To the editor:
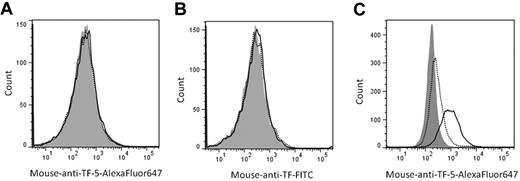
Blood coagulation is initiated by the binding of plasma factor VII/VIIa to cell surface–expressed tissue factor (TF). Normally, TF expression is confined to subendothelial tissues like fibroblasts1 and pericytes2 to limit blood coagulation to sites of vascular injury. Consistent with this notion, our laboratory demonstrated previously that platelets do not express active TF constitutively,3,4 or as a result of de novo synthesis, transfer from monocytes, or α-granule release.5 Recently, Camera et al suggested that expression of TF by platelets occurs rapidly after activation, and is transient,6 explaining our inability to observe detectable TF antigen and activity after a 2-hour platelet stimulation.5 In that study,6 3 different anti-TF antibodies (2 obtained from commercial sources and another obtained from Dr Y. Nemerson, Mt Sinai School of Medicine, New York, NY [deceased]) were used and TF antigen was detected on the platelet surface after activation with a 6-amino acid protease activated receptor-1 (PAR1) agonist peptide for 15 minutes. Using a commercially available antibody (American Diagnostica; 45-07CJ), an ∼ 50% increase in the mean fluorescence intensity (MFI) of the immunostained, activated platelets was observed when compared with unactivated platelets, or when activated platelets were stained with an isotype-matched control antibody. In contrast, no TF expression (< 10% increase in MFI) was observed after a 2-hour stimulation, consistent with our previous observation.5 In an attempt to duplicate these results, we performed analogous experiments using 2 different fluorophore-conjugated anti-TF antibodies including a specific, inhibitory anti-TF monoclonal antibody described previously (anti–TF-5)7 and the same commercially available anti-TF antibody used by Camera and colleagues (American Diagnostica, 45-07CJ).6 By flow cytometry, nearly identical levels of anti-TF-5 immunoreactivity with platelets, activated for 15 minutes (728 ± 118.3 MFI; dotted line) or 2 hours (625.3 ± 62.3 MFI; black line) with PAR 1 agonist peptide (SFLLRN), were obtained (Figure 1A). Interestingly, we observed similar results using the same anti-TF antibody used by Camera and colleagues.6 No difference in MFI between 15 minutes (5263 ± 683.5 MFI) and 2 hours (5426 ± 664.5 MFI) of platelet activation was observed (Figure 1B dotted and black lines, respectively). In both instances, the MFI were similar to those obtained when platelets were immunostained using isotype-matched control antibodies (753.8 ± 78.9 MFI and 4543 ± 568.6 MFI, respectively; shaded histograms). Control experiments performed using a lipopolysaccharide-stimulated monocyte-like cell line (THP-1) versus unstimulated cells confirmed positive immunoreactivity of the antibodies. In the absence of LPS-stimulation, few cells expressed TF (4.3 ± 0.1%) using anti–TF-5 (Figure 1C dotted line). Subsequent to LPS-stimulation, 49.3 ± 0.0% of the cells were positive for TF expression (Figure 1C black line) similar to our previous observations.3 Similar results were also obtained using the anti-TF antibody from American Diagnostica (data not shown).
No platelet tissue factor expression: short or long activation. Washed platelets were activated with PAR1 (100μM) agonist peptide for 15 minutes (dotted lines) or 2 hours (black lines) at 37°C. TF expression on platelets was determined by immunostaining with (A) anti–TF-5 conjugated to AlexaFluor647 (0.5μM); (B) a 1:10 dilution of an anti-TF antibody conjugated to FITC (American Diagnostica 45-07CJ), or by equivalent concentrations of appropriate control antibodies (shaded histograms; 15 minutes activation period), in 20mM Hepes, 0.15M NaCl (pH 7.4) containing 10 μg/mL human Fc. (C) Nonstimulated (dotted line) or LPS-stimulated (1 μg/mL, 4 hours, 37°C; black line) THP-1 cells were immunostained with anti–TF-5 or an isotype-matched control antibody (shaded histogram; nonstimulated cells) as described. Platelets or THP-1 cells (10 000), identified by their forward and side scatter, were analyzed by flow cytometry using a BD LSR II Flow Cytometer. Identical results were obtained whether platelets, stained with isotype-matched control antibodies, were activated for 15 minutes or 2 hours. Similarly, LPS-stimulation of THP-1 cells was without effect on control antibody reactivity. Representative data are shown (n = 3).
No platelet tissue factor expression: short or long activation. Washed platelets were activated with PAR1 (100μM) agonist peptide for 15 minutes (dotted lines) or 2 hours (black lines) at 37°C. TF expression on platelets was determined by immunostaining with (A) anti–TF-5 conjugated to AlexaFluor647 (0.5μM); (B) a 1:10 dilution of an anti-TF antibody conjugated to FITC (American Diagnostica 45-07CJ), or by equivalent concentrations of appropriate control antibodies (shaded histograms; 15 minutes activation period), in 20mM Hepes, 0.15M NaCl (pH 7.4) containing 10 μg/mL human Fc. (C) Nonstimulated (dotted line) or LPS-stimulated (1 μg/mL, 4 hours, 37°C; black line) THP-1 cells were immunostained with anti–TF-5 or an isotype-matched control antibody (shaded histogram; nonstimulated cells) as described. Platelets or THP-1 cells (10 000), identified by their forward and side scatter, were analyzed by flow cytometry using a BD LSR II Flow Cytometer. Identical results were obtained whether platelets, stained with isotype-matched control antibodies, were activated for 15 minutes or 2 hours. Similarly, LPS-stimulation of THP-1 cells was without effect on control antibody reactivity. Representative data are shown (n = 3).
These combined data confirm our previous observation indicating the absence of TF on/in platelets5 and challenge the notion that expression of TF by activated platelets is a dynamic event.6 Recently, a report by Aass and colleagues8 demonstrated that many commonly used, commercially available immune reagents, including the TF antibody used in the study by Camera et al, can contain fluorescent antibody aggregates that form particles large enough to be quantifiable as monocyte-derived microparticles by flow cytometry, yet possess no measurable TF activity. Centrifugation of these antibodies before use resulted in disappearance of these fluorescent events.8 It is also possible that some of these aggregates may be mistaken for platelets, which are typically 2-3 μm in diameter. These combined observations support our previous contention that differences in the assays and the quality of reagents used to quantify TF between laboratories, specifically, differences in antibody handling, may explain some of the apparent discrepancies.
Authorship
Acknowledgments: This work was supported by National Institutes of Health grants HL46703 (Project 2, to S.B.) and HL091111 (to B.A.B.).
Contribution: B.A.B. designed experiments, collected, analyzed and interpreted data, and wrote the manuscript; J.K.-A. participated in monocyte experiments; and S.B. conceived of research, designed experiments, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests
Correspondence: Saulius Butenas, Department of Biochemistry, University of Vermont, 208 S Park Dr, Suite 2, Rm T227B, Colchester, VT 05446; e-mail: sbutenas@uvm.edu.
References
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal