Abstract
Apoptosis in megakaryocytes results in the formation of platelets. The role of apoptotic pathways in platelet turnover and in the apoptotic-like changes seen after platelet activation is poorly understood. ABT-263 (Navitoclax), a specific inhibitor of antiapoptotic BCL2 proteins, which is currently being evaluated in clinical trials for the treatment of leukemia and other malignancies, induces a dose-limiting thrombocytopenia. In this study, the relationship between BCL2/BCL-XL inhibition, apoptosis, and platelet activation was investigated. Exposure to ABT-263 induced apoptosis but repressed platelet activation by physiologic agonists. Notably, ABT-263 induced an immediate calcium response in platelets and the depletion of intracellular calcium stores, indicating that on BCL2/BCL-XL inhibition platelet activation is abrogated because of a diminished calcium signaling. By comparing the effects of ABT-263 and its analog ABT-737 on platelets and leukemia cells from the same donor, we show, for the first time, that these BCL2/BCL-XL inhibitors do not offer any selective toxicity but induce apoptosis at similar concentrations in leukemia cells and platelets. However, reticulated platelets are less sensitive to apoptosis, supporting the hypothesis that treatment with ABT-263 induces a selective loss of older platelets and providing an explanation for the transient thrombocytopenia observed on ABT-263 treatment.
Introduction
All nucleated cells in multicellular organisms are genetically programmed to undergo apoptosis to remove unnecessary or damaged cells from the whole organism. This program has been recognized as the central mechanism of platelet production from megakaryocytes.1 However, the role of apoptosis in anuclear, mature platelets is less well characterized, with apoptotic-like changes seen in both aging platelets and in the formation of procoagulant microparticles after agonist stimulation.
Two main pathways lead to the execution of apoptosis: the extrinsic and the intrinsic (or mitochondrial) pathways. Both converge into the activation of caspases, which are proteases that cleave > 500 cellular targets and induce typical morphologic changes associated with apoptosis in nucleated cells. A critical step in the intrinsic pathway is the loss of mitochondrial membrane potential (MMP) and the release of cytochrome c into cytosol, where it triggers the activation of caspase-9. Therefore, the release of cytochrome c from mitochondria needs to be tightly regulated: a function that is fulfilled by the BCL2 protein family, which consists of proapoptotic and antiapoptotic members that promote or block the release of cytochrome c, respectively.2,3 The proapoptotic family members BAX and BAK play an essential role in directly mediating the release of cytochrome c by forming a pore in the outer mitochondrial membrane. Antiapoptotic BCL2 proteins, including BCL2, BCL-XL, BCL-w, MCL1, and BCL2A1, prevent the activation of BAX and BAK. Besides their function in regulating mitochondrial cytochrome c release, BCL2 proteins have also been implicated in the regulation of intracellular calcium homeostasis at the endoplasmic reticulum (ER), possibly by interacting with inositol triphosphate receptors.4,5
Because of their key role, the antiapoptotic BCL2 proteins are attractive targets for anticancer therapy, with several small molecule inhibitors currently in preclinical testing or early clinical trials.6,7 Among these, the most promising and specific inhibitors are ABT-263 (Navitoclax) and ABT-737.8,9 ABT-737 shows promising antitumor activity in animal models of leukemia and lymphoma. A related compound, ABT-263, is metabolically more stable and currently in phase 1 and 2 clinical trials for leukemia and other malignancies.10 Both compounds have often been regarded as interchangeable because they bind with high affinity to BCL2, BCL-XL, and BCL-w but do not inhibit MCL1 or BCL2A1.11 Early results from the clinical trials with ABT-263, and from animal studies with ABT-737, show that thrombocytopenia is the major toxicity of these drugs.10,12,13 As this thrombocytopenia is reversible and platelet counts recover rapidly after cessation of treatment, it appears that these drugs induce platelet apoptosis directly without affecting megakaryocyte function.13
In an intriguing study by Mason et al, ABT-737-induced thrombocytopenia was shown to be a mechanism-based toxicity explained by a dependence of platelets on BCL-XL for survival.14 Although some BCL2 proteins and caspases are expressed in platelets,15–17 their roles in platelet physiology are poorly understood. The view that apoptosis and phosphatidylserine (PS) externalization may play a role in the clearance of senescent platelets by the mononuclear phagocyte system receives some support from in vitro investigations that show a time-dependent induction of apoptosis in stored platelet preparations.18 Furthermore, during storage at 37°C, the protein expression of BCL-XL decreases, further indicating a critical antiapoptotic role for BCL-XL in platelets.19 Data from knockout mice confirm the importance of BCL-XL in platelet survival because loss of BCL-XL, but not of BCL2, BCL-w, or MCL1 resulted in thrombocytopenia.14,20
The role of BAX and BAK in platelet apoptosis is less clear. Mason et al14 concluded that BAK is essential for platelet apoptosis and needs to be inhibited by BCL-XL to prevent apoptosis. During platelet aging, the levels of BCL-XL decrease because of protein degradation, leading to a reduction in BCL-XL-mediated inhibition of BAK and resulting in apoptosis.14 The proposed model of the ratio of BCL-XL and BAK as a “molecular clock” may provide a useful explanation of how platelet life span is regulated in vivo. Support for an important role for BCL-XL in the regulation of platelet ageing was provided by the observation that platelets from BCL-XL mutant mice have a life span of 11 hours in vivo, compared with 68 hours in wild-type control mice.21
BCL2 proteins also play a role in platelet activation by thrombin.22 The signaling pathways that distinguish platelet apoptosis and platelet activation are not clearly separated because both have been reported to involve a loss of MMP and externalization of PS. Some studies have addressed this question by comparing platelet activation and apoptosis.23–25 Here we investigated the effects of BCL2 inhibition on both platelet apoptosis and activation. We demonstrate that both processes are clearly distinguishable because apoptosis occurs slowly over hours and results in biochemical and ultrastructural changes that are clearly different from those seen during the more rapid process of agonist-induced activation, which occurs within minutes. Furthermore, we found that inhibition of BCL2 proteins induces an immediate calcium response, which prevents the subsequent responsiveness of platelets to physiologic agonists.
Methods
Cells
Platelets were collected from healthy volunteers or from patients with chronic lymphocytic leukemia (CLL), with written informed consent in accordance with the Declaration of Helsinki and ethical authority approval. Blood was collected in 0.085M trisodium citrate and 0.071M citric acid. Platelet-rich plasma (PRP) was separated by centrifugation for 20 minutes at 140g. Washed platelets were isolated from PRP by centrifugation for 15 minutes at 600g in the presence of 200 ng/mL prostacyclin (Sigma-Aldrich) and resuspended in HEPES-buffered saline (HBS: 10mM HEPES, 150mM NaCl, 5mM KCl, 1mM MgSO4, pH 7.4).
Chemicals
ABT-737 and ABT-263 were purchased from Selleck Chemicals. Human serum albumin, A23187, and thrombin receptor activator peptide (TRAP) were from Sigma-Aldrich. Cross-linked collagen-related peptide (CRP-XL)26 was provided by Richard Farndale (Department of Biochemistry, University of Cambridge, United Kingdom). The broad-spectrum caspase inhibitors z-VAD.fmk and Q-VD-OPh were from MP Biomedicals.
Analysis of apoptosis
To assess the differential toxicity of BCL2-antagonists on platelets and leukemia cells, citrated blood of CLL patients was incubated with different concentrations of ABT-737 or ABT-263 for 4 hours. Apoptosis of CLL cells was investigated in whole blood, and apoptosis of platelets was investigated in plasma or on preparations of washed platelets incubated at 2 × 107 cells/mL. PS externalization was assessed by flow cytometry after incubation for 15 minutes in HBS and 1.8mM CaCl2 (pH 7.4) with the pan-platelet antibody CD41/phycoerythrin (Invitrogen) and annexin V/FITC.
Platelet activation assays
Calibrated automated thrombography was used to measure thrombin generation in platelet suspension essentially as described previously.27 Washed platelets were pretreated for 2 hours at 37°C with or without ABT-737 or ABT-263 and then stimulated for 10 minutes with CRP-XL (2 μg/mL), TRAP (1 × 10−4M), or A23187 (1 × 10−5M). Samples were mixed with an equal volume of autologous platelet-poor plasma (PPP) that had been collected in the presence of 18.3 μg/mL corn trypsin inhibitor (Enzyme Research) to inhibit contact phase activation, and filtered through a 0.2-μm acrodisc filter (Pall Corporation). Samples were incubated with PPP reagent (1pM tissue factor) and the fluorescent substrate ZGGA-AMC (both from Diagnostica Stago) analyzing fluorescence release for 60 minutes at 37°C in a fluorescent plate reader (Fluoroskan Ascent; Thermo Scientific) equipped with Thrombinoscope VI software (Synapse BV). Surface exposure of P-selectin in response to CRP-XL (2 μg/mL) and TRAP (1 × 10−4M) was assessed by flow cytometry using a P-selectin/FITC monoclonal antibody (R&D Systems) after incubation of washed platelets for 2 hours at 37°C in HBS with ABT-737 or ABT-263. Binding of fibrinogen and platelet aggregation in response to CRP-XL (2 μg/mL−1) and TRAP (1 × 10−4M) were assessed in PRP after incubation of whole blood with drugs for 2 hours at 37°C. PRP was then isolated by centrifugation at 160g for 20 minutes and the platelet count adjusted to 1.5 × 108 mL with autologous filtered PPP. For flow cytometry, 5 μL of PRP or platelet suspension was diluted 1:10 with HBS and incubated with 2 μL of fibrinogen/FITC (Dako United Kingdom) for 20 minutes before analysis. Aggregation in response to CRP-XL and TRAP was assessed in parallel in the ABT-737/-263-treated PRP samples using a PAP-8E aggregometer (Bio/Data Corporation), measuring aggregation over 6 minutes at 37°C, with a stir speed of 1200 rpm.
Calcium release assays
Washed platelets were incubated for 2 hours in HBS with or without ABT-263 and z-VAD.fmk before staining with 1μM Fluo4/AM (Invitrogen) for an additional 30 minutes. The intracellular calcium levels were assessed continuously at a FACSCalibur after establishing a stable baseline fluorescence signal for 1 minute. A23187 or ABT-263 was added and fluorescence analyzed for additional 4 minutes.
Thiazole-orange staining
The proportion of reticulated platelets was assessed by staining with thiazole-orange as described previously.28 Washed platelets were incubated in annexin buffer (10mM HEPES, 150mM NaCl, 5mM KCl, 1mM MgCl2,1.8mM CaCl2, pH 7.4) plus 50 ng/mL thiazole-orange and ABT-263 for 2 hours before addition of annexin/allophycocyanin and analysis by flow cytometry.
Microscopy
For confocal microscopy, washed platelets were allowed to settle on glass coverslips for 30 minutes before fixing for 15 minutes with 4% paraformaldehyde in PBS. Platelets were permeabilized for 10 minutes using 0.5% Triton-X 100 in PBS with 3% BSA, and incubated with mouse anti–cytochrome c, rabbit anti–active caspase 3 (both from BD Biosciences), or antitubulin antibody (Sigma-Aldrich) at 1:500 in PBS with 10% goat serum at 4°C overnight. Coverslips were washed and incubated with goat anti–mouse or rabbit secondary antibody/Alexa-546 or Alexa-488 (Invitrogen). For staining of actin, after antibody staining, platelets were incubated for 30 minutes with phalloidin/Alexa-488 (Invitrogen). Coverslips were mounted and observed using a Zeiss LSM510 confocal microscope with a 63× oil immersion lens, using a 6× zoom. Ultrathin sections for electron microscopy were prepared as described previously29 and recorded using a Megaview 3 digital camera and iTEM software 5.1 (Build 1276; Olympus Soft Imaging Solutions) in a Jeol 100-CXII electron microscope (Jeol United Kingdom).
MMP and cytochrome c release
The loss of MMP was assessed by flow cytometry, staining with 50nM tetramethylrhodamine ethyl ester (TMRE, Invitrogen) for 10 minutes at 37°C. For live cell imaging, platelets were incubated with 25nM TMRE and 1:100 CD41/FITC during the 30-minute period of settling onto coverslips, and imaged immediately. To assess the release of mitochondrial cytochrome c, platelets were washed in cold PBS and resuspended at 1 × 108 in 30 μL of mitochondrial isolation buffer (250mM sucrose, 20mM HEPES, pH 7.4, 5mM MgCl2, and 10mM KCl) containing 0.05% digitonin. Cells were left on ice for 10 minutes followed by centrifugation at 16 000g for 3 minutes. Subsequently, supernatant and pellets were analyzed by Western blotting.
Western blotting and immunoprecipitation
For analysis of protein expression, 1 × 108 platelets were lysed in 0.5% Triton-X 100, 150mM NaCl, 20mM Tris-HCl, pH 8, and protease inhibitor cocktail (Roche Diagnostics). Western blot analysis was performed using mouse anti–gelsolin (BD Biosciences), mouse anti–fodrin (Millipore), and mouse anti–cytochrome c (BD Biosciences) rabbit anti-BCL-XL (BD Biosciences), mouse anti-BCL2 (Dako) rabbit anti-BCL2A1 (kindly provided by J. Borst, The Netherlands Cancer Institute, Amsterdam), rabbit anti-MCL1 (Santa Cruz Biotechnology) rabbit anti-BAK or anti-BAX (Upstate Biotechnology), rabbit anti-BID (Cell Signaling Technology), rabbit anti-BIM (Calbiochem), mouse anti-NOXA (Calbiochem), rabbit anti-PUMA (Cell Signaling Technology), mouse anti-BAD (BD Biosciences), and mouse anti–α-tubulin (Merck). Enhanced chemiluminescence was used for detection (GE Healthcare). For immunoprecipitation, 1 × 109 washed platelets were exposed to ABT-263 (100nM) and z-VAD.fmk (50μM) for 2 hours before lysis in 1% 3(3-cholamidopropyl) dimethylammonio-1-propane sulfonate, 150mM NaCl, 20mM Tris-HCl, pH 8, and protease inhibitor cocktail. Rabbit anti-BCL-XL (BD Biosciences) was crosslinked with ProtA-dynabeads using 20mM dimethylpimelinediimidate (Sigma-Aldrich). Crosslinked antibodies were incubated with platelet lysate for 2 hours at 4°C. Beads were washed with lysis buffer containing 1% 3(3-cholamidopropyl) dimethylammonio-1-propane sulfonate before elution in sodium dodecyl sulfate-loading dye and Western blotting. Quantification of BAK binding to BCL-XL was performed using ImageJ 1.43u software.
Statistical analysis
The half-maximal effective concentration (see Figure 1) was calculated in GraphPad Prism 5 software. Comparison between 2 variables (see Figures 1,7) was performed using paired t test in Microsoft Excel. Comparison between multiple variables (see Figure 3B-D) was performed using analysis of variance and Bonferroni multiple comparison test in GraphPad Prism.
