Abstract
Advancing novel therapeutic agents for the treatment of malignancy into the marketplace is an increasingly costly and lengthy process. As such, new strategies for drug discovery are needed. Drug repurposing represents an opportunity to rapidly advance new therapeutic strategies into clinical trials at a relatively low cost. Known on-patent or off-patent drugs with unrecognized anticancer activity can be rapidly advanced into clinical testing for this new indication by leveraging their known pharmacology, pharmacokinetics, and toxicology. Using this approach, academic groups can participate in the drug discovery field and smaller biotechnology companies can “de-risk” early-stage drug discovery projects. Here, several scientific approaches used to identify drug repurposing opportunities are highlighted, with a focus on hematologic malignancies. In addition, a discussion of the regulatory issues that are unique to drug repurposing and how they impact developing old drugs for new indications is included. Finally, the mechanisms to enhance drug repurposing through increased collaborations between academia, industry, and nonprofit charitable organizations are discussed.
Introduction
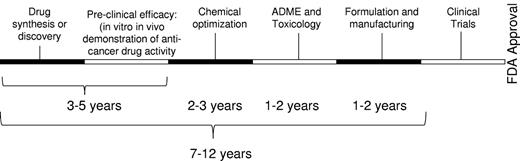
Advancing novel therapeutic agents for the treatment of malignancy into the marketplace is an increasingly costly and lengthy process. Cost estimates for de novo drug discovery range from $500 million to $2 billion,1 depending on the company undertaking the development and/or the therapy itself, with average costs cited at approximately $800 million.1,2 In addition, it often requires 10 to 17 years to obtain regulatory approval for a new drug (Figure 1), in part, given the necessary regulatory requirements regarding safety and efficacy,3 which involve testing in preclinical animal models and multiple phases of human clinical trials. During the de novo drug development pathway, > 90% of drugs fail to obtain regulatory approval, with failure occurring at every phase of testing. Highlighting this point, only 212 new molecular entities (new drugs) were approved by the United States Food and Drug Administration (FDA) between 2000 and 2009. Of these, only 24 were developed as cancer therapeutics, 14 of which had indications for hematologic malignancies (Table 1). Thus, the de novo drug development pipeline requires a significant amount of time, has a substantial failure rate, is expensive, and has realized a limited number of approved new molecules for oncology indications in the last 10 years.
Time line for de novo drug discovery. When developing a new drug, the compound requires complete preclinical characterization, including ADME profiling, and toxicology testing. Suitable compounds require formulation and manufacturing before advancing into clinical trials.
Time line for de novo drug discovery. When developing a new drug, the compound requires complete preclinical characterization, including ADME profiling, and toxicology testing. Suitable compounds require formulation and manufacturing before advancing into clinical trials.
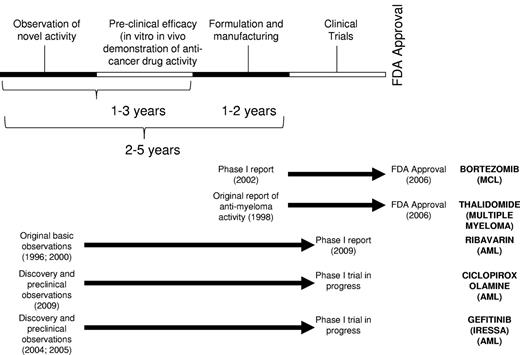
Given the low success rate of traditional drug discovery approaches, new strategies are needed. One such strategy is drug repurposing, in which a new indication for an existing drug is identified. In this approach, known on-patent or off-patent drugs with unrecognized anticancer activity can be rapidly advanced into clinical trial for this new indication by leveraging their prior ADME-tox (Adsorption, Distribution, Metabolism, Elimination-Toxicology) properties. As such, much or all of the medicinal chemistry, pharmacology, pharmacokinetic, and toxicology study requirements can be circumvented by reliance on previously published and readily available data (Figure 2).
Time line for drug repurposing. Drug repurposing leverages the prior toxicology and pharmacology testing of the compounds to enable rapid progression into clinical trial. At times, new formulation and manufacturing are required. Representative drug repurposing examples are shown along with their time lines.
Time line for drug repurposing. Drug repurposing leverages the prior toxicology and pharmacology testing of the compounds to enable rapid progression into clinical trial. At times, new formulation and manufacturing are required. Representative drug repurposing examples are shown along with their time lines.
Drug repurposing also provides a strategy to “de-risk” preclinical anticancer drug development. Smaller biotechnology companies need to raise significant amounts of external capital to advance a drug into phase 1 clinical testing. With the recent downturn in the economy, this capital investment is becoming more difficult to secure. As such, drug repurposing is of interest among smaller biotechnology companies as it will increase the likelihood of preclinical drug candidates advancing into phase 1 clinical testing, offer an early go/no-go determination on the therapeutic strategy, reduce the time needed to enter phase 1 trial, and reduce the early costs of drug development. In some cases, such as the development of thalidomide for the treatment of myeloma, the repurposed drug is successful in obtaining approval for its new indication. In other cases, the repurposed drug does not advance to the point of receiving a new label indication but provides evidence for proof of concept and proof of mechanism. As such, these early studies provide the rationale to continue to develop the therapeutic strategy and support the development of more potent novel chemical entities as second-generation analogs.
Academic groups are also becoming increasingly interested in drug discovery and development. These institutions face obstacles similar to small biotechnology companies. Obtaining peer-reviewed grant support for ADME-tox studies, formulation, and manufacturing is often difficult. Thus, for both academics and small biotechnology companies interested in drug discovery, strategies that lower the risk of failure at the late stage of preclinical development and significantly reduce or eliminate the cost of ADME-tox studies are important.
In this review, we highlight the scientific approaches used to identify drug repurposing opportunities, with a focus on the treatment of hematologic malignancies. In addition, we discuss the unique issues related to regulatory science that impact advancing these opportunities into clinical trial.
Strategies to identify drug repurposing opportunities
Clinical trials
Although often not viewed as a formal drug discovery approach, oncologists frequently use drug repurposing to develop new therapies for their patients. When a chemotherapeutic agent is approved for one indication, it is rapidly tested for efficacy in a variety of other malignancies. In many cases, the evaluation of a newly approved chemotherapeutic agent in another disease site is not based on extensive preclinical laboratory and mechanistic studies but rather early signals in phase 1 clinical trials that include patients with diverse tumor types. For example, shortly after demonstrating efficacy in myeloma, the first-in-class proteasome inhibitor bortezomib (Velcade) was tested in a series of phase 2 clinical trials for patients with diverse malignancies, including mantle cell lymphoma and acute myelogenous leukemia (AML).4 Ultimately, these trials demonstrated that single-agent bortezomib produced response rates of 40% to 50% in patients with mantle cell lymphoma. Subsequently, bortezomib was ultimately approved for this new indication.5-8 In contrast, the drug was ineffective as a single agent in the treatment of other malignancies, including AML. Currently, it remains largely unknown why bortezomib is effective as a single agent in mantle cell lymphoma but not in AML, although proteasome inhibitors induce death of AML cells in culture and delay AML growth in mouse models. Although bortezomib has limited activity when used as monotherapy, combination trials continue to be undertaken, and the drug may ultimately find a niche in other diseases, such as AML.
Many other successful repurposing indications have also arisen out of early clinical trials. For example, the use of sildanefil (Viagra) for the treatment of erectile dysfunction emerged from studies of the drug for cardiac disorders. Likewise, the ABL kinase inhibitor imatinib has been studied as a therapeutic agent for the treatment of rheumatoid arthritis. The rationale for these trials is based in part on clinical observations demonstrating improved rheumatoid symptoms in patients who received imatinib for their coexisting chronic myelogenous leukemia.9 As shown in Table 2, 11 known drugs received a new anticancer indication between 2000 and 2009. Except for the use of thalidomide for the treatment of multiple myeloma, the other 10 drugs represented label extensions of known chemotherapeutic agents. Thus, identification of new indications through clinical evaluation is the most common form of drug repurposing. Yet, it also suggests that other opportunities for drug repurposing remain undiscovered. It is noteworthy that identifying new anticancer indications for a known chemotherapeutic agent, such as bortezomib, is a different form of drug repurposing than identifying anticancer activity for a drug, such as thalidomide, that was never previously used for the treatment of malignancy.
A series of fortunate events: thalidomide and myeloma
Perhaps the best known and most successful example of drug repurposing for the treatment of hematologic malignancies is the identification of thalidomide as a novel therapeutic agent for the treatment of multiple myeloma. The success of thalidomide illustrates how a degree of serendipity is often useful in drug discovery and development. Thalidomide was used in the 1950s as a sedative hypnotic and as a treatment for nausea during pregnancy. However, the drug was subsequently withdrawn from the market because of its teratogenic effects.10 Decades later, Celgene resurrected thalidomide and began evaluating its potential therapeutic value for the treatment of several diseases, including discoid lupus erythematosis, aphthous ulcers in HIV syndromes, and Behçet disease.11,12 Around that time, the wife of a cardiologist with refractory myeloma was reviewing the medical literature, searching for a potential therapy for her husband. She contacted Dr Judah Folkman in Boston and discussed the antiangiogenic properties of thalidomide and the potential use of this drug in myeloma. Based on this discussion, Celgene was contacted and thalidomide was obtained for compassionate use in this patient with myeloma. Although the drug did not work in her husband, it was tried in 4 other patients. The results of this early pilot study were reported by Singhal et al, who treated patients with refractory myeloma with thalidomide on a compassionate-use protocol.13 One of these patients, with a very large tumor burden, who did not respond to 2 prior cycles of high-dose chemotherapy, had a nearly complete remission within 3 months of initiating thalidomide therapy. Singhal et al13 followed this observation up with a phase 2 study in 84 patients with previously treated and progressive myeloma. Patients were initially treated with 200 mg oral thalidomide daily, with fortnightly increases of 200 mg, for a total of 6 weeks and a final treatment dose of 800 mg. In this study, 10% of patients had a complete or nearly complete remission; 32% had reduction of serum or urine paraprotein levels by at least 25%. These and subsequent studies led to the approval of thalidomide for the treatment of myeloma.14-16 Later studies also demonstrated efficacy in myelodysplasia.17,18 Encouraged by these reports of efficacy, the more potent second-generation analog lenolidomide was developed and has largely replaced thalidomide in the treatment of hematologic malignancies.19
Although initially evaluated in myeloma because of its potential antiangiogenic effects, thalidomide's antimyeloma mechanism of action is much more complex and includes altering cell adhesion molecules and cytokine expression as well as modulation of cell-mediated immunity.20 Indeed, its full mechanism of action in both myeloma and myelodysplasia remains ill understood.
