Abstract
APRIL (A proliferation-inducing ligand) is a TNF family member that binds two TNF receptor family members, TACI and BCMA. It shares these receptors with the closely related TNF family member, B-cell activating factor (BAFF). Contrary to BAFF, APRIL binds heparan sulfate proteoglycans (HSPGs), which regulates cross-linking of APRIL and efficient signaling. APRIL was originally identified as a growth promoter of solid tumors, and more recent evidence defines APRIL also as an important survival factor in several human B-cell malignancies, such as chronic lymphocytic leukemia (CLL). To target APRIL therapeutically, we developed two anti–human APRIL antibodies (hAPRIL.01A and hAPRIL.03A) that block APRIL binding to BCMA and TACI. Their antagonistic properties are unique when compared with a series of commercially available monoclonal anti–human APRIL antibodies as they prevent in vitro proliferation and IgA production of APRIL-reactive B cells. In addition, they effectively impair the CLL-like phenotype of aging APRIL transgenic mice and, more importantly, block APRIL binding to human B-cell lymphomas and prevent the survival effect induced by APRIL. We therefore conclude that these antibodies have potential for further development as therapeutics to target APRIL-dependent survival in B-cell malignancies.
Introduction
A proliferation inducing ligand (APRIL) is a TNF family ligand that is expressed as type II transmembrane protein. APRIL has high sequence homology with BAFF (B-cell activating factor belonging to the TNF family), but distinct from BAFF and most TNF family members, APRIL is cleaved in the Golgi apparatus by a furin convertase and secreted as a soluble ligand.1,2 APRIL and BAFF both bind 2 members of the TNF receptor family: B-cell maturation antigen (BCMA) and transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI).3-6 BAFF binds another TNF family receptor, BAFF-Receptor7 (BAFF-R). In contrast, APRIL binds heparan sulfate proteoglycans8,9 (HSPGs), and this interaction provides a platform for retention and multimerization of secreted APRIL, which is necessary to deliver an effective signal via BCMA and TACI.10,11 In addition, binding of APRIL to TACI and HSPG has been suggested to induce signaling through HSPG as well.12
APRIL expression was initially observed in solid tumors and transformed cell lines and was shown to stimulate proliferation of such cells both in vitro and in vivo.4,13 Since then, studies have revealed a physiologic role for APRIL in humoral immunity (reviewed in Kimberly et al1 and Dillon et al14 ), which is, despite the overlap in receptor interaction, clearly distinct from the role of BAFF. BAFF knockout mice reveal a nonredundant role for BAFF in B-cell maturation and conversely, BAFF transgenic expression results in B-cell expansion that leads to autoimmunity.15 In contrast, APRIL transgenic mice (APRIL-Tg)16 as well as APRIL-deficient mice17 do not exhibit abnormalities in B-cell development. These differences indicate that BAFF, mainly through BAFF-R, serves as an important survival factor for immature B cells, while APRIL is dispensable for B-cell development. However, APRIL appears to be a critical factor for long-term survival of activated B cells in bone marrow and mucosal-associated lymphoid tissue (MALT).18 Moreover, APRIL is involved in driving antibody class switch recombination (CSR).19 In the gut, APRIL that is produced by intestinal epithelial cells (IECs) and dendritic cells (DCs) drives CSR to IgA2.20 Consistent with these findings, APRIL-Tg mice elicit enhanced IgA responses especially toward thymus-independent antigens, while APRIL-deficient mice largely lack this response.21
Besides a physiologic role in B-cell responses, APRIL and BAFF have been implicated in several lymphoid malignancies that include chronic lymphocytic leukemia (CLL), multiple myeloma, follicular lymphoma, and diffuse large B-cell lymphoma 22-26 as well as in autoimmune diseases, such as systemic lupus erythematosus and Sjögren syndrome.27 Although both BAFF and APRIL can stimulate B-cell survival in vitro, recent findings suggest distinct roles of the 2 ligands in lymphoma progression. Whereas APRIL levels in serum are increased and correlate with prognosis, BAFF levels do not clearly associate with prognosis in CLL patients.28,29 Similarly, interaction among APRIL, HSPG, and TACI is suggested to be critical for survival of multiple myeloma, which expresses high surface levels of the HSPG, syndecan-1.30 The apparent lymphoma-supporting role of APRIL in CLL is underscored by our own observation in APRIL-Tg mice that develop a CLL-like phenotype characterized by expansion of peritoneal CD5+ B1 B cells, which on aging results in infiltration of spleen, kidney and liver.25
To date, molecules that target both APRIL and BAFF (TACI-Fc, branded as Atacicept) or BAFF alone (Belimumab or Lymphostat-B) have been characterized and have entered clinical trials for treatment of autoimmune diseases and B-cell malignancies.31,32 However, the crucial role for BAFF in normal B-cell development may potentially limit the use of such drugs as they lead to erosion of the mature B-cell compartment.33 This therefore warrants development of APRIL antagonists that selectively target APRIL pro-survival responses in human B-cell malignancies. Here, we report the development and characterization of 2 anti–human APRIL monoclonal antibodies, which effectively block binding of APRIL to both BCMA and TACI and prevent in vitro and in vivo APRIL-induced B-cell activation. These 2 antibodies inhibit the APRIL-dependent CLL-like phenotype in mice, and more importantly, prevent APRIL-induced survival of primary CLL in vitro.
Methods
Production and purification of anti-APRIL antibodies
Antibodies were generated using preselection of B cells and mini-electrofusion method.34 In short, 7- to 8-week-old female BALB/C mice were immunized with gold bullets coated with plasmids pCI-neo-hAPRIL, mFlt3L and mGM-CSF (Aldevron) in a 2:1:1 ratio in both ears using a Helios Gene gun (BioRad) for 4-5 cycles. Mice with high serum titers against recombinant FLAG-hAPRIL11 were immunized for a final round and killed 4 days later. Erythrocyte-depleted splenocytes were subjected to a negative panning (anti–FLAG-M2, Sigma-Aldrich), and a positive panning (anti–FLAG-M2 bound to FLAG-hAPRIL), using tosyl-activated Dynabeads M-450 (Invitrogen). Antigen-specific B cells were cultured as described.34 After 8 days APRIL-reactive supernatants were screened for inhibition of FLAG-hAPRIL binding to BCMA-Fc. B-cell clones were selected and immortalised by mini-electrofusion to generate hybridomas. Selected hybridomas were cultured in DMEM/F12-modified media (Sigma-Aldrich) containing 10% BCS, 2mM l-glutamine, penicillin/streptomycin, HT supplement, and 1% (vol/vol) T24 conditioned media (IL-6 containing supernatant [CM]). For purification, hybridomas were cultured in serum-free EX-CELL CD hybridoma media (SAFC Biosciences) containing penicillin/streptomycin, 10mM l-glutamine, SyntheChol NSO Supplement (Sigma-Aldrich), and 1% serum-free T24 CM in CELLine bioreactors (Integra-Biosciences). After 7-10 days in culture, supernatants were harvested, filtered, and purified with ProteinG HiTrap columns (GE Healthcare). Antibodies were eluted with 0.1M Glycine pH = 2.7 and neutralized with 3M tris(hydroxymethyl)aminomethane. Buffer was exchanged for PBS using PD-10 gel-filtration columns (GE Healthcare). Antibodies were concentrated with Amicon Ultra-15 centrifugal filter units (Millipore) and quantified using spectrophotometry. Purity was analyzed by SDS-PAGE and Coomassie staining.
APRIL binding and blocking ELISA
Mice sera, hybridoma supernatants, and purified or commercial anti-APRIL antibodies were tested for APRIL binding in an ELISA. Plates (96-well Nunc Maxisorp) were coated with 50 ng of rabbit–anti-FLAG (Sigma-Aldrich) overnight at 4°C. All subsequent incubation steps were carried out at room temperature for 1 hour, alternating with washes with PBST (PBS/0.1% Tween 20). Plates were blocked with 10% goat serum/PBS/0.01% Tween and incubated with supernatant (1:4 in PBS) containing soluble FLAG-hAPRIL, followed by incubations with mouse sera, hybridoma supernatants or purified antibodies. Bound antibodies were detected using goat anti–mouse IgG–HRP (1:2000, Southern Biotech) and visualized using OptiEIA TMB substrate (BD Biosciences). To assess antagonism of purified hAPRIL.01A and hAPRIL.03A, a blocking ELISA was used. For comparison, a panel of commercially available anti-hAPRIL antibodies were tested: Aprily-1, Aprily-2, Aprily-5, Aprily-8, Sacha-1, Sacha-2 (Alexis Biochemicals), LS-C18658, LS-C18659, LS-C18687 (Lifespan Biosciences), TNFSF13 monoclonal antibody (M02) clone G3 (ABNOVA), Human APRIL/TNFSF13 MAb clone 101115 (R&D Systems) and Clone T3-6 (Biolegend). 96-well plates were coated with 50 ng of recombinant (R&D Systems) or in-house purified BCMA-Fc or TACI-Fc and incubated at 4°C overnight. After blocking, FLAG-hAPRIL supernatant was pre-incubated with dilutions of antibody and then added to receptor-Fc-coated plates. Bound FLAG-hAPRIL was detected with 1μg/mL of anti–FLAG-M2–HRP and visualized using BD OptEIA TMB substrate or ABTS (Sigma-Aldrich).
Kinetic analysis by bio-light interferometry
Bio-light interferometry using Octet (ForteBio) was performed by coupling purified hAPRIL.01A and hAPRIL.03A antibodies to amine-reactive biosensors (Fortebio) using standard amine chemistry. Association of recombinant hAPRIL (R&D Systems) was observed by immersing biosensors in wells containing 1 or 2μg/mL APRIL and monitoring interferometry. Dissociation was measured after transfer of the biosensors into PBS. The observed on/off rates (kass/kdiss) were analyzed using a 1:1 binding global fit model, and the equilibrium binding constant, KD, was calculated.
Cells and animals
Cell lines were obtained from ATCC or provided by Dr Marcel Spaargaren (Department of Pathology, Academic Medical Center [AMC], Amsterdam, The Netherlands) and cultured in RPMI (Gibco Life Technologies) containing 8% FCS, 2mM l-glutamine, penicillin/streptomycin (complete medium) and maintained at 37°C with 5% CO2. RAJI, Ramos, and Namalwa are Burkitt lymphoma lines, while UM-1, L363 and RPMI-8226 are multiple myeloma lines. C57BL/6 APRIL-Tg16 and wild-type (WT) mice were used in our experiments. Mice were bred in the AMC and all experiments were approved by the AMC institutional animal ethical committee.
CLL samples
Peripheral blood samples were obtained from CLL patients under supervision at the AMC. The study was approved by the institutional medical ethical review board and informed consent was obtained from patients in agreement with the Helsinki Declaration of 1975, revised in 1983. Peripheral blood mononuclear cells from CLL patients were isolated via density-gradient centrifugation and contained > 90% CD5+/CD19+ cells. Cells were frozen in IMDM/15% FCS/10% DMSO, and after freeze-thaw, viability was > 95%. The cells were subsequently cultured in IMDM supplemented with FCS, Glutamine and penicillin/streptomycin.
Mouse B-cell assays
All mouse primary cells were maintained at 37°C with 5% CO2. Mouse B cells were purified from splenocytes using magnetic activated cell separation with CD45R/B220 magnetic-activated cell sorting beads (Miltenyi Biotec) and cultured in 96-well round-bottomed microtiter plates (2 × 105 /well). To measure proliferation, anti-IgM–treated B cells (5μg/mL; Jackson ImmunoResearch Laboratories) were incubated with FLAG-hAPRIL-conditioned medium or purified FLAG-hBAFF (500ng/mL; Alexis Biochemicals) in combination with a panel of antibodies and recombinant proteins (5μg/mL). Cross-linking anti-FLAG M2 monoclonal antibody (Sigma-Aldrich) was added at a final concentration of 1μg/mL. The cells were incubated at 37°C for 48 hours and pulsed with 0.3μCi 3H thymidine (GE Healthcare) for 18 hours before harvesting. To measure IgA production, mouse B cells were cultured without anti-IgM stimulation and treated as described in the preceding sentences. After 6 days, supernatant was collected and assayed for IgA by ELISA. Coated 96-well plates (2μg/mL anti–mouse-Ig; Southern Biotech), were blocked with PBS/5% BSA and, afterwashes with PBST, incubated at 37°C for 1 hour with collected supernatants. Bound IgA was detected with HRP-labeled anti–mouse-IgA (Southern Biotech) and ABTS (Sigma-Aldrich).
In vivo experiment to block APRIL function
APRIL-Tg and WT littermate mice (8-10 weeks, 5 mice/group) were injected intraperitoneally with either PBS (200μL), hAPRIL.01A, hAPRIL.03A, TACI-Fc, or msIgG1 (200μg/200μL PBS). Treatment started 3 days before 4-hydroxy-nitrophenacetyl-Ficoll (NP-Ficoll; Biosearch Technologies) immunization (day 0; 100μL intraperitoneally with 250μg of the immunogen) and injections were administered twice a week for 4 weeks. Blood was collected on days −1, 3, 7, 13, and 28 and assayed by ELISA for anti–NP-specific antibodies (IgM, IgG, and IgA) using diluted sera (1:100 for IgA; 1:500 for IgG and 1:2000 for IgM) as described.21 One-way ANOVA test was used to check statistical significance between groups. To study the effect of antibody or receptor-Fc treatment on splenic B cells, mice were killed on day 30 after immunization (NP-Ficoll). Splenocytes were counted and stained with anti-CD3–antigen-presenting cell (eBioscience)/anti–B220-FITC or with anti–IgM-phycoerythrin/anti–IgD-FITC (BD Biosciences). After washes with PBS-BSA-1%, cells were analyzed by FACS. To examine B1 B-cell expansion, 8-9 weeks old APRIL-Tg mice were injected intraperitoneally twice a week (100 μg in 100 μL PBS) with either hAPRIL.01A, msIgG1, TACI-Fc or PBS. WT littermates were treated with PBS. After 3 months of treatment, mice were killed and peritoneal exudate cells (PECs) were stained with anti–IgD-FITC/anti–CD43-PE (BD Biosciences) to discriminate B1 and B2 B cells. Anti-CD3–antigen-presenting cells (eBioscience) was used to gate out T cells.
APRIL binding and APRIL/BAFF receptors expression and internalization
To assay for APRIL/BAFF receptor expression, 5 × 105 cells in a 96-well plate were incubated with conditioned medium containing FLAG-hAPRIL or control medium (MOCK) with or without 1.5μg/mL of cytosine guanine dinucleotide (Invivogen), and in the absence or presence of the blocking anti-APRIL antibody hAPRIL.01A. After 24 hours, cells were stained with anti–TACI-PE (BD Biosciences), anti–BCMA-FITC (Alexis, Vicky-1) and/or anti–BR-3–PE (BD Biosciences) for 30 minutes at 4°C, washed with PBS/BSA 1%, and analyzed by FACS. Isotype-matched antibodies were used as negative staining controls. To test for APRIL binding, a purified FLAG-tagged mutant form of APRIL that does not bind HSPG11 was incubated at 5μg/mL in PBS/1% BSA with 3 × 105 cells/well. After 1 hour incubation on ice, bound APRIL was detected with anti–FLAG-Biotin (Sigma-Aldrich), followed by Streptavidin–antigen-presenting cell (BD Biosciences). Samples were analyzed by FACS. To determine receptor internalization on ligand interaction, cells were labeled with the directly conjugated anti–TACI-PE antibody and incubated with APRIL or MOCK conditioned medium at 37°C. After 1 hour cells were placed on ice and treated 1 minute with either 0.154M NaCl pH 2 (to strip off detection antibodies) or PBA (PBS/1% BSA/Azide), then analyzed for the presence of remaining phycoerythrin label. In the case of acid treatment the remaining signal represents internalized antibody-receptor complexes.
CLL proliferation and survival
CLL patient samples were labeled for 10 minutes in 1mL PBS with 5μM of CFSE (Invitrogen), and the reaction was stopped with ice-cold medium containing 10% FCS. The cells were spun down, resuspended in culture medium with APRIL or mock conditioned medium (1:4 dilution), and plated in a 48-well plate at a density of 105 cells/well. Viability was assessed at different time points using PI staining to detect dead cells, and proliferation was assessed by CFSE dilution. Apoptotic cells were detected by antigen-presenting cell–labeled annexin V and 7-amino-actinomycin D (Becton Dickinson) following manufacture's instructions.
Detection of APRIL in serum of APRIL TG mice
Human APRIL was immunoprecipitated out of 10 μL of mouse serum using Aprily-5 antibody coupled to protein-G (Santa Cruz Biotechnology). After 2 hours of incubation, bound APRIL was pulled down and loaded on gel for SDS-PAGE. Human APRIL was revealed by biotinylated Aprily-5 followed by Streptavidin-HRP.
Results
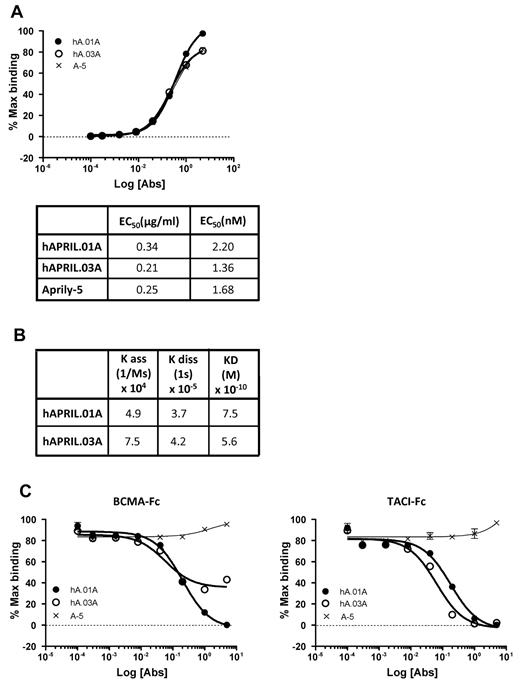
hAPRIL.01A and hAPRIL.03A bind APRIL with high affinity and block APRIL binding to BCMA and TACI
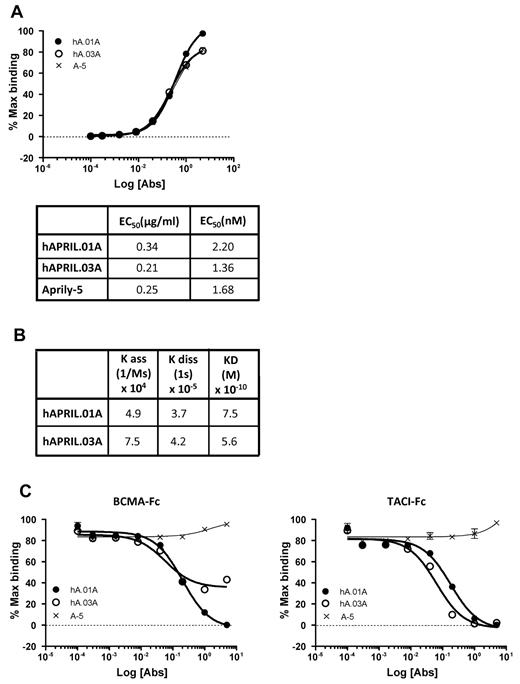
To generate APRIL antagonistic antibodies, mice were immunized with plasmids that encode human APRIL and murine immunostimulatory signals. APRIL-reactive B cells were isolated using magnetic beads coated with hAPRIL and were immortalized by mini-electrofusion with myeloma cells as described.34 We isolated 2 hybridomas, hAPRIL.01A (hA.01A) and hAPRIL.03A (hA.03A) that bind adsorbed human APRIL by ELISA with EC50 values of 2.2 and 1.4nM, respectively, which is in the same order of magnitude as the anti-APRIL antibody Aprily-5 (Figure 1A). To further elucidate binding kinetics and calculate equilibrium binding constants, both antibodies were also profiled using bio-light interferometry using the Octet system (Figure 1B). This confirmed a high affinity for APRIL, marginally higher than the reported affinity of APRIL for its receptors,35 and suggested that the antibodies have the required affinity to act antagonistically. To determine this directly, a competitive ELISA was designed to measure binding of FLAG-hAPRIL to either coated TACI-Fc or BCMA-Fc. This demonstrated that both antibodies effectively blocked the interaction of APRIL with TACI-Fc at a range of concentrations, while binding to BCMA-Fc was blocked by hAPRIL.01A, but somewhat less effectively by hAPRIL.03A (Figure 1C). Importantly, their antagonistic properties are unique when compared with a series of commercially available monoclonal anti–human APRIL antibodies (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Purified hAPRIL.01A and hAPRIL.03A bind APRIL with high affinity to APRIL and block binding to its receptors BCMA and TACI. (A) An ELISA in which soluble FLAG-hAPRIL was captured by rabbit anti-FLAG antibody and reactivity for purified Aprily-5 (A-5), hAPRIL.01A (hA.01A) and hAPRIL.03A (hA.03A) purified was measured. 50% effective concentration values were extrapolated from this ELISA. (B) Binding constants and equilibrium constants for both antibodies were analyzed using bio-light interferometry using the Octet system. (C) ELISA to examine the ability of hAPRIL.01A and hAPRIL.03A to block the binding of soluble FLAG-hAPRIL to adsorbed BCMA-Fc or TACI-Fc (both coated at 0.5 μg/mL). Aprily-5, a nonantagonistic anti-APRIL antibody, was used as a control. Antibodies were titrated by 5-fold dilution steps starting from 5 μg/mL.
Purified hAPRIL.01A and hAPRIL.03A bind APRIL with high affinity to APRIL and block binding to its receptors BCMA and TACI. (A) An ELISA in which soluble FLAG-hAPRIL was captured by rabbit anti-FLAG antibody and reactivity for purified Aprily-5 (A-5), hAPRIL.01A (hA.01A) and hAPRIL.03A (hA.03A) purified was measured. 50% effective concentration values were extrapolated from this ELISA. (B) Binding constants and equilibrium constants for both antibodies were analyzed using bio-light interferometry using the Octet system. (C) ELISA to examine the ability of hAPRIL.01A and hAPRIL.03A to block the binding of soluble FLAG-hAPRIL to adsorbed BCMA-Fc or TACI-Fc (both coated at 0.5 μg/mL). Aprily-5, a nonantagonistic anti-APRIL antibody, was used as a control. Antibodies were titrated by 5-fold dilution steps starting from 5 μg/mL.
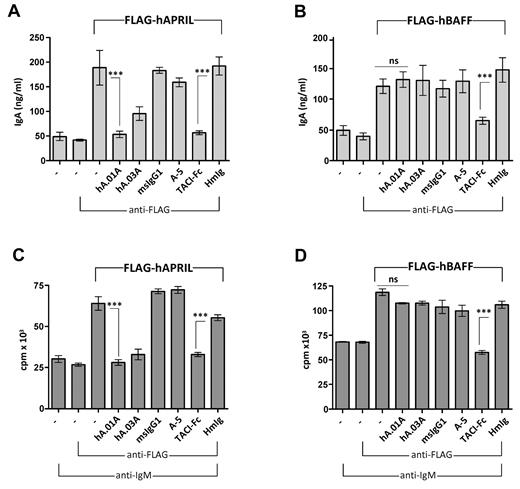
hAPRIL.01A and hAPRIL.03A prevent APRIL-driven IgA production and proliferation of mouse B cells
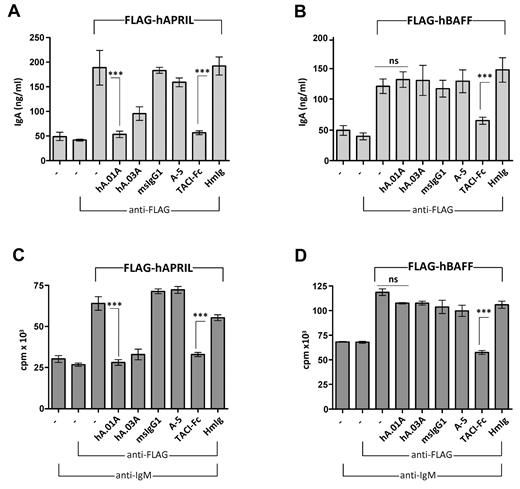
To show that both antibodies can functionally block APRIL responses in vitro, mouse B cells were used to examine APRIL-driven IgA production or APRIL-induced costimulation of anti-IgM–treated mouse B cells. Both hAPRIL.01A and TACI-Fc were able to fully block the APRIL-induced response, while Aprily-5, a noncompetitive APRIL binder, or the negative isotype controls (mIgG1 and hIg) showed no inhibitory effect (Figure 2A). Interestingly, hAPRIL.03A was marginally less effective than hAPRIL.01A at blocking IgA production (Figure 2A). As expected, only TACI-Fc, which binds both APRIL and BAFF, was able to inhibit IgA production of BAFF-stimulated B cells (Figure 2B), confirming that the antagonistic antibodies act specifically via APRIL. Similar inhibition profiles were observed when hAPRIL or hBAFF-induced costimulation of B-cell proliferation was analyzed (Figure 2C,D), indicating that hAPRIL.01A and hAPRIL.03A effectively and selectively block APRIL-mediated responses in vitro.
hAPRIL.01A and hAPRIL.03A block APRIL driven IgA production and proliferation of mouse B cells. Mouse B220+ B cells were stimulated with conditioned medium containing soluble FLAG-hAPRIL (A) or soluble FLAG-hBAFF (B) for 6 days and the supernatants assayed by ELISA for IgA production. The same cells were treated with anti-IgM (5μg/mL), costimulated with medium containing FLAG-hAPRIL (C) or soluble FLAG-hBAFF (D) for 2 days and pulsed with tritiated thymidine for 18 hours before harvesting. Cross-linking condition (via the addition of anti-FLAG antibody) was used to optimize the APRIL and BAFF signal. Aprily-5 (A-5) was used as a nonantagonistic control, mouse IgG1 (msIgG1) was used as an isotype control for hA.01A/hA.03A and human IgG (HmIg) as control for TACI-Fc. Each sample was analyzed in triplicate. ***P < .001, 1-way ANOVA; error bars = SD.
hAPRIL.01A and hAPRIL.03A block APRIL driven IgA production and proliferation of mouse B cells. Mouse B220+ B cells were stimulated with conditioned medium containing soluble FLAG-hAPRIL (A) or soluble FLAG-hBAFF (B) for 6 days and the supernatants assayed by ELISA for IgA production. The same cells were treated with anti-IgM (5μg/mL), costimulated with medium containing FLAG-hAPRIL (C) or soluble FLAG-hBAFF (D) for 2 days and pulsed with tritiated thymidine for 18 hours before harvesting. Cross-linking condition (via the addition of anti-FLAG antibody) was used to optimize the APRIL and BAFF signal. Aprily-5 (A-5) was used as a nonantagonistic control, mouse IgG1 (msIgG1) was used as an isotype control for hA.01A/hA.03A and human IgG (HmIg) as control for TACI-Fc. Each sample was analyzed in triplicate. ***P < .001, 1-way ANOVA; error bars = SD.
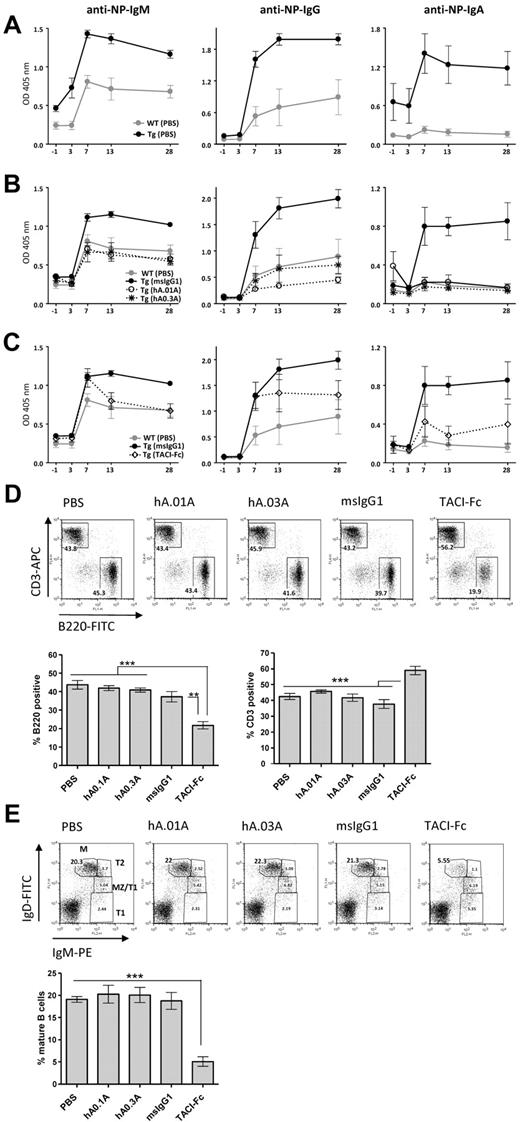
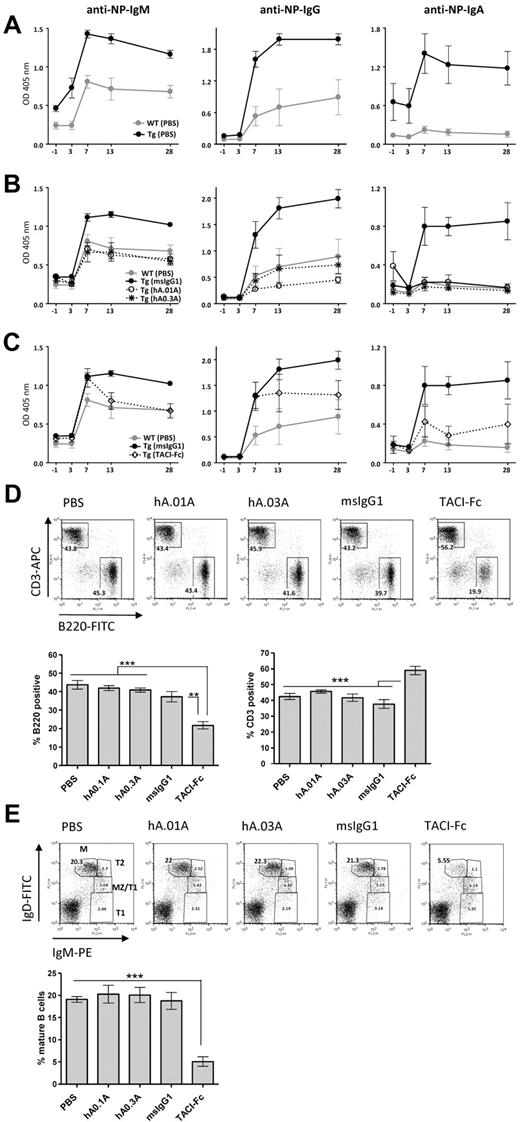
hAPRIL.01A and hAPRIL.03A decrease thymus-independent Type II antibody responses in APRIL transgenic mice
To demonstrate an in vivo blocking effect of the antibodies on APRIL function, we examined their ability to block the previously reported21 enhanced humoral response against NP-Ficoll in APRIL-Tg mice, (Figure 3A). Administration of both hAPRIL.01A and hAPRIL.03A to APRIL-Tg mice reduced thymus-independent B-cell response to the same levels found in WT-littermates, as shown by measurement of 3 different anti-NP antibody isotypes: IgM, IgG and IgA (Figure 3A). As expected, APRIL-Tg mice treated with mouse IgG1 as an isotype-matched control did not show any reduction of the anti-NP titres (Figure 3B). In contrast, TACI-Fc did show antagonistic activity, but this was less pronounced than hAPRIL0.1A and hAPRIL0.3A (Figure 3C), pointing to a higher efficacy of the antibodies in blocking APRIL in comparison with TACI-Fc. Treatment of mice with TACI-Fc has previously been reported to massively affect B-cell development in an APRIL-independent way through inhibition of BAFF.33,36 In agreement, analysis of splenic B-cell populations of TACI-Fc–treated mice showed a marked reduction in the overall number of splenic B cells and, as a consequence, a relative increase in the percentage of T cells (Figure 3D). The maturation of B cells in this group of animals, was impaired beyond the T1 stage of maturation displaying a strong decrease in mature B cells (Figure 3E), consistent with the nonredundant role of BAFF in this step of B-cell development. Treatment with either hAPRIL.01A or hAPRIL.03A did not show any alteration in the B-cell compartments analyzed (Figure 3D-E).
