At the beginning of the 2000, Rauch et al proposed that thrombosis occurring on plaque rupture does not necessarily require the exposure of vessel wall–derived tissue factor (TF).1 Platelets can be a source of active TF, the so-called “blood-borne” TF or “circulating” TF, which can sustain activation of the blood coagulation on the edge of a growing thrombus. Since then more than 10 papers have documented the presence of functionally active TF in human platelets,2-12 with at least 3 mechanisms implicated for the presence of TF in platelets: (1) the microparticle transfer mechanism, as firstly proposed by Rauch1 ; (2) the storage within the α-granule, as suggested by Muller4 ; and (3) the de novo protein synthesis from the specific TF mRNA, as proved by Schwertz et al and Panes et al.7,8 We believe that these pathways are not mutually exclusive, and one mechanism may dominate over the other depending on the pathophysiologic conditions.
Despite all this evidence, Bouchard et al still do not find TF expression in stimulated platelets.13 Based on their paper, the following considerations can be made.
First, the antibody from American Diagnostica that we have been using, and whose specificity was previously questioned,13 works well also in the hands of Bouchard since, as the authors assert, it is able to recognize the TF expressed on LPS-stimulated THP-1 cells.
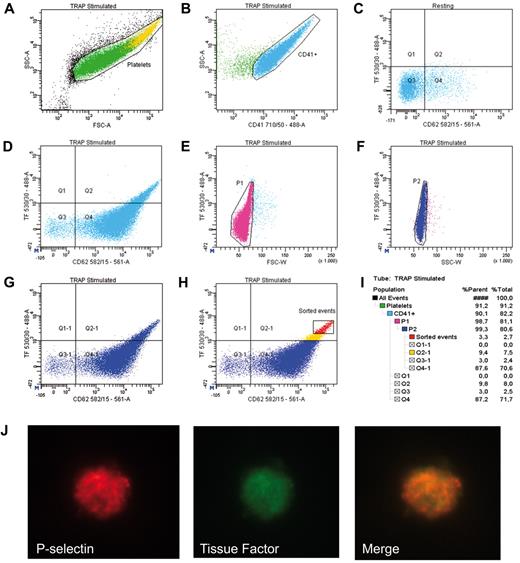
Second, it is quite hard to believe that the events that we observe with this antibody are antibody's aggregates that we confuse, as proposed by Bouchard, with microparticles. Microparticles are indeed much smaller (100 nm-1 μm) than platelets (2-3 μm) and, more importantly, TF localizes on the biggest activated platelets (Figure 1A,H). However, even if by mistake we would consider these antibody's aggregates as microparticles or platelets, it would be quite strange that they were positive also for CD41, a marker of platelet population. Indeed, as always specified in our papers5,12,14 and by all the other authors who have documented the presence of TF in platelets, TF positive events are analyzed in a cell population identified by antigenic (CD41, platelet specific) and physical parameters (side scatter [SSC] or forward scatter [FSC]; Figure 1).
Identification of TF positive platelets by flow cytometry and confocal microscopy. Platelet rich plasma from a healthy volunteer was activated with TRAP-6 for 15 minutes at room temperature in the presence of CD41-PerCPcy5.5 (BD Biosciences), CD62P-PE (P-selectin, BD Biosciences) and TF-FITC (American Diagnostica) mAbs, analyzed and sorted by BD FACSAria IIU (561-488-633-355 laser equipped) and BD FACSDiva software. The following gating strategy for the identification and sorting of TF+ platelet was used (population hierarchy in panel I). Platelets were identified according to their physical parameters, FSC and SSC (panel A) and to the expression of the platelet marker, CD41 (panel B). The quadrant gate in panel C defines the CD62P/TF negative population in resting platelets. This marker has been copied on subsequent dot plots. Panel D shows TF/CD62P positivity on activated CD41+ cells. Cross excitation risk was avoided by using 488 and 561 lasers for FITC and PE, respectively. TF+ platelet aggregates were further removed through doublets exclusion in FSC/TF and SSC/TF dot plots (panels E and F) and double positive TF/CD62P single events are shown in panel G. Highly fluorescent events (P3, panel H) were sorted and analyzed by confocal microscopy (panel J; LSM 710 Zeiss, objective Plan-Apochromat 100×/1.4 oil).
Identification of TF positive platelets by flow cytometry and confocal microscopy. Platelet rich plasma from a healthy volunteer was activated with TRAP-6 for 15 minutes at room temperature in the presence of CD41-PerCPcy5.5 (BD Biosciences), CD62P-PE (P-selectin, BD Biosciences) and TF-FITC (American Diagnostica) mAbs, analyzed and sorted by BD FACSAria IIU (561-488-633-355 laser equipped) and BD FACSDiva software. The following gating strategy for the identification and sorting of TF+ platelet was used (population hierarchy in panel I). Platelets were identified according to their physical parameters, FSC and SSC (panel A) and to the expression of the platelet marker, CD41 (panel B). The quadrant gate in panel C defines the CD62P/TF negative population in resting platelets. This marker has been copied on subsequent dot plots. Panel D shows TF/CD62P positivity on activated CD41+ cells. Cross excitation risk was avoided by using 488 and 561 lasers for FITC and PE, respectively. TF+ platelet aggregates were further removed through doublets exclusion in FSC/TF and SSC/TF dot plots (panels E and F) and double positive TF/CD62P single events are shown in panel G. Highly fluorescent events (P3, panel H) were sorted and analyzed by confocal microscopy (panel J; LSM 710 Zeiss, objective Plan-Apochromat 100×/1.4 oil).
Third, the fact that Bouchard et al do not detect TF in washed activated platelets,13 even if the antibody works on THP-1 cells, raises the suspicion that the problem lies in platelet preparation. Indeed, particular care should be used to avoid non specific platelet activation. Flow cytometry analysis of P-selectin and/or activated GpIIbIIIa should be always carried out together with TF analysis to be sure that platelet separation has been performed correctly, without procedural activation, and that the isolated platelets are functionally active and able to respond to exogenously added stimuli (Figure 1).
In conclusion, we agree with Bouchard et al that their failure to detect TF in platelets is because of some methodologic problem. They should also consider that platelet associated TF can be easily detected in whole blood,5,14 where no platelet separation steps are required, using, as stated above, population specific markers.
Authorship
Acknowledgments: The authors thank Angelo Pluderi and Fabio Villa, Carl Zeiss S.p.A. Italy; and Daniele Manganaro, BD Biosciences Italia, for technical assistance.
Contribution: M.C. conceived of research, designed experiments, analyzed data, and wrote the paper; M.B. conceived of research, designed experiments, analyzed data, and reviewed the manuscript; D.B., L.F., P.C., and L.R. performed experiments, analyzed data, and reviewed the manuscript; and V.T. and E.T. conceived of research and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marina Camera, PhD, Department of Pharmacological Sciences, Università degli Studi di Milano, Via Balzaretti 9, 20133 Milan, Italy; e-mail: marina.camera@unimi.it or marina.camera@ccfm.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal