The reciprocal translocation t(9;22) (q34;q11) that generates the Philadelphia (Ph) chromosome is present in most chronic myeloid leukemia (CML) patients. However, in 5% to 10% of newly diagnosed patients, a cytogenetic variant is observed.1
In this issue of Blood, Marzocchi et al (on behalf of the GIMEMA [Italian Group for Hematologic Malignancies of the Adults] CML Working Party) report for the first time a large systematic analysis of the role of variant Ph translocations in newly diagnosed CML patients in chronic phase. These variant translocations implicate another breakpoint.2 The mechanisms of generation of the translocations are not fully understood. In addition, the prognostic implication of these cytogenetic abnormalities has been studied by several groups with no uniform and clear conclusions.3,4 The analysis by Marzocchi and colleagues included 559 patients all in chronic phase treated with either 400- or 800-mg doses of imatinib. All these patients were included in prospective clinical trials. Patients were followed with real-time quantitative PCR (RQ-PCR) every 3 to 6 months and by cytogenetic and FISH analysis. Marzocchi et al established a very experienced network of cytogenetic laboratories that were involved in this analysis. FISH analysis was performed on 200 to 300 nuclei, with most of the laboratories using the same probes. The molecular analysis was centralized at a single laboratory (Bologna). Of the 559 patients enrolled in the study, 30 (5%) showed variant translocation. The majority of cases showed a 3-way translocation. Additional information was provided by FISH analysis to investigate the mechanism of the genesis of the variant translocation. The one-step mechanism occurred more frequently (75%) than the 2-step or complex mechanism.
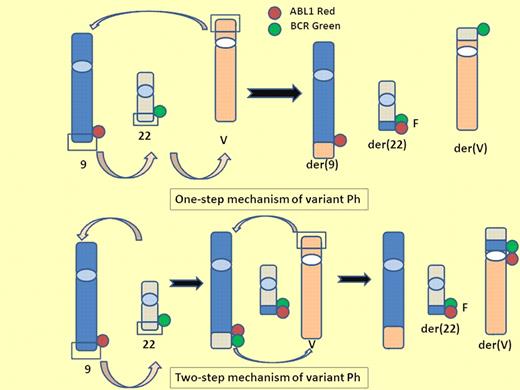
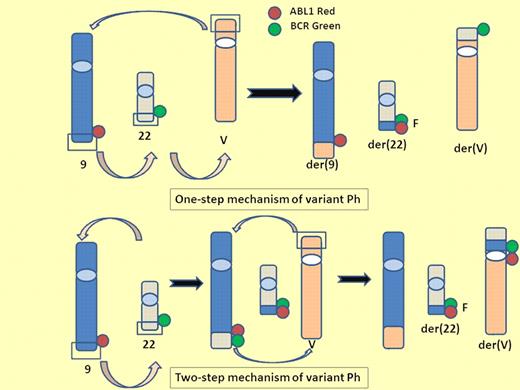
One-step and 2-step mechanisms of variant Ph. A variant Philadelphia chromosome (Ph) is generated from translocation of 1 or more partner chromosomes in addition to chromosomes 9 and 22. One-step mechanism of variant Ph: 3 breaks and reciprocal rejoining occur at the same time on chromosome 9, 22, and partner chromosome. Two-step mechanism of variant Ph: the standard t(9;22) is formed and then the segment of der9 exchanges material with the variable partner chromosome. V indicates variable partner chromosome; G, green signal; R, red signal; and F, fusion signal.
One-step and 2-step mechanisms of variant Ph. A variant Philadelphia chromosome (Ph) is generated from translocation of 1 or more partner chromosomes in addition to chromosomes 9 and 22. One-step mechanism of variant Ph: 3 breaks and reciprocal rejoining occur at the same time on chromosome 9, 22, and partner chromosome. Two-step mechanism of variant Ph: the standard t(9;22) is formed and then the segment of der9 exchanges material with the variable partner chromosome. V indicates variable partner chromosome; G, green signal; R, red signal; and F, fusion signal.
The key results of analysis by Marzocchi and colleagues is that patients who have a variant translocation responded to imatinib frontline as well as those without a variant translocation. Progression-free survival, event-free survival, failure-free survival, and overall survival were identical in the 2 groups of patients. In addition, patients with or without variant translocation achieved similar rates of complete hematologic response, complete cytogenetic response, and major molecular response. Of note, the median duration of cytogenetic responses was the same with or without variant translocation. These results are in contrast to previously published reports.5 For example, Stagno et al suggested that variant translocation patients have an unfavorable clinical outcome. However, their 10 variant patients were not compared with a control group as Marzocchi et al did. A report from the Houston group supports the results produced by Marzocchi and colleagues, although the patients were previously treated with interferon.6
The second message of this paper is that deletion of the derivative chromosome 9 (der9) has no influence on the response. Large, submicroscopic deletions adjacent to the breakpoint on der9 have been reported in 10% to 15% of patients with CML.7 The deletions were detected by FISH and confirmed by microsatellite PCR and locus-specific probes for chromosome 9 and 22 sequences. These deletions are seen more frequently in cases of variant translocation (30%-40%). der9 deletions do not correlate with clinical features, laboratory findings, Sokal and Euro scoring systems, or disease phase.8 Marzocchi et al already investigated the prognostic value of der9 deletions in a large group of patients treated with frontline doses of imatinib of 400 mg or 800 mg.9 It was concluded that when detected by FISH, der9 deletions are not a prognostic factor in patients with early chronic phase CML treated with imatinib. The present study confirms these finding. In addition, the FISH analysis provided information on the mechanism of the translocation. The investigators suggested that the deletion on der9 is the result of a 2-step or multistep mechanisms. The European Leukemia Net recommendations do not suggest for a separate group for patients with variant translocation.10 The analysis of Marzocchi and colleagues confirms this recommendation. Finally, it would be of interest to investigate the relationship between second-line tyrosine kinase inhibitors and the prognostic value of variant translocation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■