hAPRIL.01A and hAPRIL.03A decrease thymus-independent type II antibody responses in APRIL transgenic mice without affecting the splenic B-cell compartment. Mice (5 per group) were immunized with NP-Ficoll at day 0 and treated with the indicated proteins for 1 month from the start of injection. Anti–NP-specific antibody titres (IgM-IgG-IgA) were measured by ELISA at the indicated time points. (A) Anti-NP antibody titres in APRIL-Tg and WT littermates treated with PBS. (B) Effect of hAPRIL.01A and hAPRIL.03A on anti-NP antibody titers in APRIL-Tg mice. (C) Effect of TACI-Fc on anti-NP antibody titres in APRIL Tg mice. Please note: Some of the control lines are shown in multiple figures. The TACI-Fc and antibody inhibition were performed within one experiment, but are shown in separate graphs for clarity. Y-axis represents OD measured in the ELISA and therefore represents a relative measure of NP-specific antibodies. (D) Representative FACS profiles (and relative histograms) to show the percentage of T (CD3 positive) and B (B220 positive) lymphocytes in the spleen of APRIL Tg mice treated with the indicated proteins. (E) Splenocytes from the same mice were also screened for different B-cell subsets: M indicates mature; MZ, marginal zone; T2, transitional 2; and T1, transitional 1 B cells. Percentages of mature B cells are plotted on the histogram. ***P < .001, **P < .01, 1-way ANOVA.
hAPRIL.01A and hAPRIL.03A decrease thymus-independent type II antibody responses in APRIL transgenic mice without affecting the splenic B-cell compartment. Mice (5 per group) were immunized with NP-Ficoll at day 0 and treated with the indicated proteins for 1 month from the start of injection. Anti–NP-specific antibody titres (IgM-IgG-IgA) were measured by ELISA at the indicated time points. (A) Anti-NP antibody titres in APRIL-Tg and WT littermates treated with PBS. (B) Effect of hAPRIL.01A and hAPRIL.03A on anti-NP antibody titers in APRIL-Tg mice. (C) Effect of TACI-Fc on anti-NP antibody titres in APRIL Tg mice. Please note: Some of the control lines are shown in multiple figures. The TACI-Fc and antibody inhibition were performed within one experiment, but are shown in separate graphs for clarity. Y-axis represents OD measured in the ELISA and therefore represents a relative measure of NP-specific antibodies. (D) Representative FACS profiles (and relative histograms) to show the percentage of T (CD3 positive) and B (B220 positive) lymphocytes in the spleen of APRIL Tg mice treated with the indicated proteins. (E) Splenocytes from the same mice were also screened for different B-cell subsets: M indicates mature; MZ, marginal zone; T2, transitional 2; and T1, transitional 1 B cells. Percentages of mature B cells are plotted on the histogram. ***P < .001, **P < .01, 1-way ANOVA.
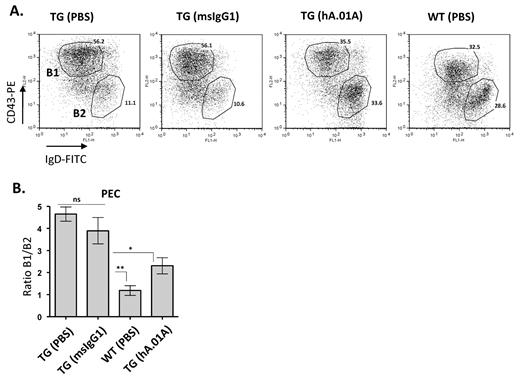
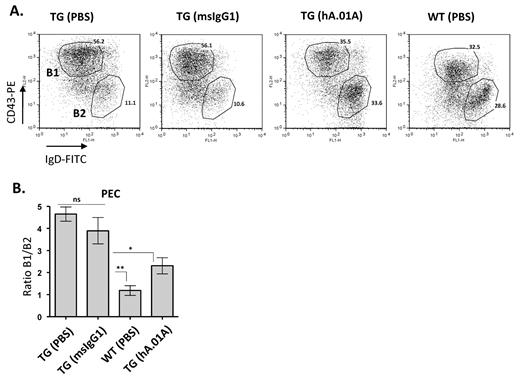
hAPRIL.01A inhibits peritoneal B-cell expansion
The exacerbated thymus independent immune response in APRIL-Tg mice is thought to be the result of hyper-stimulation of B1 B cells in the peritoneal cavity, which are the main responders to NP-Ficoll. In agreement, we previously showed that APRIL-Tg mice are characterized by an age-related expansion of peritoneal B1 B cells.25 The gradual accumulation of B1 B cells leads to mesenteric lymph node and Peyer patch hyperplasia and eventually to splenic as well as kidney and liver infiltration.25 B1 B cell–derived hyperplasia in mice has been compared with human CLL, because both diseases develop as a result of slowly expanding CD5-positive B-cell populations. In addition, increased serum APRIL levels is associated with poor prognosis in human CLL. To investigate whether inhibition of APRIL by hAPRIL.01A would interfere with the hyperplastic B1 B-cell expansion, APRIL-Tg mice were injected with antibodies once a week for a period of 3 months and subsequently analyzed for B1 and B2 B-cell populations in the peritoneal cavity. APRIL-Tg mice treated with hAPRIL.01A showed a significant reduction in B1 B-cell expansion compared with mice treated with the isotype matched control (Figure 4A and 4B). Importantly, hAPRIL.01A-treated mice showed a significantly reduced ratio of B1/B2 cells, indicating that the antagonistic antibody could prevent long-term effects of transgenic APRIL expression and therefore effectively suppress the CLL-like phenotype.