Results
Platelets are more sensitive to ABT-263 than to ABT-737
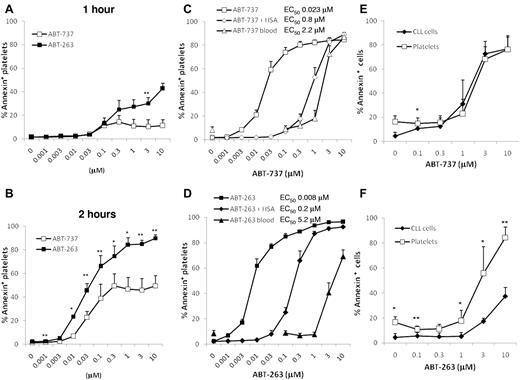
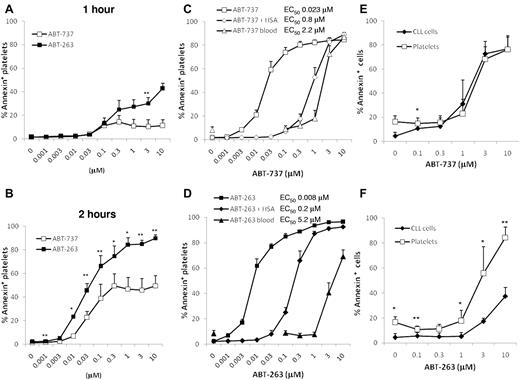
To investigate apoptosis induced by inhibition of BCL2 proteins, washed human platelets were incubated in HBS with ABT-263 or ABT-737. After 1 hour of exposure, only low levels of apoptosis were detectable as assessed by PS externalization (Figure 1A), but after 2 and 4 hours of exposure (Figure 1B-D) extensive platelet apoptosis was induced in a time- and concentration-dependent manner. These data indicate that the induction of apoptosis by BCL2 inhibition occurs much more slowly than platelet activation, which occurs within minutes of agonist exposure. The half-maximal effective concentrations for ABT-737 and ABT-263 at 4 hours were in the low nanomolar range, demonstrating that platelets are intrinsically very sensitive to BCL2 inhibition. Notably, in clinical trials for ABT-263, plasma concentrations up to approximately 6μM were achieved.30 At all time points measured, ABT-263 was more potent at inducing platelet apoptosis than ABT-737 (at 4 hours, the half-maximal effective concentration for ABT-737 was 23nM and 8nM for ABT-263). To investigate the effects of BCL2 inhibition in a more physiologic environment, whole blood of healthy volunteers was incubated with ABT-737 or ABT-263 before isolation of PRP and measurement of platelet apoptosis. In whole blood, micromolar concentrations of both drugs were required to induce platelet apoptosis; and surprisingly, ABT-263 was now less potent than ABT-737 (Figure 1C-D). We have previously shown that the presence of albumin decreases the efficacy of both compounds to induce apoptosis of CLL cells and that ABT-263 has a higher affinity for albumin and is therefore more efficiently sequestered by this plasma protein.31 To investigate whether the binding of drug to albumin was responsible for the loss of sensitivity in whole blood, we incubated washed platelets in HBS containing 3% human serum albumin. The addition of albumin resulted in a loss of sensitivity to ABT-737 and ABT-263, confirming that albumin sequesters both drugs. Interestingly, in the case of ABT-737 (Figure 1C), the addition of albumin accounted for most of the reduced sensitivity in whole blood, whereas for ABT-263 (Figure 1D) the addition of albumin caused only a partial loss of sensitivity compared with whole blood. These data indicate that other factors in the blood, besides albumin binding, diminish the efficacy of ABT-263, but not of ABT-737, to induce platelet apoptosis.
ABT-263 and ABT-737 induce platelet apoptosis. (A-B) Washed human platelets were incubated in HBS with different concentrations of ABT-737 (open boxes) or ABT-263 (filled boxes) for 1 hour (A) or 2 hours (B). Apoptosis was assessed by PS externalization and staining with annexin V/FITC before analysis by flow cytometry. *P < .05. **P < .01. (C-D) Washed platelets were incubated in HBS with (♦,♢) or without (■,□) 3% human serum albumin and different concentrations of ABT-737 (C, open symbols) or ABT-263 (D, filled symbols) for 4 hours. Alternatively, whole blood (▴,▵) was incubated with different concentrations of ABT-737 (C) or ABT-263 (D) for 4 hours before analysis of platelet apoptosis by PS externalization and staining with annexin V/FITC. Data are mean ± SD (n = 6). (E-F) Citrated blood of patients with CLL was incubated with different concentrations of ABT-737 (E) or ABT-263 (F) for 4 hours. Apoptosis of CLL cells (♦) was investigated in whole blood by staining with CD5/CD19 antibodies and annexin V/allophycocyanin. Apoptosis of platelets (□) was investigated in plasma by staining with CD41 antibodies and annexin V/FITC. Data are mean ± SD (n = 4). *P < .05. **P < .01.
ABT-263 and ABT-737 induce platelet apoptosis. (A-B) Washed human platelets were incubated in HBS with different concentrations of ABT-737 (open boxes) or ABT-263 (filled boxes) for 1 hour (A) or 2 hours (B). Apoptosis was assessed by PS externalization and staining with annexin V/FITC before analysis by flow cytometry. *P < .05. **P < .01. (C-D) Washed platelets were incubated in HBS with (♦,♢) or without (■,□) 3% human serum albumin and different concentrations of ABT-737 (C, open symbols) or ABT-263 (D, filled symbols) for 4 hours. Alternatively, whole blood (▴,▵) was incubated with different concentrations of ABT-737 (C) or ABT-263 (D) for 4 hours before analysis of platelet apoptosis by PS externalization and staining with annexin V/FITC. Data are mean ± SD (n = 6). (E-F) Citrated blood of patients with CLL was incubated with different concentrations of ABT-737 (E) or ABT-263 (F) for 4 hours. Apoptosis of CLL cells (♦) was investigated in whole blood by staining with CD5/CD19 antibodies and annexin V/allophycocyanin. Apoptosis of platelets (□) was investigated in plasma by staining with CD41 antibodies and annexin V/FITC. Data are mean ± SD (n = 4). *P < .05. **P < .01.
ABT-737 and ABT-263 lack selective toxicity in CLL
In initial clinical studies with ABT-263 as a potential anticancer agent, thrombocytopenia is the unwanted and dose-limiting toxicity. To compare directly the susceptibility of the therapeutic target (leukemia cells) with that of platelets, we incubated whole blood of patients with CLL in the presence of ABT-737 or ABT-263 and assessed apoptosis in the different cell types. ABT-737 induced similar levels of apoptosis in CLL cells and in platelets, whereas ABT-263 was less potent at inducing CLL cell apoptosis than platelet apoptosis (Figure 1E-F), demonstrating that, at least during the first treatment course, ABT-263 would not be expected to display selective toxicity against leukemia cells.
BCL2/XL inhibition, but not platelet activation, induces apoptotic signaling
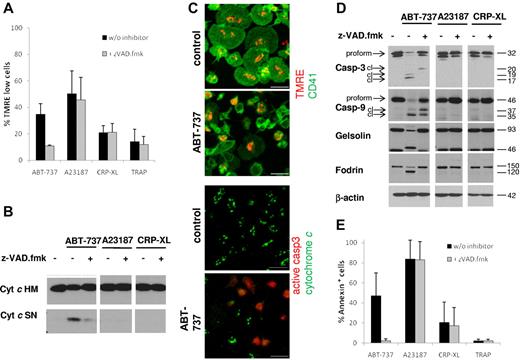
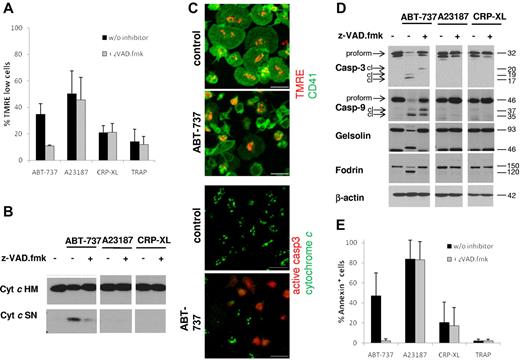
There has been considerable discussion on the involvement of apoptotic signaling events in platelet activation.25,32 Therefore, we investigated apoptosis pathways after exposure to platelet agonists or BCL2 inhibition. Exposure to ABT-737 induced loss of MMP (Figure 2A,C), release of cytochrome c (Figure 2B-C), and caspase cleavage (Figure 2C-D), in agreement with activation of the intrinsic apoptotic pathway. Loss of MMP was also seen after exposure to the calcium ionophore A23187, but not the platelet agonists CRP-XL or TRAP. At the time points investigated, caspase-3 and caspse-9 were clearly not activated by platelet agonists or by A23187 (Figure 2D). Apoptosis is defined as a caspase-dependent process that is blocked or delayed by caspase inhibitors. Addition of the caspase inhibitor z-VAD.fmk completely blocked the ABT-737-induced cleavage of the caspase substrates gelsolin and fodrin (Figure 2D) and PS externalization (Figure 2E). Interestingly, ABT-737-induced loss of MMP was also severely inhibited by z-VAD.fmk (Figure 2A), indicating that, in contrast to many other cell types, the loss of MMP in platelets occurred primarily through a caspase-driven amplification process. Taken together, these data demonstrate that caspases are essential for platelet apoptosis but not activation. Our data do not exclude that caspase activation or release of cytochrome c may occur at later times after platelet activation, but these events are clearly not required for the initiation of platelet activation.
Platelet apoptosis, but not platelet activation, depends on caspase activation. Washed human platelets were incubated in HBS with ABT-737 (100nM) for 2 hours or A23187 (10μM), CRP-XL (2 μg/mL), or TRAP (100μM) for 15 minutes with or without the caspase inhibitor z-VAD.fmk (50μM). (A) Loss of mitochondrial membrane potential was assessed by staining with TMRE (50nM) before analysis by flow cytometry. Data are mean ± SD (n = 4). (B) Release of mitochondrial cytochrome c was assessed by lysis in 0.05% digitonin and differential centrifugation. The heavy membrane fraction (HM) containing mitochondria and the supernatant (SN) containing cytosol were analyzed for cytochrome c content by Western blotting. (C) Release of mitochondrial cytochrome c and loss of mitochondrial membrane potential after exposure to ABT-737 (100nM) for 2 hours were assessed by immunohistochemistry and staining with TMRE (red, top panels) and anti-CD41/FITC (green, top panels), or anti–cytochrome c (green, bottom panels) and anti–active-caspase-3 (red, bottom panels). Bar represents 5 μm. Note that control cells display spreading on glass slides, whereas ABT-737-treated cells do not. (D) Cleavage of caspases and caspase targets (fodrin, gelsolin) was analyzed by Western blotting. cl indicates cleaved caspase fragments. (E) Apoptosis was assessed by PS externalization and staining with annexin V/FITC before analysis by flow cytometry. Data are mean ± SD (n = 4).
Platelet apoptosis, but not platelet activation, depends on caspase activation. Washed human platelets were incubated in HBS with ABT-737 (100nM) for 2 hours or A23187 (10μM), CRP-XL (2 μg/mL), or TRAP (100μM) for 15 minutes with or without the caspase inhibitor z-VAD.fmk (50μM). (A) Loss of mitochondrial membrane potential was assessed by staining with TMRE (50nM) before analysis by flow cytometry. Data are mean ± SD (n = 4). (B) Release of mitochondrial cytochrome c was assessed by lysis in 0.05% digitonin and differential centrifugation. The heavy membrane fraction (HM) containing mitochondria and the supernatant (SN) containing cytosol were analyzed for cytochrome c content by Western blotting. (C) Release of mitochondrial cytochrome c and loss of mitochondrial membrane potential after exposure to ABT-737 (100nM) for 2 hours were assessed by immunohistochemistry and staining with TMRE (red, top panels) and anti-CD41/FITC (green, top panels), or anti–cytochrome c (green, bottom panels) and anti–active-caspase-3 (red, bottom panels). Bar represents 5 μm. Note that control cells display spreading on glass slides, whereas ABT-737-treated cells do not. (D) Cleavage of caspases and caspase targets (fodrin, gelsolin) was analyzed by Western blotting. cl indicates cleaved caspase fragments. (E) Apoptosis was assessed by PS externalization and staining with annexin V/FITC before analysis by flow cytometry. Data are mean ± SD (n = 4).
Platelet apoptosis triggers thrombin generation
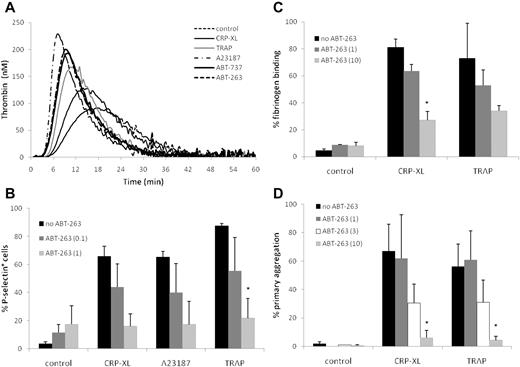
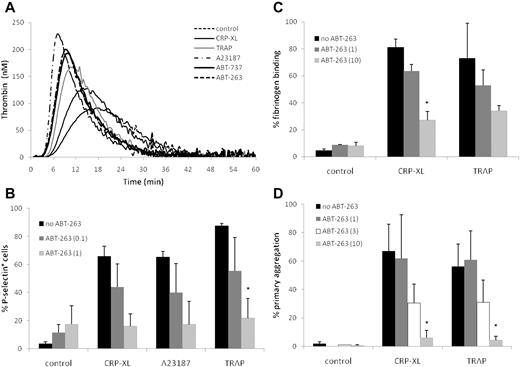
The externalization of PS is a common feature of both platelet activation and apoptosis. Because PS exposure on the platelet surface facilitates the formation of the prothrombinase complex,33 we investigated whether platelets exposed to ABT-263 or ABT-737 promote the generation of thrombin at time points when the PS exposure was clearly evident (2 hours). Although the PS externalization occurred much more slowly (Figure 1), both ABT-737 and ABT-263 induced the generation of thrombin at a faster rate (as indicated by shorter lag times and time to peak) and more strongly (as indicated by a greater level of peak thrombin) than CRP-XL or TRAP and nearly as strongly as A23187 (Figure 3A), confirming that the PS exposure during apoptosis triggers the formation of the prothrombinase complex.
ABT-263 induces thrombin generation but inhibits platelet activation and aggregation. (A) Washed human platelets were incubated for 2 hours at 37°C in HBS with ABT-263 (1μM), ABT-737 (1μM), or for 10 minutes with CRP-XL (2 μg/mL), TRAP (100μM), or A23187 (10μM). Thrombin generation over time was measured by calibrated automated thrombography. Data are shown from one representative donor typical of 3 examined. (B) Washed platelets were incubated in HBS with ABT-263 (0, 0.1, or 1μM) for 2 hours before exposure to the platelet agonists CRP-XL, A23187, or TRAP for 10 minutes. Platelet activation was assessed by exposure of P-selectin and flow cytometry. (C-D) Whole blood was incubated with ABT-263 (0, 1, 3, or 10μM) for 2 hours. PRP count was adjusted with autologous filtered PPP to 1.5 × 108/mL and either (C) fibrinogen binding was assessed by flow cytometry or (D) aggregation was measured using an aggregometer. Data are mean ± SD (n = 3, except 3μM ABT-263 in panel D, when n = 2). Statistical significance was tested using analysis of variance and comparison of “no ABT-263” with “ABT-263 (0.1)” and “ABT-263(1)” in panel B and with “ABT-263(1)” and “ABT-263(10)” in panels C and D. *P < .05.
ABT-263 induces thrombin generation but inhibits platelet activation and aggregation. (A) Washed human platelets were incubated for 2 hours at 37°C in HBS with ABT-263 (1μM), ABT-737 (1μM), or for 10 minutes with CRP-XL (2 μg/mL), TRAP (100μM), or A23187 (10μM). Thrombin generation over time was measured by calibrated automated thrombography. Data are shown from one representative donor typical of 3 examined. (B) Washed platelets were incubated in HBS with ABT-263 (0, 0.1, or 1μM) for 2 hours before exposure to the platelet agonists CRP-XL, A23187, or TRAP for 10 minutes. Platelet activation was assessed by exposure of P-selectin and flow cytometry. (C-D) Whole blood was incubated with ABT-263 (0, 1, 3, or 10μM) for 2 hours. PRP count was adjusted with autologous filtered PPP to 1.5 × 108/mL and either (C) fibrinogen binding was assessed by flow cytometry or (D) aggregation was measured using an aggregometer. Data are mean ± SD (n = 3, except 3μM ABT-263 in panel D, when n = 2). Statistical significance was tested using analysis of variance and comparison of “no ABT-263” with “ABT-263 (0.1)” and “ABT-263(1)” in panel B and with “ABT-263(1)” and “ABT-263(10)” in panels C and D. *P < .05.
Inhibition of BCL2 proteins prevents platelet activation
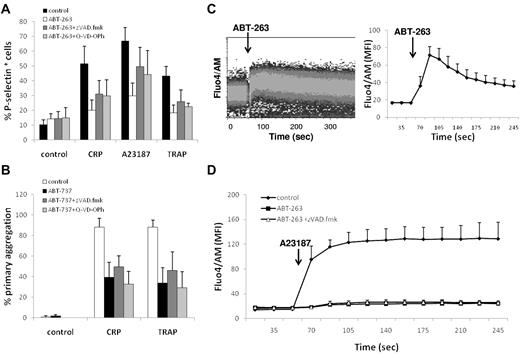
Incubation of platelets for 2 hours with ABT-263 did not induce surface expression of P-selectin (Figure 3B), binding of fibrinogen to activated αIIbβ3 (Figure 3C), or aggregation (Figure 3D), demonstrating that BCL2 inhibition did not contribute to platelet degranulation or activation of integrins. This contrasts with platelets treated with CRP-XL, TRAP, or A23187, which demonstrated exposure of P-selectin, fibrinogen binding, and platelet aggregation (Figure 3B-D). However, if platelets were preexposed to ABT-263 for 2 hours before incubation with CRP-XL, TRAP, or A23187, there was a marked, concentration-dependent inhibition of all 3 measures of platelet activation, indicating that platelets in which BCL2 proteins have been inhibited have a diminished capacity to be activated via agonist receptor-mediated pathways (CRP-XL and TRAP) or by a sustained rise in intracellular calcium (A23187). Comparable results were obtained with ABT-737 (data not shown). Taken together, these data show that, despite PS exposure and thrombin generation, BCL2 inhibition prevents, rather than facilitates, platelet activation.
Inhibition of BCL2 proteins results in a depletion of intracellular calcium
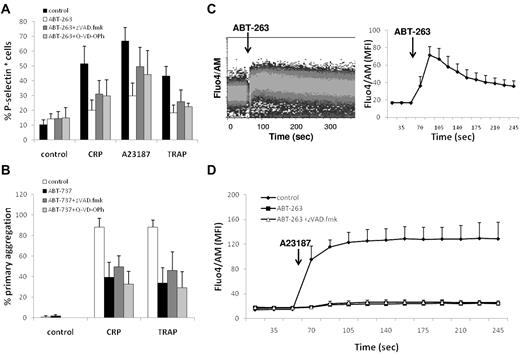
Next, we investigated whether the repression of platelet activation on BCL2 inhibition was the result of a caspase-mediated event. However, addition of the caspase inhibitors z-VAD.fmk or Q-VD-OPh to ABT-263 or ABT-737 did not restore the platelets' ability to respond to agonist or calcium ionophore (Figure 4A-B), indicating that events upstream of caspase activation are responsible for the repression of platelet activation induced by BCL2 inhibition. BCL2 proteins have been implicated in the regulation of intracellular calcium homeostasis; and because the release of calcium from the ER is an important signaling event shared by platelet agonists and the calcium ionophore, we subsequently asked whether inhibition of BCL2 proteins by ABT-737 or ABT-263 has a direct effect on calcium homeostasis in platelets. Notably, exposure to ABT-263 induced an immediate increase in intracellular calcium (Figure 4C) in a concentration-dependent manner (data not shown). In addition, pretreatment with ABT-263 for 2 hours resulted in a depletion of intracellular calcium stores, as assessed using the response to A23187 (Figure 4D), suggesting an initial release of calcium from ER stores by ABT-263 and subsequent depletion of intracellular calcium in this virtually calcium-free environment. The depletion of intracellular calcium stores was not a caspase-mediated effect because addition of z-VAD.fmk did not prevent the calcium depletion. Taken together, these data demonstrate that inhibition of BCL2 proteins in platelets has a direct effect on the intracellular calcium stores, thereby providing an explanation for the repression of agonist-induced platelet activation on ABT-263 exposure.
ABT-263 depletes intracellular calcium stores. (A) Washed platelets were incubated in HBS with ABT-263 (1μM) and caspase inhibitors (z-VAD.fmk, 100μM, Q-VD-POh, 50μM) for 2 hours before exposure to the platelet agonists CRP-XL (2 μg/mL), A23187 (10μM), or TRAP (100μM) for 10 minutes. Platelet activation was assessed by exposure of P-selectin and flow cytometry. (B) Whole blood was incubated with ABT-737 (10μM) and caspase inhibitors (z-VAD.fmk, 100μM, Q-VD-POh, 50μM) for 2 hours. PRP count was adjusted with autologous filtered PPP to 1.5 × 108/mL, and aggregation was measured using an aggregometer. Data are mean ± SD (n = 3). (C) Washed human platelets were stained with Fluo4/AM in HBS for 30 minutes. Intracellular calcium levels were continuously monitored by flow cytometry. After establishing a baseline fluorescence signal for 1 minute, ABT-263 (10μM) was added and the calcium response was monitored for an additional 4 minutes. (Left panel) Representative graph. (Right panel) Mean ± SD of the geometric mean fluorescence (MFI) (n = 3). (D) Washed human platelets were incubated with or without ABT-263 (1μM) and z-VAD.fmk (100μM) in HBS for 2 hours before staining with Fluo4/AM for an additional 30 minutes. After establishing a baseline fluorescence signal for 1 minute, A23187 (5μM) was added and the calcium response was monitored for an additional 4 minutes. Data are mean ± SD of the geometric mean fluorescence (n = 3).