Drug repurposing opportunities through the understanding of disease biology
Drug repurposing opportunities can also arise as a focused effort to target molecular defects in malignant cells. For example, activating mutations of the KIT tyrosine kinase were reported in sporadic and hereditary gastrointestinal stromal tumors (GISTs).21 When the ABL kinase inhibitor imatinib was shown to cross-react with the KIT kinase, preclinical studies were conducted to evaluate imatinib in GIST.22 Imatinib induced cell death in GIST cells in culture through a mechanism that appeared related to KIT inhibition.22 Based on this and similar preclinical evidence, phase 2 clinical trials of imatinib were conducted in patients with unresectable or metastatic GIST. Imatinib demonstrated clinical efficacy in these patients with response rates of 33% to 43% for 400- and 600-mg doses, respectively. As such, imatinib was subsequently approved by the FDA for the treatment of GIST.23 Using a similar rationale, imatinib was also evaluated in combination with reinduction chemotherapy in patients with KIT-positive relapsed AML, where promising results have been seen in early-phase clinical trials.24,25 Likewise, as imatinib cross-reacts with the platelet-derived growth factor receptor kinase, it has also been repurposed for the treatment of dermatofibrosarcoma and systemic mastocytosis.26,27
Recent clinical trials of the antiviral ribavarin in patients with refractory AML also developed from a focused effort to target the eukaryotic translation initiation factor 4E (eIF4E) in AML. Briefly, 4E is involved in mRNA transport from nucleus to cytoplasm of a select class of oncogenic mRNAs28 (eg, cyclin D1), and its overexpression elevates this transport, thus increasing cyclin D1 at the level of protein translation.29 Subsequent studies indicated that 4E binding to the methyl-7-guanine cap of mRNA is required for 4E-dependent transport of mRNA into the cytosol.30 Furthermore, 4E levels are elevated in M4 and M5 AML and in blast crisis chronic myelogenous leukemia patient samples but not in normal hematopoietic cell samples.31 Ribavarin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide, also known as Virazole) is a guanosine analog, with efficacy as a therapeutic in a number of viral-mediated diseases, including infections with Lassa fever virus, respiratory syncytial virus, hepatitis C virus, and severe acute respiratory syndrome coronavirus. Strikingly, ribavarin was shown to bind 4E and inhibit its activity.32 This effect of ribavirin on 4E coupled with the biologic role of this protein in AML prompted preclinical studies of this drug in AML. In preclinical studies, ribavarin decreased clonogenic growth of CD34+ cells extracted from AML M5 patients, with a 50% inhibitory concentration of approximately 1μM, with no concomitant effects on AML M1 blasts or normal hematopoietic cells. Given these data, a phase 1 clinical trial was conducted where patients with AML M4 or M5 received escalating doses of ribavirin.33 Of the 11 evaluable patients, the best responses observed included one complete remission and 2 partial remissions. Molecular responses (2- to 10-fold decrease in eIF4E expression) were also observed in 10 of 11 evaluable patients, concomitant with relocalization of eIF4E from the nucleus to cytoplasm. Thus, ribavarin is potentially efficacious as a therapy for AML patients who overexpress eIF4E and is an example of repurposing compound developed through an initial understanding of mRNA transport and translation.
Finally, data on biomarkers from clinical trials and subsequent laboratory evaluation can also identify new drug indications. For example, clinical and laboratory studies demonstrated the efficacy of the BRAF inhibitor PLX4032 in patients with melanoma and a BRAFV600E mutation.34 As a result of these studies, PLX4032 may also have efficacy in other malignancies with BRAFV600E mutations, such as papillary thyroid cancer and colon cancer
High-throughput screening for drug repurposing opportunities
Although the aforementioned strategies and case studies highlight the benefits of drug repurposing, they share a reliance on prior clinical observations and/or a detailed understanding of disease biology. Thus, candidate agents are identified one at a time and one disease at a time. High-throughput screening of known drug libraries permits the evaluation of a large number of drugs in an unbiased approach. For example, our laboratory recently screened a library of off-patent drugs and chemicals for agents with potential activity in leukemia and myeloma. From this screen, we identified the topical antifungal agent ciclopirox olamine.35 Ciclopirox olamine displayed in vitro and in vivo activity against leukemia cells and stem cells as well as myeloma cells at concentrations that appeared pharmacologically achievable. Mechanistically, ciclopirox olamine functioned as an intracellular iron chelator that inhibited iron-dependent enzymes, including ribonucleotide reductase. The preclinical antileukemia and antimyeloma efficacy of ciclopirox olamine, combined with its prior safety record and pharmacokinetic profile, suggested that ciclopirox olamine could be rapidly advanced into a phase 1 clinical trial for patients with refractory hematologic malignancies. In partnership with the Leukemia & Lymphoma Society (LLS), an oral formulation was developed and Good Manufacturing Practice (GMP)–quality drug was manufactured. Within 2 years of identification of this hit in our screen, a phase 1 dose escalation study of oral ciclopirox olamine was initiated in patients with relapsed and refractory hematologic malignancy (www.clinicaltrials.gov; #NCT00990587). At the time of writing, the fifth dose level is being evaluated. To date, pharmacodynamic effects of the drug have been observed, demonstrating proof of mechanism and early signals of clinical response have also been seen. Thus, this work serves as a useful model for high-throughput approaches to identify compounds with specific biologic effects, which can be repurposed to treat hematologic malignancies.