hAPRIL.01A blocks Peritoneal B1 B-cell expansion Eight- to 9-week-old mice (n = 5) were treated intraperitoneally once a week for 3 months with 100μg (in 100 μL of PBS) with the indicated proteins and subsequently analyzed for their lymphocyte composition in the peritoneal cavity. (A) Representative scatter plots in which Peritoneal Exudates Cells (PECs) of 5-month-old mice were stained for IgD (FITC) and CD43 (PE) to discriminate B1 and B2 B cells. T cells were gated out using CD3 (antigen-presenting cell). (B) Bar graph showing the average of B1 B-cell expansion (%B1/%B2). **P < .01, *P < .05, 1-way ANOVA.
hAPRIL.01A blocks Peritoneal B1 B-cell expansion Eight- to 9-week-old mice (n = 5) were treated intraperitoneally once a week for 3 months with 100μg (in 100 μL of PBS) with the indicated proteins and subsequently analyzed for their lymphocyte composition in the peritoneal cavity. (A) Representative scatter plots in which Peritoneal Exudates Cells (PECs) of 5-month-old mice were stained for IgD (FITC) and CD43 (PE) to discriminate B1 and B2 B cells. T cells were gated out using CD3 (antigen-presenting cell). (B) Bar graph showing the average of B1 B-cell expansion (%B1/%B2). **P < .01, *P < .05, 1-way ANOVA.
hAPRIL.01A prevents APRIL receptor interaction and APRIL-induced survival of CLL
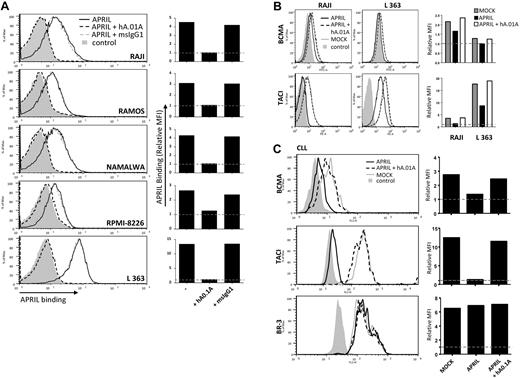
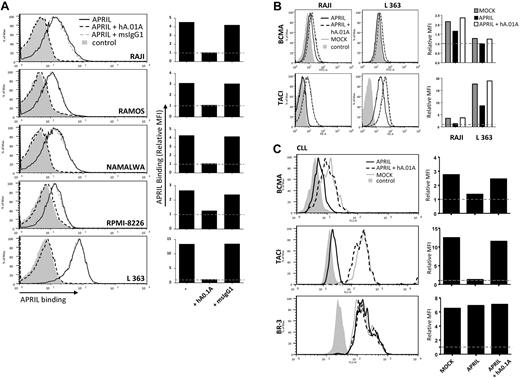
APRIL is a known survival factor for different types of B-cell lymphomas and has in some cases a clear prognostic role for disease progression. The ultimate goal in the development of APRIL antagonistic antibodies therefore is ablation of this survival effect. To test the efficacy of our antagonistic antibodies on human B-cell lymphomas, we first analyzed whether these antibodies could prevent interaction of APRIL with its receptors on B-cell lymphoma cell lines. Some of these lines express low to very low levels of TACI, while L363 showed high levels of this receptor (supplemental Figure 2). BCMA expression appeared consistently low on these cells. To exclude binding to HSPG expressed on the lymphoma cells, we made use of an APRIL variant that can only interact with BCMA and TACI, but not HSPG.11 Incubation of lymphoma lines with this mutant FLAG-hAPRIL showed clear binding to all cell lines and this was completely blocked with the hAPRIL.01A antibody (Figure 5A), confirming antagonism on human lymphoma cells. FLAG-hAPRIL stimulation of lymphoma cells resulted in receptor internalization (supplemental Figure 3). In agreement, we observed a clear decrease in the level of BCMA and TACI on the cell surface after APRIL treatment. This decrease was completely prevented by hAPRIL.01A (Figure 5B). Combined, this indicates that the antagonistic antibodies prevent APRIL from interacting with its receptors and stimulating B-cell lymphomas.
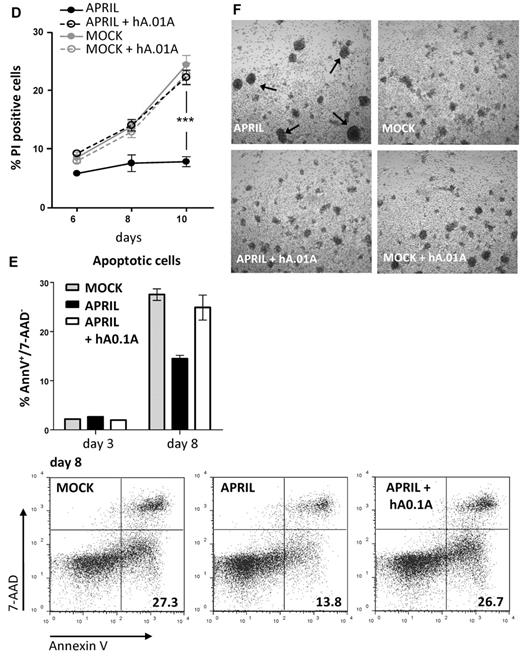
hAPRIL0.1A prevents APRIL binding to lymphoma cell lines and its survival effect on peripheral blood–derived CLL cells. (A) Five different lymphoma cell lines are shown to bind APRIL (black line) and this effect is blocked by hAPRIL0.1A (black dashed line). (B) The highest TACI/BCMA expressing cell lines (RAJI and L363, supplemental Figure 2) were screened for receptor detection after 24 hours incubation in the absence or presence of FLAG-hAPRIL. Loss of receptor detection after FLAG-hAPRIL stimulation is fully prevented when hAPRIL.1A is added to the overnight culture. (C) TACI and BCMA in cytosine guanine dinucleotide treated CLL (CLL 2, supplemental Figure 3) are down-regulated by the binding of FLAG-hAPRIL. hAPRIL.01A (hA.01A) blocks FLAG-hAPRIL binding and therefore blocks receptor down regulation of TACI and BCMA. BR-3 is taken along as a nonAPRIL binding control. (D) cytosine guanine dinucleotide treated CLL cells expressing both TACI and BCMA (CLL 2, supplemental Figure 3) were cultured in the absence or presence of FLAG-hAPRIL and tested for cell survival at different time-points. The effect of hAPRIL.01A was analyzed on control (MOCK) and FLAG-hAPRIL treated cells (APRIL). Each sample was analyzed in triplicate; ***P < .001 1-way ANOVA; error bars = SD. (E). Apoptosis of CLL cells measured after 3 and 8 days in the absence or presence of APRIL and in the presence of APRIL plus hAPRIL01.A. Apoptosis is measured using annexinV staining and counterstaining for dead cells (late apoptotic) with 7-AAD. (F). Representative pictures (magnified 5×) of the cells treated as indicated in panel D. Experiments (A-C) are accompanied by histograms that represent the relative mean fluorescence intensity (MFI) determined by the ratio between the background and the specific MFI. Basal value (MFI = 1) is pointed out by the gray dotted line.