ABT-263 depletes intracellular calcium stores. (A) Washed platelets were incubated in HBS with ABT-263 (1μM) and caspase inhibitors (z-VAD.fmk, 100μM, Q-VD-POh, 50μM) for 2 hours before exposure to the platelet agonists CRP-XL (2 μg/mL), A23187 (10μM), or TRAP (100μM) for 10 minutes. Platelet activation was assessed by exposure of P-selectin and flow cytometry. (B) Whole blood was incubated with ABT-737 (10μM) and caspase inhibitors (z-VAD.fmk, 100μM, Q-VD-POh, 50μM) for 2 hours. PRP count was adjusted with autologous filtered PPP to 1.5 × 108/mL, and aggregation was measured using an aggregometer. Data are mean ± SD (n = 3). (C) Washed human platelets were stained with Fluo4/AM in HBS for 30 minutes. Intracellular calcium levels were continuously monitored by flow cytometry. After establishing a baseline fluorescence signal for 1 minute, ABT-263 (10μM) was added and the calcium response was monitored for an additional 4 minutes. (Left panel) Representative graph. (Right panel) Mean ± SD of the geometric mean fluorescence (MFI) (n = 3). (D) Washed human platelets were incubated with or without ABT-263 (1μM) and z-VAD.fmk (100μM) in HBS for 2 hours before staining with Fluo4/AM for an additional 30 minutes. After establishing a baseline fluorescence signal for 1 minute, A23187 (5μM) was added and the calcium response was monitored for an additional 4 minutes. Data are mean ± SD of the geometric mean fluorescence (n = 3).
Ultrastructural changes during platelet apoptosis
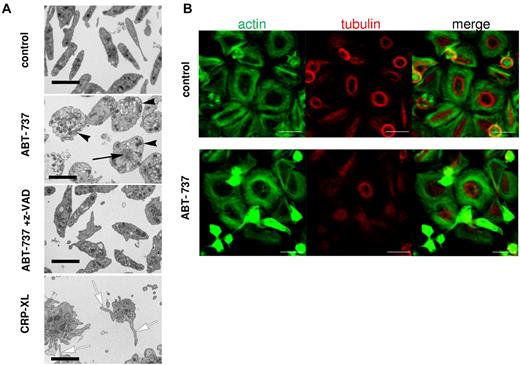
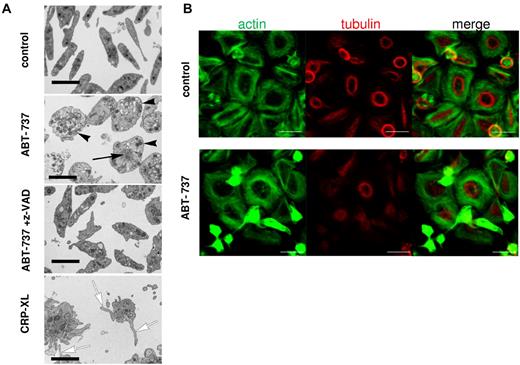
Because the ultrastructural changes during apoptosis of non-nucleated platelets have not been well characterized, we examined platelet morphology after exposure to ABT-737. Resting platelets displayed the typical discoid shape with a diffuse distribution of granules and organelles, whereas platelets exposed to ABT-737 were distended and displayed a peripheral distribution of most granules (Figure 5A), consistent with a disruption of the cytoskeleton. After ABT-737 exposure, disorganized cytoskeletal structures also accumulated at the center of the cell. All ultrastructural changes induced by ABT-737 were mediated by caspases as they were inhibited by z-VAD.fmk (Figure 5A). Similar ultrastructural changes were observed on exposure to ABT-263 (data not shown). By contrast, platelet activation by CRP-XL resulted in the characteristic opening of the surface connecting canalicular system and a distinct morphology mainly characterized by filopodia and microparticle formation (Figure 5A). Further characterization of the ABT-737-induced cytoskeletal changes was performed on glass coverslips, which induced a spontaneous activation and spreading of the control platelets but not of ABT-737-treated platelets. Activated control platelets displayed a tubulin ring close to the cell center with actin fibers surrounding it (Figure 5B top panels). After exposure to ABT-737, a marked condensation of actin structures and inhibition of tubulin ring formation were observed in those cells that were apoptotic (Figure 5B bottom panels). These changes, together with those observed by electron microscopy, indicate that caspases activated during apoptosis caused a dramatic change in cell morphology clearly distinct from the morphology observed during platelet activation.
Platelet apoptosis is characterized by ultrastructural changes distinct from platelet activation. (A) Washed human platelets incubated in HBS retained their normal discoid shape and ultrastructure, whereas treatment with ABT-737 (100nM) for 2 hours resulted in their distension with displacement of granules toward the periphery of the rounded-up cells (black arrowheads). Many of these cells also displayed focal aggregations of cytoskeletal material (black arrow). These changes were all prevented by the incorporation of z-VAD.fmk (50μM) into the medium. Treatment with CRP-XL (2 μg/mL) for 15 minutes resulted in a completely different morphology with the proliferation of numerous filopodia (white arrows) and microparticles. Bars represent 2 μm. (B) Platelets were incubated in HBS with or without ABT-737 (100nM) for 2 hours before analysis by immunohistochemistry and confocal microscopy. Actin was stained using phalloidin/Alexa-488 (green), and tubulin was stained using anti–tubulin/phycoerythrin (red). Bar represents 5 μm.
Platelet apoptosis is characterized by ultrastructural changes distinct from platelet activation. (A) Washed human platelets incubated in HBS retained their normal discoid shape and ultrastructure, whereas treatment with ABT-737 (100nM) for 2 hours resulted in their distension with displacement of granules toward the periphery of the rounded-up cells (black arrowheads). Many of these cells also displayed focal aggregations of cytoskeletal material (black arrow). These changes were all prevented by the incorporation of z-VAD.fmk (50μM) into the medium. Treatment with CRP-XL (2 μg/mL) for 15 minutes resulted in a completely different morphology with the proliferation of numerous filopodia (white arrows) and microparticles. Bars represent 2 μm. (B) Platelets were incubated in HBS with or without ABT-737 (100nM) for 2 hours before analysis by immunohistochemistry and confocal microscopy. Actin was stained using phalloidin/Alexa-488 (green), and tubulin was stained using anti–tubulin/phycoerythrin (red). Bar represents 5 μm.
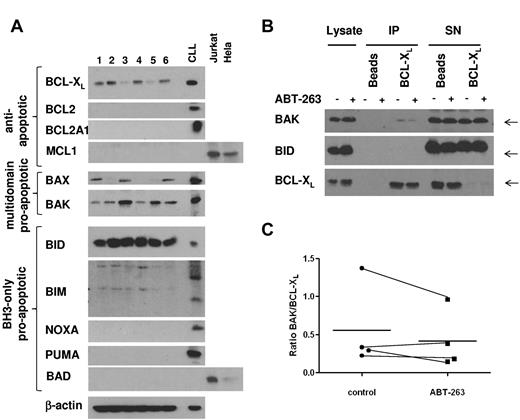
BCL-XL sequesters BAK in resting platelets
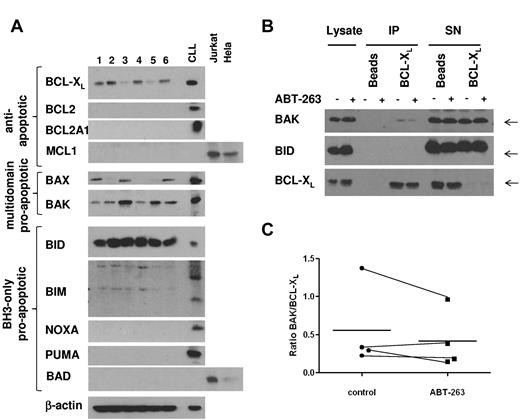
To investigate which members of the BCL2 family were expressed in platelets, we analyzed platelet lysates from 6 different healthy donors (Figure 6A). Among the antiapoptotic BCL2 proteins, only BCL-XL was expressed at detectable levels in platelets, suggesting that BCL-XL is the main target of ABT-737 and ABT-263 in platelets. BAX and BAK were both expressed, although to various levels in the individual samples. Among the BCL2 homology domain 3 (BH3)-only proteins, BID was highly expressed, whereas NOXA, BAD, and PUMA were not detectable. There was some reactivity with the BIM antibody; however, the resulting bands did not correspond to the BIM bands routinely detected in our studies with CLL cells. To elucidate the mechanism of ABT-263-induced apoptosis in platelets, we investigated the interaction of BCL-XL and proapoptotic BCL2 proteins by immunoprecipitation. To exclude any downstream effects mediated by caspases, this experiment was carried out in the presence of z-VAD.fmk. In resting platelets, BAK was sequestered by BCL-XL; and in some experiments (2 of 4), we detected minor displacement of BAK from BCL-XL on treatment with ABT-263 (Figure 6B-C) under conditions that induced platelet apoptosis (2 hours of exposure to 100nM ABT-263). Our data do not exclude that higher concentrations or longer incubation with ABT-263 might displace more BAK from BCL-XL. Notably, no interaction between BCL-XL and the BH3-only protein BID was detected (Figure 6B), despite high expression of BID (Figure 6A). Taken together, our data indicate that BCL-XL sequesters BAK in platelets, but whether displacement of BAK from BCL-XL may contribute to apoptosis induced by ABT-263 remains to be demonstrated.
BCL-XL directly interacts with BAK in resting platelets. (A) Lysates from washed platelets (20 μg) from 6 healthy volunteers were analyzed by Western blotting for expression of BCL2 proteins. A lysate from CLL cells cultured for 24 hours on CD154-expressing fibroblasts, as well as lysates from Jurkat or HeLa cells, were used as positive controls. (B) Washed platelets from a healthy volunteer were exposed to ABT-263 (100nM) in the presence of z-VAD.fmk (50μM) for 2 hours before lysis and immunoprecipitation of BCL-XL. Interaction of BCL-XL with BAK or BID was assessed by staining with anti-BAK or anti-BID antibody, respectively. (C) Binding of BAK to BCL-XL was quantified using densitometry on lanes 5 and 6 of Western blots as shown in panel B and expressed as a ratio of BAK/BCL-XL in 4 individual samples together with the mean (−).
BCL-XL directly interacts with BAK in resting platelets. (A) Lysates from washed platelets (20 μg) from 6 healthy volunteers were analyzed by Western blotting for expression of BCL2 proteins. A lysate from CLL cells cultured for 24 hours on CD154-expressing fibroblasts, as well as lysates from Jurkat or HeLa cells, were used as positive controls. (B) Washed platelets from a healthy volunteer were exposed to ABT-263 (100nM) in the presence of z-VAD.fmk (50μM) for 2 hours before lysis and immunoprecipitation of BCL-XL. Interaction of BCL-XL with BAK or BID was assessed by staining with anti-BAK or anti-BID antibody, respectively. (C) Binding of BAK to BCL-XL was quantified using densitometry on lanes 5 and 6 of Western blots as shown in panel B and expressed as a ratio of BAK/BCL-XL in 4 individual samples together with the mean (−).
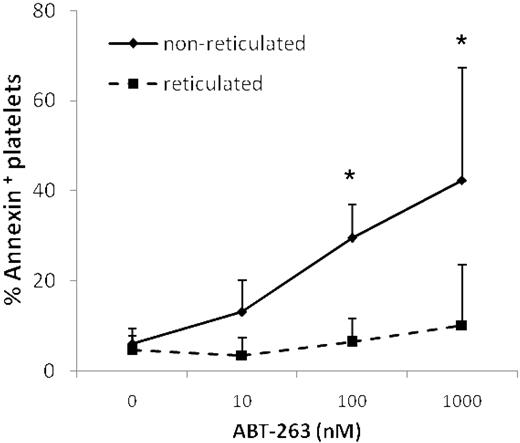
Reticulated platelets are resistant to ABT-263-induced apoptosis
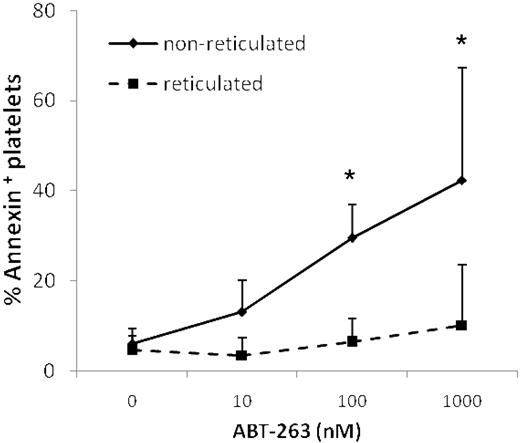
Treatment of mice with ABT-737 increased the number of reticulated “young” platelets, as assessed by staining with thiazole-orange,14 suggesting that ABT-737 selectively kills older platelets, leaving younger platelets unaffected. Thus, prolonged exposure to ABT-263 might result in the generation of an overall younger platelet population. Costaining with thiazole-orange and annexin V demonstrated that younger reticulated platelets were significantly more resistant to ABT-263-induced apoptosis (Figure 7), supporting the hypothesis that mature platelets are more sensitive to ABT-263 and providing an explanation for the generation of a less sensitive, and possibly younger, platelet population on treatment with ABT-263.
Reticulated platelets are resistant to ABT-263-induced apoptosis. Washed platelets of healthy volunteers or CLL patients were incubated in annexin buffer plus 50 ng/mL thiazole-orange and different concentrations of ABT-263 for 2 hours. Apoptosis was assessed in the thiazole-orange-positive (reticulated) or -negative (nonreticulated) platelet population by staining with annexin V/allophycocyanin and gating by flow cytometry. Data are mean ± SD (n = 4). *P < .05.
Reticulated platelets are resistant to ABT-263-induced apoptosis. Washed platelets of healthy volunteers or CLL patients were incubated in annexin buffer plus 50 ng/mL thiazole-orange and different concentrations of ABT-263 for 2 hours. Apoptosis was assessed in the thiazole-orange-positive (reticulated) or -negative (nonreticulated) platelet population by staining with annexin V/allophycocyanin and gating by flow cytometry. Data are mean ± SD (n = 4). *P < .05.
Discussion
In this study, we investigated the consequences of BCL2 inhibition in platelets. Because of the shared characteristic of PS exposure, platelet apoptosis and activation are often considered related processes. In contrast to apoptosis induced by BCL2 inhibition, platelet activation induced by the thrombin receptor agonist TRAP, the collagen peptide-mimetic CRP-XL, or the calcium ionophore A23187 led to PS exposure and was accompanied by aggregation, increased fibrinogen binding, and rapid exposure of P-selectin, but did not involve caspase activation or cytochrome c release (Figures 2,3). Our results indicate that mitochondrial changes and caspase activation are not required for platelet activation, although we cannot exclude that these may occur later during prolonged activation or as bystander effects. Apoptosis is defined as a strictly caspase-dependent process.34 Our data do not support an involvement of apoptosis in platelet activation but rather show that platelet apoptosis as induced by BCL2 inhibition is clearly distinct from platelet activation, in line with a previous report.24 Notably, PS externalization resulting from ABT-263- and ABT-737-induced apoptosis or from platelet activation both resulted in an increased thrombin generation (Figure 3A), confirming that PS exposure is directly linked to promotion of hemostasis irrespective of the pathways leading to PS externalization. However, the lack of P-selectin expression and the deregulation of integrin-ligand interactions after BCL2 inhibition suggest that platelets would not become actively incorporated into a thrombus; therefore, their thrombotic effect will be marginal in vivo.
To emphasize further the different pathways resulting in platelet activation or apoptosis, our data demonstrate unequivocally that, despite the increased thrombin generation (Figure 3A), apoptosis inhibits the subsequent response of platelets to their physiologic agonists (Figure 3B-D). It is interesting to consider these findings in the context of observations made in stored platelets. In platelet concentrates stored for transfusion, apoptosis is increased over storage time.19 Simultaneously, platelets become less responsive to their physiologic agonists, a phenomenon referred to as the platelet storage lesion.35 Interestingly, the depletion of intracellular calcium observed on BCL2 inhibition (Figure 4) provides a novel mechanistic explanation for the repressed platelet activation. Several BCL2 proteins, such as BCL-XL, localize to the ER and have been implicated in calcium homeostasis in multiple ways. Notably, inhibition of BCL2 proteins has been previously shown to result in calcium depletion in pancreatic and leukemia cells.36,37 Because ABT-737-induced apoptosis in platelets was not affected by chelation of extracellular calcium,24 it is possible that the effect of BCL2/BCL-XL inhibition on calcium is unrelated and independent of its apoptosis-inducing effects.
The ultrastructural characteristics of apoptotic platelets are not, as yet, well defined. Cytoplasmic condensation and budding, to yield microparticles, have been associated with apoptosis in aging platelets in stored concentrates,18 but these platelets have also undergone a degree of activation during their preparation, which increased during storage in parallel with their decline into an apoptotic-like state.38 However, morphologic changes similar to those observed in our study were also reported during apoptosis in platelets aged in vitro, which was also accompanied by a reduced aggregatory response.39
Exposure of patients to ABT-263 results in thrombocytopenia during the first 4 weeks of treatment.12,13 In the current clinical studies, this problem is circumvented by giving the patients a low priming dose of ABT-263 (100 mg) for 7 to 14 days before starting with the full therapeutic dose (eg, 250 mg). This priming dose is sufficient to induce a mild thrombocytopenia because of selective apoptosis of older platelets and supposedly results in the generation of an overall younger platelet population, more resistant to the drug thereby allowing dose escalation.12,14 In this study, we show, for the first time, that reticulated human platelets are significantly less susceptible to ABT-263-induced apoptosis (Figure 7), providing an explanation for the generation of an overall younger platelet population after treatment with ABT-263. Younger platelets are generally thought to be more active than older platelets,40 although direct evidence for this comes mainly from studies in animal models.41 It is possible that, in patients treated with ABT-263, the lack of platelet numbers and the inhibition of platelet activation in apoptotic platelets might be compensated by a higher activation response in the remaining younger platelets.
The reduced susceptibility of young platelets may be the result of an enhanced expression of the drug target itself, BCL-XL. However, younger platelets most probably show increased expression of multiple proteins that might contribute to the observed resistance. In nucleated cells, the most common resistance factor for ABT-737 or ABT-263 is the antiapoptotic BCL2 protein MCL1, which can compensate for loss of BCL-XL function and is not targeted by either ABT-737 or ABT-263. Therefore, in nucleated cells, overexpression of MCL1 or another related antiapoptotic BCL2 protein, BCL2A1, protects cells from ABT-737-induced apoptosis.42–44 Although neither MCL1 nor BCL2A1 was expressed at detectable levels in platelets from healthy donors (Figure 6A), these short-lived proteins might be expressed selectively in reticulated platelets and thus contribute to the resistance of young platelets.
To date, little is known about the molecular mechanism of ABT-263– or ABT-737–induced apoptosis in platelets. We provide evidence for the interaction of BAK and BCL-XL in platelets, supporting the notion that BCL-XL ensures platelet survival by sequestering BAK. However, we detected only minor displacement of BAK after exposure to ABT-263 in 2 of 4 samples (Figure 6B-C), thus not convincingly demonstrating whether ABT-263–induced cytochrome c release is mediated by BAK displacement. Besides BAK, the proapoptotic protein BAX may mediate cytochrome c release.45 Although BAX is clearly expressed, we found no evidence for a direct interaction between BAX and BCL-XL in platelets. Other probable candidates that could mediate ABT-263–induced apoptosis are the BH3-only proteins, which have also been shown to be displaced from antiapoptotic BCL2 proteins by ABT-263 or ABT-737.46 There is a great paucity of data on which, if any, BH3-only proteins are present in platelets. Our data suggest that BID (and possibly BIM) is the sole BH3-only protein expressed at detectable protein levels in platelets. This is reinforced by detection of transcripts for BID, but not BIM (BCL2L11) in both platelets and megakaryocytic cells, as well as transcripts for BCL-XL (BCL2L1), BCL-w (BCL2L2), BAK1, and BAX.47,48 Interestingly, recent experiments in mice suggest that neither BIM nor BID is important for the regulation of platelet life span or ABT-737–induced apoptosis.45
A desirable property for any anticancer agent is selective toxicity exhibited against the tumor cells compared with the cells/tissue displaying dose-limiting toxicity. In this regard, no difference in sensitivity of CLL cells and platelets to ABT-737 was observed, whereas platelets were clearly more sensitive to ABT-263 than CLL cells (Figure 1E-F). However, this apparent lack of selectivity is in part overcome by the administration of a low priming dose of ABT-263 to patients as discussed herein. In CLL cells, the main target of ABT-737 and ABT-263 is BCL2 because circulating CLL cells display very little BCL-XL but overexpress BCL2. In platelets, however, the main target of these drugs is BCL-XL because BCL2 and BCL-w are not expressed at detectable levels (Figure 6A). Hence, a selective antagonist of BCL2 that does not inhibit BCL-XL could potentially circumvent thrombocytopenia and be used at higher doses, which might be more effective in leukemia therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank R. Walewska, A. Majid, and M. Ahearne for helping to acquire blood samples from healthy volunteers and M. Mahaut-Smith for helpful discussions of the calcium experiments.
This work was supported by funding from the Medical Research Council (United Kingdom) and Leukaemia and Lymphoma Research (United Kingdom). H.A.H. was supported by the Saudi Cultural Bureau (studentship).
Authorship
Contribution: M.V. performed most experiments and wrote the manuscript; H.A.H. carried out the platelet activation assays; X.-M.S. and R.T.S. helped with the flow cytometric analysis; E.T.W.B. and D.D. performed the microscopic analysis; and M.J.S.D., A.H.G., and G.M.C. designed the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerald M. Cohen, Medical Research Council Toxicology Unit, Hodgkin Bldg, University of Leicester, PO Box 138, Lancaster Rd, Leicester, LE1 9HN, United Kingdom; e-mail: gmc2@le.ac.uk.
References
Author notes
A.H.G. and G.M.C. contributed equally to this study.