In addition to identifying single drugs with previously unrecognized activity in hematologic malignancies, high-throughput screening of known drug libraries has also been used to identify novel drug combinations for potential use in the treatment of malignancy. For example, Rickles et al36 recently reported a high-throughput screen to identify known drugs that synergized with dexametha sone to induce cell death in myeloma cells. Although only 2841 drugs were evaluated, they were tested in binary combinations using a full 6 × 6 matrix of drug concentrations. As such, more than 100 000 assays per cell line were conducted, and almost 650 combinations were tested using this matrix in a panel of 4 myeloma cell lines. From this large screening effort, combinations synergistic with dexamethasone were identified. Among the promising combinations were the phosphodiesterase inhibitor papaverine and the adenosine receptor agonist chloro-IB-MECA, which both acted synergistically with dexamethasone to induce cell death in myeloma cells. Of note, none of these combinations could have been predicted to have activity in myeloma solely through an understanding of disease biology or available clinical data.
In silico analyses to identify repurposing opportunities
Recent advances in bioinformatics have also been applied to identify potential drug repurposing opportunities by analyzing gene expression databases, text mining of databases of drug side effects, and in silico docking studies. For example, an in silico analysis of gene expression in AML led to the identification of the epidermal growth factor receptor inhibitor gefitinib (Iressa) as a potential therapy for this disease. This study used a bioinformatic approach to identify a gene signature unique to myeloid differentiation, which could be measured by a multiplex polymerase chain reaction assay. The authors then screened 1739 known drugs, to identify small molecules that would promote the myeloid differentiation of HL60 cells. This screen identified epidermal growth factor receptor inhibitors as drugs capable of triggering myeloid differentiation.37 Follow-up studies demonstrated that the FDA-approved epidermal growth factor receptor inhibitor gefitinb (Iressa) was capable of inducing myeloid differentiation and reducing the viability of both AML cell lines and primary samples, without concomitant effect on normal hematopoietic cells.38 These preclinical studies have led to an ongoing clinical trial of gefitnib in patients with relapsed or refractory AML (www.clinicaltrials.gov; #NCT00130702).
Animal models in drug repurposing
Demonstrating preclinical efficacy in mouse models is generally a prerequisite for advancing a new anticancer drug into human clinical trials. In the case of drug repurposing, one might question the need to demonstrate antitumor efficacy in mice before initiating human trials. When a drug's pharmacokinetics differ substantially between mouse and human, dosing schedules to mimic the human situation may not be feasible. As such, decisions to proceed to human clinical trial based only on preclinical efficacy in mice may not be justified.
Rather than using animal models solely to demonstrate efficacy, these models may also serve other purposes in drug repositioning. For example, using primary and secondary transplants of primary AML cells engrafted into immunodeficient mice, one can assess the ability of the drug to target leukemia stem cells. Alternatively, one can use mouse models to assess the impact of the microenvironment on the anticancer activity of the drug.
Regulatory issues related to drug repurposing as a drug discovery strategy
The attractiveness of drug repurposing as a drug discovery strategy relies on the ability to leverage prior toxicology and pharmacology data related to the drug to advance rapidly into clinical trial and ultimately approval for a new indication. As such, regulatory considerations are closely intertwined with drug repurposing.
In the United States, there are 3 common paths available to obtain approval for drug products: 505(b)(1), 505(j), and 505(b)(2). The 505(b)(1) path pertains to approval of novel chemical entities and requires extensive nonclinical and clinical pharmacology and toxicology testing. The 505(j) pathway is used for generic drugs and requires clinical bioavailability/bioequivalence studies to show that 2 drug products are equivalent. The 505(b)(2) pathway focuses on a new formulation or new use of an already approved drug product. In this pathway, the previous findings of safety and efficacy of known drugs can be leveraged so that only studies necessary to support the safety and/or efficacy of the new indication need to be conducted. Therefore, using the 505(b)(2) mechanism, the sponsor may be able to capitalize on the prior pharmacology and toxicology studies related to the drug rather than repeating these studies. The application, in this case, can reference published literature, approved product labels, or product monographs. Of note, a similar mechanism also pertains to the filing of investigational new drug applications for clinical trials of known drugs for new indications. In this regard, even on-patent drugs can be evaluated for new indications without the need to submit the chemistry and manufacturing files in the investigational new drug or even the approval of the original owners if the drug product is used in compliance with the approved product label. However, patent considerations are relevant for drugs approval under 505(b)(2) mechanisms, as approval under a 505(b)(2) application may be delayed because of patent or exclusivity protection. In other regions, including Canada, Australia, and Europe, regulatory paths similar to the 505(b)(2) mechanism exist. Like the United States, the regulatory agencies will accept data from the published literature and drug product monographs to support trials of drug repositioning.
Related to the regulatory issues pertaining to drug repurposing are considerations of the intellectual property surrounding the new use for known drugs. The discovery of a new indication for an old drug can form the basis for a use patent if the discovery is novel, unexpected, and potentially beneficial clinically. The new use claims can apply to both on-patent and off-patent drugs if the new use has not been previously disclosed or covered in the original patents pertaining to the drug. However, by definition, there is not an opportunity for a composition of matter claim as the drug is already a known entity. Strategies to achieve market exclusivity include the development of a new formulation in conjunction with the new use claim.
Achieving marker exclusivity for repurposing drugs targeting hematologic malignancies can often be aided by the Orphan Drug Act (ODA). The ODA in the United States encourages pharmaceutical companies to develop compounds for the treatment of rare (“orphan”) diseases, where orphan is defined as a prevalence of less than 200 000 people in the United States. As such, many hematologic malignancies, including AML, multiple myeloma, and chronic myelogenous leukemia, fall within this designation. Similar legislation has been enacted in other regions, including Europe, Japan, and Australia. This legislation offers economic and other incentives to develop therapies for rare disease. For example, when reviewing orphan drug applications, agencies, such as the FDA or the European Medicines Agency (EMA), will accept smaller cohort sizes for registration trials and will waive certain fees associated with the development and approval of orphan drugs. In addition, approval of orphan drugs provides market exclusivity for 7 years in the United States and for 10 years in the European Union. Some of the incentives offered by the EMA and FDA to develop orphan drugs are summarized in Table 3.
Before the introduction of the ODA in the United States in 1983, only 38 treatments were approved by the FDA for orphan indications. In contrast, since passage of this legislation, more than 350 new treatments have been approved for orphan indications. Thus, this act has successfully encouraged the development of new therapies for rare diseases. Although not the intention of this legislation, the ODA has also encouraged some manufacturers to intentionally position drugs for orphan indications and then rely on off-label prescribing for nonorphan indications. In addition, it has allowed some manufacturers to obtain orphan designation for known drugs already widely used but unapproved for an orphan designation. On approval, the holder of the orphan designation significantly increased the drug price. For example, Biomarin recently received orphan drug designation in Europe for the treatment of Lambert Eaton myasthenic syndrome with amifampridine, which was widely used for the treatment of this condition. Although widely used, the formulation was not standardized and formal clinical trials to support the use had not been conducted. On approval and orphan designation, the price of amifampridine rose significantly. The high price was very controversial, however, and the company came under pressure to lower the price.39 Thus, the lower cost of developing a drug via a repositioning approach is not always passed on to the consumer. In another example, URL Pharma received orphan drug designation for colchicine for the treatment of familial Mediterranean fever and a label indication for the treatment of gout. Although colchicine had been used for many years for the treatment of these conditions, randomized phase 3 data were not available to support the indication. In exchange for producing the data demonstrating clinical efficacy, the FDA granted URL Pharma 3 years of market exclusivity for the treatment of gout had 7 years of market exclusivity for the treatment of familial Mediterranean fever. To recoup the cost of the clinical trials, the price of colchicine rose 50-fold after these approvals.40 These examples were probably not the intended outcome of the drug legislation and are fortunately unusual events. Nonetheless, these examples highlight how economic incentives are powerful drivers of drug development strategies.
Off-label use and drug repurposing
Thalidomide was initially licensed for the treatment of erythema nodosum leprosum in 1998, and it was not until 2006 that thalidomide was approved for the treatment of myeloma10 (www.fda.gov). Yet, in this time period, more than 720 000 thalidomide prescriptions were written, with only 0.1% of prescriptions for the label indication of erythema nodosum leprosum.41 The initial off-label use of thalidomide for the treatment of myeloma after approval for leprosy highlights a broader issue in drug repositioning related to the need to obtain a labeled indication. The potential new use for an old drug could be rapidly and widely disseminating through publications and presentations. If the drug is currently available in an appropriate formulation, off-label prescribing could lead to wide adoption of this therapy in the absence of formal regulatory approval. One might then question the need to obtain formal approval and a new label. The new label might offer economic benefit for the developers and provide a mechanism for patients to obtain the drug if their payers only provide coverage for on-label use. A significant concern, however, to adopting new indications for known drugs and off-label prescribing is that the clinical data supporting the new indication may be weak and the drug may, indeed, not be beneficial for the new indication. Perhaps off-label drug repurposing could be addressed through a partnership between industry, regulatory agencies, and academia. Through this partnership, off-label indications could be scientifically evaluated and recommendations short of a new label designation provided. A precedent for such discussions might be a recent roundtable with academia, industry, and FDA that sought to define clinical trial metrics and designs for new drug approval.42
Future directions and conclusions
If one considers that, of the 212 new molecular entities (new drugs) approved between 2000 and 2009, only 24 had application as cancer therapeutics and only 14 of those were indications for hematologic malignancies, it is clear that novel approaches to drug discovery are needed. Drug repurposing represents an opportunity to rapidly advance new therapeutic strategies into clinical trials at a relatively low cost. As such, it offers an opportunity for academic groups to participate in the drug discovery field and the opportunity for smaller biotechnology companies to de-risk early-stage drug discovery. Even large pharmaceutical companies are establishing divisions to look at repurposing compounds in their pipelines.
Potentially, additional repurposing opportunities could be advanced through close collaborations between pharmaceutical companies, academic groups, and not-for-profit charitable foundations. The pharmaceutical industry could provide academic groups access to their approved drugs as well as “shelved” compounds in their libraries, which can also serve as viable repurposing candidates. This would expand the libraries of drugs available for academics to test by having access to drugs that were previously evaluated by industry in clinical trials but did not advance to market. Potentially, these drugs could be resurrected for treating new indications. Finally, disease philanthropies, such as the LLS, could offer resources for the development of these new therapeutic strategies.
Indeed, such collaborative approaches are already underway. For example, the Pharmaceutical Assets Portal was recently developed by an academic consortium led by the University of California Davis. Here, compounds from participating pharmaceutical companies that have been published in the literature can be accessed using this on-line system. Through greater partnerships between industry and academia, additional drug repositioning opportunities could be identified. For example, if pharmaceutical companies would donate compounds that failed to progress for reasons other than toxicity, a unique library with compounds from different pharmaceutical companies could be constructed and screened by academic groups. Hits from these screens could be developed jointly by academia and industry and could even include evaluation of analogs in the company's portfolio. Access to such a library could be facilitated through a mechanism similar to the Pharmaceutical Assets Portal. However, development and access of this library would first require understandings around Intellectual Property rights and ownership.
Not-for-profit groups can also contribute to this drug discovery strategy by providing resources to advance promising therapies into clinical trial related to their disease area of interest. For example, The Therapy Acceleration Program (TAP) was recently developed by the LLS to speed the development of blood-cancer treatments and supportive diagnostics and has supported drug repurposing projects.
TAP is composed of 3 divisions: The Biotechnology Accelerator division, the Clinical Trials division, and the Academic Concierge division. The Biotechnology Accelerator division supports biotechnology and small pharmaceutical companies that have potential blood cancer therapies but lack expertise and/or resources to develop those therapies for blood cancer patients. The Clinical Trials division has established a partnership with the Cleveland Clinic to develop a community-based approach to accruing more patients to clinical trials. The Academic Concierge division complements the LLS's $63 million annual investment in research through its grant programs by assisting potential therapies to move from late-stage preclinical testing, such as ADME-tox and formulation, through manufacturing and into clinical trial. In addition to providing financial resources, LLS staff with expertise in drug development also provide guidance and expertise to the project. Of note, TAP funds are not grants but rather business alliances with agreed on time lines, milestones, and go/no-go decision points. Potential partners for TAP can find more information, including contacts, at the LLS TAP website (www.lls.org/#/researchershealthcareprofessionals/drugdevelopment/therapyacceleration).
In addition to the TAP program by LLS, the National Institutes of Health also offers resources to help academic investigators advance potential therapeutic agents, including drug repurposing opportunities. For example, the Therapeutics for Rare and Neglected Disease program in the National Institutes of Health will engage in collaborations with academic investigators to advance therapies for rare and neglected diseases toward clinical trial. This branch selects projects for assistance through a competitive on-line application process via proposalCENTRAL (www.proposalcentral.altum.com). Other National Institutes of Health programs, such as the National Cancer Institute Experimental Therapeutics Program and Rapid Access to Interventional Development, also help advance potential therapies through late-stage preclinical testing, drug manufacturing, and early clinical trials. Similar to the Therapeutics for Rare and Neglected Disease and TAP programs, these are not grants but rather allow investigators to access resources within the National Institutes of Health to facilitate drug development. Access to these programs is also competitive.
In conclusion, drug repositioning represents a strategy to rapidly advance new therapies from preclinical testing to clinical trial. The rapid evaluation in the clinic allows for early determination of proof of mechanism and proof of concept. In some cases, the repurposing opportunity can lead to a new approved indication. In other cases, it provides the rationale to develop more potent and specific second-generation compounds. Given the high cost of traditional drug discovery pathways, this rapid approach is highly appealing to biotechnology companies as well as academic groups. Increasing the number of drug repositioning opportunities could be realized by expanding the collaborative networks between academia, industry, and charitable organizations.
Acknowledgments
This work was supported in part by the Leukemia & Lymphoma Society and the Ontario Ministry of Health and Long Term Care. A.D.S. is a Leukemia & Lymphoma Society Scholar in Clinical Research. M.A.S. and P.A.S. are Canadian Institutes of Health Research Postdoctoral Fellows. M.A.S. is also an Ontario Ministry of Research and Innovation Post-Doctoral Fellow. P.A.S. is also supported by the Lady Tata Memorial Trust.
The views expressed do not necessarily reflect those of the Ontario Ministry of Health and Long Term Care.
Authorship
Contribution: All authors contributed to the writing and editing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Aaron D. Schimmer, 610 University Ave, Toronto, ON, Canada, M5G 2M9; e-mail: aaron.schimmer@utoronto.ca.