hAPRIL0.1A prevents APRIL binding to lymphoma cell lines and its survival effect on peripheral blood–derived CLL cells. (A) Five different lymphoma cell lines are shown to bind APRIL (black line) and this effect is blocked by hAPRIL0.1A (black dashed line). (B) The highest TACI/BCMA expressing cell lines (RAJI and L363, supplemental Figure 2) were screened for receptor detection after 24 hours incubation in the absence or presence of FLAG-hAPRIL. Loss of receptor detection after FLAG-hAPRIL stimulation is fully prevented when hAPRIL.1A is added to the overnight culture. (C) TACI and BCMA in cytosine guanine dinucleotide treated CLL (CLL 2, supplemental Figure 3) are down-regulated by the binding of FLAG-hAPRIL. hAPRIL.01A (hA.01A) blocks FLAG-hAPRIL binding and therefore blocks receptor down regulation of TACI and BCMA. BR-3 is taken along as a nonAPRIL binding control. (D) cytosine guanine dinucleotide treated CLL cells expressing both TACI and BCMA (CLL 2, supplemental Figure 3) were cultured in the absence or presence of FLAG-hAPRIL and tested for cell survival at different time-points. The effect of hAPRIL.01A was analyzed on control (MOCK) and FLAG-hAPRIL treated cells (APRIL). Each sample was analyzed in triplicate; ***P < .001 1-way ANOVA; error bars = SD. (E). Apoptosis of CLL cells measured after 3 and 8 days in the absence or presence of APRIL and in the presence of APRIL plus hAPRIL01.A. Apoptosis is measured using annexinV staining and counterstaining for dead cells (late apoptotic) with 7-AAD. (F). Representative pictures (magnified 5×) of the cells treated as indicated in panel D. Experiments (A-C) are accompanied by histograms that represent the relative mean fluorescence intensity (MFI) determined by the ratio between the background and the specific MFI. Basal value (MFI = 1) is pointed out by the gray dotted line.
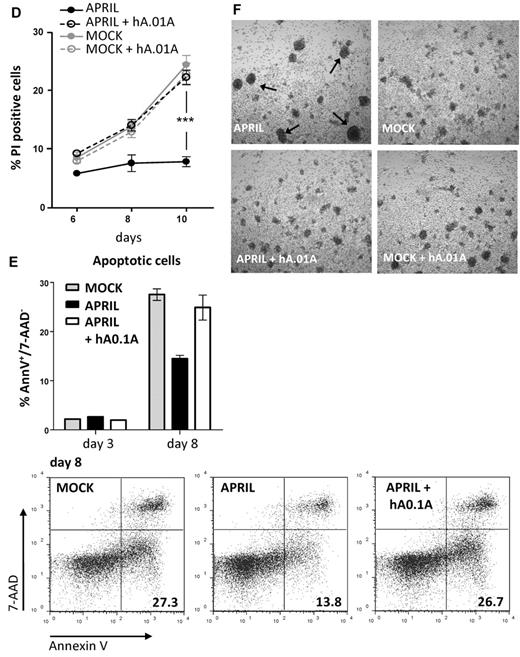
Survival of CLL has been suggested to rely on the production of APRIL by the surrounding microenvironment, which may also provide relevant signals for up-regulation of TACI and BCMA. In vitro, this dependency can be mimicked by coculture with nurse-like cells (NLCs) or by addition of APRIL.37 To directly test whether the antagonistic antibodies had an inhibitory effect on survival elicited by APRIL on lymphoma cells we obtained freshly isolated peripheral blood mononuclear cell's from blood of CLL patients, which contain > 90% of CLL cells. Isolated CLL cells express variable levels of TACI and BCMA, but stimulation with cytosine guanine dinucleotide, a TLR9 ligand, activated CLL B cells and led to an enhanced expression of TACI, while BCMA showed variable induction (supplemental Figure 4). As observed with the lymphoma cell lines, we found that FLAG-hAPRIL stimulation induced a decrease in TACI and BCMA on the surface of these primary lymphoma cells as well, while no reduction was observed for BR-3 (Figure 5C). Also under these conditions hAPRIL.01A was able to fully block APRIL-induced decrease in BCMA and TACI cell surface expression (Figure 5C). This indicates that APRIL binds its predicted targets and that hAPRIL.01A fully prevents binding to naturally occurring membrane-bound receptors on lymphoma cell lines and primary CLL.
The same patient-derived CLL cells were also tested for an APRIL-mediated survival benefit. Stimulated CLL cells with hFLAG-APRIL in the presence of cytosine guanine dinucleotide displayed only a mild increase in proliferation (visible at day 6, supplemental Figure 5). In contrast, APRIL stimulation induced a reproducible survival advantage over time (Figure 5D), that was a result of decreased apoptosis in the APRIL-treated CLL cells (Figure 5E). The presence of APRIL in CLL cultures also led to the formation of larger, easily distinguishable cell clusters (Figure 5F). Importantly, these effects of APRIL were completely reverted by the addition of hAPRIL.01A (Figure 5D-F, supplemental Figure 5). In conclusion, our data indicate that hAPRIL.01A can interfere with the activity of APRIL toward its TNF-like receptors TACI and BCMA and prevents APRIL-induced survival of malignant human B cells. These findings warrant further development of these antibodies for therapeutic purposes.
Discussion
APRIL is a crucial factor regulating the survival of B cells under physiologic and pathologic settings. Here we show that blocking APRIL binding to its receptors, TACI and BCMA, has an inhibitory effect on known effects of APRIL both in vitro and in vivo. APRIL antagonism prevents APRIL-induced effects in mouse B-cell assays and blocks B-cell expansion seen in aged transgenic mice representing a model for CLL. Importantly, mice treated with APRIL antagonistic antibodies display significant reduction in APRIL serum levels after 2 injections and undetectable levels after 4 weeks of antibody treatment (supplemental Figure 6). To demonstrate therapeutic capacity, we extended our study to examine the effect of APRIL antagonism on the survival of lymphoma cells. Importantly, we confirmed that APRIL binding and stimulation of lymphoma cells could be blocked by our antibodies, as well as APRIL-induced survival of malignant CLL cells in vitro. These primary CLL cells do not have an intrinsic resistance to apoptosis and normally undergo spontaneous apoptosis when cultured in vitro (Figure 5E). Coculture with NLCs or addition of recombinant APRIL to the culture enhances their survival.37 Triggering TLR9 induces an up-regulation of TACI in murine B cells,38 an effect that is mirrored in primary CLL, where TACI, and in some cases BCMA, is up-regulated (supplemental Figure 4), rendering them receptive to APRIL survival signals (Figure 5). Our observations therefore confirm the survival benefit APRIL provides to CLL/B1 B cells, and illustrate the activity of the anti-APRIL antibodies in blocking this.
BAFF and APRIL both serve an important role in B-cell development and immunity. Despite the overlap in receptors, both ligands regulate different aspects of B-cell biology. This can be explained in part by the unique binding of BAFF to BR-3, which is crucial for the development of B cells. However, a partly unexplained bias toward one or the other ligand is evident during different phases of humoral immunity. Under physiologic conditions, APRIL is indispensible for survival of bone marrow plasma cells as well as for longevity of the response in mucosal immunity. Importantly, BAFF can clearly stimulate survival of plasma cells under in vitro conditions, yet appears superfluous in the bone marrow in vivo. This is in part suggested to be the result of syndecan-1 expression on plasma cells, which provides a high affinity HSPG binding site for APRIL and enhances the efficacy of the ligand. Moreover, it appears that selective local production of APRIL in the bone marrow skews the in vivo dependence of this survival response toward APRIL. This latter idea is further supported by the recent observation that megakaryocytes, which produce significant amounts of APRIL,39 are a crucial constituent of the bone marrow niche for plasma cells.40 Such a selective local production of APRIL may be crucial for the survival of lymphoid malignancies in the bone marrow.
Although both BAFF and APRIL have been suggested to have pro-survival effects in lymphoid malignancies, such observations are frequently based on in vitro studies using recombinant ligands. If APRIL is selectively produced locally, the differential usage of the 2 ligands in physiology might be followed by a differential contribution in cancer progression. In this light it is interesting to note that multiple myeloma localizes to the bone marrow and receives crucial survival signals from the local microenvironment. Both BAFF and APRIL can induce the survival of multiple myeloma in vitro, but in vivo evidence comparing TACI-Fc and BAFF-R-Fc led Yaccoby et al41 to conclude that the role of APRIL in local cell survival is dominant. This was further supported by the observations of the group of Klein, which suggests that an interdependency between APRIL, TACI and the HSPG syndecan-1 determines the survival benefit in multiple myeloma and thereby prognosis.30 That is, patients with high levels of all 3 will have a poor prognosis because of selective survival signaling in the bone marrow. This clearly points to a crucial role for APRIL in the survival of multiple myeloma cells in the bone marrow.
A similar situation appears to exist for CLL. In CLL the role of the microenvironment is particularly well established. T cells and NLCs are described to deliver important survival stimuli.42 In vitro both BAFF and APRIL, either produced by NLCs or delivered as recombinant proteins can promote survival of CLL cells.29,37 Nevertheless, the prognosis of patients has been associated with circulating levels of APRIL and not with BAFF, suggesting that APRIL also predominates in this disease.28,29 Obviously, these data do not exclude a role for locally produced BAFF that is not detected systemically. In agreement, several lines of evidence would also argue for a survival effect elicited by BAFF in vivo. However, these data do indicate a significant role for APRIL in the survival of CLL cells in patients and thus support therapeutic intervention with this survival benefit. Similarly, diffuse large B-cell lymphoma patients also appear to have a poor prognosis when their serum level of APRIL is high,26 but in these patients BAFF also clearly plays a role.43
The use of selective inhibition of APRIL as compared with combined inhibition of BAFF and APRIL with Atacicept hold promise that they will result in less side-effects. As shown in our mouse experiments, TACI-Fc treatment has rapid and detrimental effects on B-cell homeostasis. Consistent with previous findings on BAFF or BAFF-R knockout mice and TACI-Fc transgenic or TACI-Fc treated mice, we found a strong decrease in mature B-cell numbers.33,36,44 This is also observed in patients treated with Atacicept, which show a 30%-70% decrease in immunoglobulin levels and a consistent decrease in B-cell numbers.45-47 Similarly, anti-BAFF treatment (Belimumab) of systemic lupus erythematosus patients results in a strong decrease in B-cell numbers over time.48 This side-effect is unlikely to occur in anti-APRIL treated patients, because APRIL does not serve a crucial role in the development of B cells or their activation. B-cell responses are relatively normal in APRIL knockout mice, aside from a defect in IgA class switching.17,19 In addition, a decrease in long-lived plasma cells can be expected in APRIL-depleted patients as these B cells are dependent on APRIL. Nevertheless, the toxicity of APRIL antagonistic antibodies should be relatively marginal and thus a valuable option in the selective blocking of lymphoma survival signals and thus the treatment of lymphoma patients. Our mouse model for CLL provides evidence that this approach could work. Although this is clearly not a complete model for the disease CLL, we previously provided compelling evidence that a CLL-like phenotype occurs in APRIL-Tg mice25 and this selective B1 B-cell expansion that is reminiscent of CLL is prevented by treatment with hAPRIL.01A (Figure 4).
APRIL also appears to play a role in solid tumors. The original identification of APRIL suggested a role in stimulating proliferation of solid malignancies. More recent data indeed indicate a correlation between APRIL mRNA expression and prognosis in several solid malignancies,49 while we also find an important role for APRIL in colorectal cancer (M.G., J.P.M., unpublished observations, 2010). However, whether our antagonistic antibodies could serve as a treatment in solid malignancies remains to be established, because the mechanism for APRIL stimulation of solid tumors is currently unclear. Most solid tumors appear to be devoid of TACI and BCMA expression and are therefore suggested to interact with APRIL through HSPGs. However, whether this interaction provides a direct signal into tumor cells is not yet established. It appears likely that in this context HSPGs also provide a platform for binding to TACI expressed at low levels, to BCMA or to an unknown receptor. In agreement, the inhibition of colorectal tumor growth by BCMA-Fc suggests that the TNF receptor interaction site of APRIL is required for the effect observed,4 which suggests that APRIL antagonistic antibodies could be beneficial for combination treatment of solid tumors.
In conclusion, our current data provide evidence that 2 newly developed antibodies against APRIL have strong antagonistic properties and prevent binding of APRIL to TACI and BCMA. This antagonism inhibits APRIL-driven B-cell stimulation in vitro and in vivo and deprives lymphoma cells of APRIL-induced survival signals. Further development of these antibodies as therapeutic agents is therefore warranted and is ongoing.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the animal caretakers at the AMC and Greg Carven, Lilian Driessen-Engels and Sean Gurdak for technical assistance. We thank Michael Hahne for providing plasmids for the in-house expression and purification of human BCMA- and TACI-Fc.
This research was performed within the framework of project T3.112 of the Dutch Top Institute Pharma and was also supported by Dutch Cancer Society grant (2007-3750).
Authorship
Contribution: M.G., F.C.K., U.P., P.M.V., K.C. performed in vitro experiments; M.G. and H.R. performed in vivo experiments; E.E. and A.P.K. obtained CLL samples and helped designing CLL experiments. H.v.E. and U.P. planned the strategy and generated the antibodies; H.v.E. and J.P.M. designed experiments and guided the project; M.G., F.C.K. and J.P.M. wrote the paper.
Conflict-of-interest disclosure: M.G., F.C.K., U.P., H.v.E. and J.P.M. are inventors on a patent that describes the use of the antagonistic antibodies; and U.P., P.V. and H.v.E. are employees of Merck Research Laboratories, which coowns this patent. The remaining authors declare no competing financial interests.
Correspondence: Jan Paul Medema, Meibergdreef 9, 1105 AZ, Room G2-131, Amsterdam, The Netherlands; e-mail: j.p.medema@amc.uva.nl.
References
Author notes
M.G., F.C.K., and U.P. contributed equally to this study.
H.v.E. and J.P.M. contributed equally to this study.