro. APRIL, which is barely detectable in freshly purified monocytes, is actively produced at mRNA and protein level during monocytes differentiation to macrophages (Figure 2A-B); however, as quantified by ELISA of the culture supernatant (not shown), APRIL was not significantly released at any time point during the culture. HP exposure of MDM induces a time-dependent expression of APRIL mRNA (Figure 2C) in parallel with the extracellular accumulation of the related mature protein (Figure 2D). Addition of HP to MDM also triggered a time-dependent accumulation of intracellular APRIL (Figure 2E).
APRIL production during macrophage differentiation and exposure to HP or to HP-specific T cells. Monocytes exposed to M-CSF were harvested at the indicated time points and APRIL mRNA was quantified by real-time PCR (A); in parallel the APRIL protein cell content was determined by Western blot. GAPDH was used as marker of equal loading (B). M-CSF–differentiated macrophages (MDM) were exposed to HP (5 × 105 colony-forming unit/mL). At the indicated time points cells were harvested for mRNA evaluation (C) and culture supernatant collected for APRIL content determination (D); AU indicates arbitrary units. In parallel, MDM cytospins were labeled for their intracellular APRIL content (E). HP-specific Th cells isolated from MALT lymphoma patients and from chronic gastritis patients were cocultured with autologous macrophages in the presence of medium or HP antigens and the APRIL production was assessed by ELISA (F). Results represent the APRIL levels induced by T-cell clones over the APRIL production in cultures of macrophages alone. ***P < .01
APRIL production during macrophage differentiation and exposure to HP or to HP-specific T cells. Monocytes exposed to M-CSF were harvested at the indicated time points and APRIL mRNA was quantified by real-time PCR (A); in parallel the APRIL protein cell content was determined by Western blot. GAPDH was used as marker of equal loading (B). M-CSF–differentiated macrophages (MDM) were exposed to HP (5 × 105 colony-forming unit/mL). At the indicated time points cells were harvested for mRNA evaluation (C) and culture supernatant collected for APRIL content determination (D); AU indicates arbitrary units. In parallel, MDM cytospins were labeled for their intracellular APRIL content (E). HP-specific Th cells isolated from MALT lymphoma patients and from chronic gastritis patients were cocultured with autologous macrophages in the presence of medium or HP antigens and the APRIL production was assessed by ELISA (F). Results represent the APRIL levels induced by T-cell clones over the APRIL production in cultures of macrophages alone. ***P < .01
HP-specific T helper (Th) clones stimulate APRIL production from autologous macrophages
Proliferation of neoplastic B cells in MALT lymphoma is sustained by both the antigen and the tumor-infiltrating helper T cells.21 To test the hypothesis that HP-specific T cells might sustain macrophage-derived APRIL production, we studied the helper function of clones of ex vivo freshly purified T cells obtained from antral biopsies of 5 patients with uncomplicated HP chronic gastritis and 5 HP-associated gastric low-grade MALT lymphoma (see “Generation of gastric T-cell clones and assay for T-cell clone helper function for APRIL production” for details). By measuring the [3H]thymidine deoxyribose uptake, we screened our T-cell clones for HP-induced proliferation in MHC-restricted conditions and obtained 71 HP-specific Th clones from patients with HP-associated low-grade MALT lymphoma and 50 HP-specific Th clones from patients with HP chronic gastritis without MALT lymphoma. To test the helper ability of HP-specific T cells for APRIL production, HP-specific T-cell clones were cocultured for 16 hours with autologous monocyte-derived macrophages in the presence of HP antigen. Remarkably, as demonstrated by ELISA, HP-specific Th clones from chronic gastritis and, even more strongly, those purified from MALT lymphoma cases, were able to stimulate the production of variable amounts of APRIL protein in an antigen-specific manner (Figure 2F).
Discussion
In this study, we have demonstrated that HP infection in the gastric mucosa might sustain the local production of APRIL, a tumor necrosis factor superfamily member known to be important for B-cell development, maturation, and survival. In particular, numerous APRIL-producing macrophages are found in HP-infected chronic gastritis and mucosa-associated lymphoid tissue lymphoma biopsies, while they are very rare in normal mucosa. Notably, a fraction of APRIL-positive macrophages contain HP-derived products; moreover, in vitro HP infection induces expression of APRIL in monocyte-derived macrophages, thus indicating a primary role of the bacterium in promoting monocyte-derived cells to produce APRIL in infected gastric mucosa.
A large body of experimental and clinical evidence suggest that APRIL sustains B-cell transformation and progression of B-cell lymphomas.11-14 Different forms of B-cell lymphoma were found to contain APRIL-positive cells, that were represented either by neoplastic B-cells and by tumor associated immune cells,13 including macrophages.22 In keeping with these observations we have documented that APRIL+ macrophages are also abundant in gastric diffuse large B-cell lymphomas devoid of HP infection. These observations indicate that the induction of APRIL in lymphomas partially reflect the local activation of tumor-associated macrophages likely mediated by different mechanisms. Craxton et al reported that human macrophages but not monocytes or monocyte-derived dendritic cells constitutively express high levels of APRIL.23 Furtermore, the expression of APRIL has been detected in macrophage-like cell lines, monocyte-derived macrophages and inflammatory macrophages.23-27 Stimuli mediating APRIL release by macrophages are largely unknown. We found that APRIL is gradually induced but not released by macrophages during M-CSF–dependent differentiation from monocytes. However, we provide evidence that APRIL release by macrophages depends on HP, either after a direct stimulation by the bacteria, or by HP-specific T cells, especially those purified from overt MALT B-cell lymphoma.
The role of HP in gastric MALT lymphoma is well established, considering that chronic HP antigen stimulation is essential for their progression. Survival and proliferation of transformed B-cells in gastric MALT lymphoma require signals from tumor-associated T-cells or direct auto-antigen stimulation of neoplastic cells. (reviewed in Isaacson and Du3 and D'Elios et al21 ). Our data suggest that tumor-associated macrophages might sustain progression of MALT lymphoma by releasing APRIL. We propose that HP-driven gastric inflammation leads to the generation of a local pool of macrophages that on HP infection release large amount of APRIL. This functional loop can be further amplified and perpetuated by HP-specific T cells. Remarkably, as proof of principle of this postulate, APRIL-producing macrophages are dramatically reduced on lymphoma remission induced by HP eradication.
Our results provide a new piece of information to HP-dependent MALT lymphomagenesis pointing to tumor-associated macrophages as contributors of tumor progression via APRIL release. Thus, based on these findings, we suggest that, among biologic therapies targeting B cells,28 blockade of APRIL might represent a novel additional therapeutic strategy for gastric MALT lymphoma, supporting the notion that the targeting tumor-associated macrophages and their products can be particularly relevant in lymphoid malignancies.29,30
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Wilma Pellegrini, Cristina Rossini (supported by Fondazione Beretta, Brescia, Italy), Paola Bossini, and Laura Fappani for their technical support.
This work was supported by Progetto di Eccellenza Fondazione Cassa di Risparmio di Padova e Rovigo, Research Grant from the University of Padova (CPDA074121/07), Associazione Italiana per la Ricerca sul Cancro Grant Regionale 2008, to M.d.B.; Fondazione Berlucchi, Brescia, Italy, to W.V., F.F., and S.L.; Ministero dell'Istruzione, dell'Università e della Ricerca (PRIN 2008C33HRL_003) and Ente Cassa di Risparmio di Firenze to M.M.D.; Ministero dell'Istruzione, dell'Università e della Ricerca (PRIN 2007H9AWXY), Fondazione Cariverona, and Associazione Italiana per la Ricerca sul Cancro (AIRC 5839), to M.A.C. S.L. is supported by “Borsa di studio Prof Roberto Tosoni” (Garda Vita, BCC del Garda).
Authorship
Contribution: W.V., M.M.D., M.A.C., and M.d.B. designed the study and wrote the manuscript; F.M., S.L., A.A., and M.C. performed research and analyzed data; and C.D., F.F., Y.E., M.R., and M.F. provided reagents, were involved in the interpretation of the results, read the manuscript, and gave comments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William Vermi, MD, Department of Pathology, University of Brescia, Piazzale Spedali Civili, Brescia, Italy; e-mail: william.vermi@gmail.com; or Mario M. D'Elois, Department of Internal Medicine, University of Firenze, Firenze, Italy; e-mail: mariomilco.delios@unifi.it..
References
Author notes
F.M. and S.L. contributed equally to this work.

![Figure 1. APRIL production by tissue macrophages. Sections are from normal gastric mucosa (A), WI grade 5 (B,D-F) and grade 1 (G-L). Compared with normal gastric mucosa where APRIL reactivity is observed in rare large cells of the lamina propria (A), numerous APRIL+ cells are found (B-C) surrounding CD20+ neoplastic cells in MALT lymphoma (panel B inset) [red symbol in panel C indicates significance vs normal and G/HP- (#P < .01) or vs mWI-5/PT (##P < .01)]. Double staining demonstrates that APRIL+ cells coexpress CD11c (D), CD68 (E), and CD163 (F). No APRIL reactivity is observed in neoplastic B cells (B,D-F). In WI grade 1, the anti-HP antibody recognizes bacilli in the gastric mucous layer (panel G inset); HP reactivity is also observed in the cytoplasm of numerous cells in the gastric lamina propria (H). Double stain demonstrates that HP-containing cells are represented by macrophages coexpressing CD11c (I), CD163 (J), and CD68 (K) and some of them produce APRIL (L). For immunohistochemistry, sections are counterstained with Meyer haematoxylin and secondary antibodies revealed with DAB (brown, anti-APRIL in panels A,BD,E,F,L; anti-HP in panels G-K), and Ferangi blue (blue, anti-CD11c in panels D,I; anti-CD68 in panels E,K; anti-CD163 in panels F,J; APRIL in panel L). Scale bar: 100 μm (panels A,B,G) and 20 μm (panels D-F,I-L). Legend in panel C: G/HP- indicates HP-negative gastritis; mWI-5, matched WI-5; and mWI-5/PT, matched WI-5 posteradication treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/24/10.1182_blood-2010-06-293266/4/m_zh89991173110001.jpeg?Expires=1769079204&Signature=dVeFyTQ1hjT8h3TTg~7I~GeC6c529dQMZeSTpz4Lj3G4RHhIOLJKbcxoSoNMaZifl0IbuItCmFi~E2bDc0GggFhkHH4Px9Yb7xgwlHkXaNbgtc7GomT3QbS-Tt8piaLCkXjcQs7JSXWuvk6Ndk9MrAZbaGM-rwU04d1R~7SWFvkRTv8AFYuQ0kfQOWepxBXzZ-2SxToUsJMmPlhxKCz7SVsbbaOAAJgKeNtqYjHzBkNzWlpexfyoPtHc8Qs083eu7tuQiTDFR3MsZijVWjfHk0XX-oDPzUtA6U-Z7PqhhHEr~W5mpbEv5de32jn5h7CeVS9PL0psyRFpp3GZgAnyfQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal