Donor lymphocyte infusions have been effective in patients with chronic myeloid leukemia (CML) relapsing after allogeneic stem cell transplantation, but their use is associated with the risk of graft-versus-host disease. We investigated the effects of prophylactic infusion of in vitro-generated donor T cells reactive against peptides derived from CML-associated antigens. Fourteen CML patients received conditioning therapy followed by CD34+-selected peripheral blood stem cells from matched siblings (n = 7) or unrelated (n = 7) donors. Donor-derived mature dendritic cells generated in vitro from CD14+ monocytes were loaded with human leukocyte Ag-restricted peptides derived from PR1, WT1, and/or B-cell receptor–ABL and used to repetitively stimulate donor CD8+ T cells in the presence of IL-2 and IL-7. Stimulated T cells were infused 28, 56, and 112 days after transplantation. Thirteen patients are alive and 7 remain in molecular remission (median follow-up, 45 months). Interestingly, all 4 patients receiving CD8+ T cells displaying marked cytotoxic activity in vitro and detectable peptide-reactive CD8+ T cells during follow-up have not experienced graft-versus-host disease or relapse. Our study reveals that prophylactic infusion of allogeneic CD8+ T cells reactive against peptides derived from CML-associated antigens is a safe and promising therapeutic strategy. This trial was registered at www.clinicaltrials.gov as #NCT00460629.
Introduction
Despite the advent of tyrosine kinase inhibitors (TKIs), such as imatinib mesylate (IM), allogeneic stem cell transplantation (SCT) remains the only therapy with definitive curative potential for patients with chronic myeloid leukemia (CML). For patients relapsing after allogeneic SCT, donor lymphocyte infusions (DLIs) have been established as an effective therapy that may reinduce remission when used at an earlier stage in cases of molecular or cytogenetic relapse.1 Nevertheless, the risk of graft-versus-host disease (GVHD) associated with DLI remains considerable, even when it is administered in incremental doses.2
Efforts to decrease the risk of GVHD by depleting T-cell numbers in the graft have been associated with an increased incidence of relapse and subsequent treatment failure.3 Newer protocols using grafts composing CD34+-selected hematopoietic cells from matched sibling donors, and subsequent infusion of T cells in incremental doses to treat or avoid disease relapse, seem to be more promising.4,5 However, individual T-cell thresholds required to induce graft-versus-leukemia effects without relevant GVHD are difficult to predict.
CD8+ T-cell responses against the human leukocyte Ag-A2–restricted nonamer peptide PR1 derived from proteinase 3 have been shown to be associated with long-term survival of CML patients after therapy with interferon-α or allogeneic hematopoietic cell transplantation.6 Similarly, cytotoxic CD8+ T lymphocytes (CTLs) reactive against human leukocyte Ag-A2– and -A24–restricted peptides derived from Wilms' tumor antigen 1 (WT1) expressed in subtypes of myeloid leukemia have been described.7,8 WT1-specific CD8+ T cells were detected in leukemic patients, and a correlation between graft-versus-leukemia effects and detectable WT1-specific CTL was observed after allogeneic SCT for CML and acute lymphoblastic leukemia.9,10 Peptide-based vaccination trials with regard to both PR1 and WT1 have demonstrated clinical responses, and an association between therapeutic response and increased frequency of WT1-specific CTL was reported.8,11,12
In contrast to the aforementioned leukemia-associated antigens, the B-cell receptor–ABL fusion protein represents a tumor-specific antigen expressed in CML stem and progenitor cells but not in healthy hematopoietic stem cells (HSCs). Several studies have demonstrated that CD8+ T cells reactive against B-cell receptor–ABL-derived peptides can be generated in vitro and are able to lyse peptide-loaded target cells or CML cells.13,–15 Furthermore, it has been reported that B-cell receptor–ABL peptide-reactive CD8+ T cells are detectable in CML patients.10,16 B-cell receptor–ABL-derived peptides can also be used to induce specific T-cell responses in CML patients.17
Pivotal clinical studies have demonstrated that leukemia-specific CTLs can be successfully generated to treat posttransplantation relapse.18 In addition, human leukocyte Ag-restricted minor-histocompatibility antigen (mHAg)–specific CTLs have been detected in vivo after DLI, which was followed by complete remission of leukemia.19 Recently, Warren et al were able to demonstrate the safety and efficacy of CD8+ T-cell clones specific for tissue-specific mHAg when administered to patients with relapsed leukemia after allogeneic SCT.20
In the present study, donor CD8+ cytotoxic T cells reactive against PR1-, WT-1-, and/or B-cell receptor–ABL-derived peptides were generated in vitro and prophylactically administered to CML patients after allogeneic T cell–depleted SCT. Besides the feasibility and safety of this approach, we were able to demonstrate, for the first time, that the prophylactic infusion of these CD8+ T-cell preparations in allogeneic SCT is associated with durable molecular remission in 50% of patients.
Methods
Study design and procedures
We conducted a clinical trial to determine the feasibility and efficacy of the adoptive transfer of in vitro–stimulated leukemia antigen-reactive donor T cells after allogeneic transplantation of CD34+-selected peripheral blood stem cells. The trial was approved by the Institutional Review Board of the University Hospital Dresden and registered at www.clinicaltrials.gov as #NCT00460629. Patients 18 years of age or older with (1) the presence of B-cell receptor–ABL-positive CML, (2) chronic or accelerated phase, and (3) identification of an human leukocyte Ag-identical family donor or an unrelated volunteer donor matched for 9 of 10 human leukocyte Ag alleles were eligible for enrollment. Written informed consent or assent was obtained from all patients and donors. Additional inclusion criteria were either the presence of a b3a2 B-cell receptor–ABL transcript in human leukocyte Ag-A3-, -A11-, and -B8-positive patients and/or human leukocyte Ag-A2 positivity. Patients with blast phase, major organ dysfunction, or uncontrolled infection were excluded.
Primary outcome measures of this trial were the safety and feasibility of transferring leukemia-reactive donor T cells after T-depleted SCT. Secondary endpoints included the probability of surviving in molecular remission, the incidence of acute and chronic GVHD, and the rate of opportunistic infectious complications.
To compare the kinetics of donor T-cell chimerism with or without prophylactic T-cell infusion, a historical cohort of 10 patients (composed of 2 CML, 4 acute myeloid leukemia, and 4 acute lymphoblastic leukemia patients) with a similar age distribution receiving the identical conditioning regimen and T-depleted SCT without prophylactic donor T-cell infusion was selected.21
Conditioning therapy and transplantation
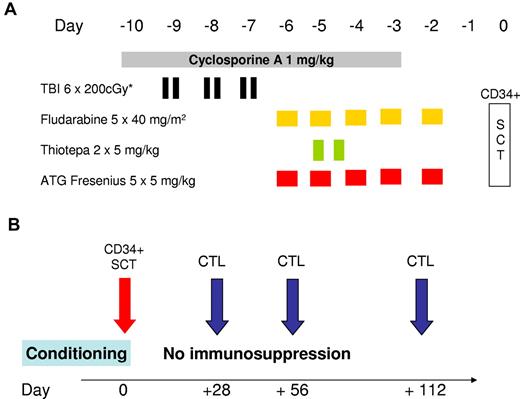
According to protocol, total body irradiation (TBI) divided into fractions of 200 cGy was performed with a total dose of 800 cGy (day −8 to day −7) in patients older than 40 years (n = 9) and with 1200 cGy (day −9 to day −7) in younger patients (n = 5). The regimen also included intravenous fludarabine (40 mg/m2 over 5 days, day −6 to day −2, total dose 200 mg/m2), thiotepa 2 × 5 mg/kg on day −6 (total dose 10 mg/kg), antithymocyte globulin (ATG)–Fresenius 5 mg/kg over 5 days (day −5 to day −1, total dose 25 mg/kg), and cyclosporine A at 1 mg/kg per day from day −10 to day −3. CD34+-selected donor cells were infused on day 0 (Figure 1). No further posttransplantation GVHD prophylaxis was performed.
Conditioning regimen and schedule for CTL infusion. (A) The conditioning regimen used before allogeneic SCT. The regimen for patients 18 to 40 years of age consisted of hyperfractionated TBI with 6 × 200 cGy from day −9 to day −7, with lung shielding (maximum lung dose of 800 cGy). Patients older than 40 years received only 4 × 200 cGy. Fludarabine was infused at a daily dose of 40 mg/m2 from day −6 to day −2. On the same days, 5 mg/kg antithymocyte globulin (ATG)–Fresenius was infused over 4 hours. Two doses of thiotepa (5 mg/kg) were infused over 4 hours on day −5. Treatment with cyclosporine A was initiated on day −10 at a daily dose of 1 mg/kg until day −3. CD34+-selected peripheral blood stem cells were infused on day 0 (SCT). (B) The planned schedule of infusion of in vitro-generated CD8+ T-cell preparations. Whenever possible, in vitro-cultured donor CD8+ T cells were infused on days 28, 56, and 112. No postgrafting immunosuppressive therapy was administered.
Conditioning regimen and schedule for CTL infusion. (A) The conditioning regimen used before allogeneic SCT. The regimen for patients 18 to 40 years of age consisted of hyperfractionated TBI with 6 × 200 cGy from day −9 to day −7, with lung shielding (maximum lung dose of 800 cGy). Patients older than 40 years received only 4 × 200 cGy. Fludarabine was infused at a daily dose of 40 mg/m2 from day −6 to day −2. On the same days, 5 mg/kg antithymocyte globulin (ATG)–Fresenius was infused over 4 hours. Two doses of thiotepa (5 mg/kg) were infused over 4 hours on day −5. Treatment with cyclosporine A was initiated on day −10 at a daily dose of 1 mg/kg until day −3. CD34+-selected peripheral blood stem cells were infused on day 0 (SCT). (B) The planned schedule of infusion of in vitro-generated CD8+ T-cell preparations. Whenever possible, in vitro-cultured donor CD8+ T cells were infused on days 28, 56, and 112. No postgrafting immunosuppressive therapy was administered.
Cell processing and generation of activated CD8+ T cells
Clinical-scale immunomagnetic CD34+ selection using the CliniMacs device (Miltenyi Biotec) was performed as previously described.22 Immunomagnetic CD8+ T-cell isolation (Miltenyi Biotec) was subsequently performed using the leukapheresis product of the second day or the CD34− fraction of the aforementioned positive selection procedure. CD14+ monocytes were also immunomagnetically isolated from an aliquot of the first or second leukapheresis product at a purity of more than 90% according to the manufacturer′s instructions (Miltenyi Biotec). To generate immature dendritic cells (DCs), the selected CD14+ cells were incubated at 37°C in 5% CO2 at a density of 2 to 3 106/mL in X-Vivo 15 medium (Bio-Whittaker) supplemented with IL-4 (1000 IU/mL; R&D Systems), granulocyte-macrophage colony-stimulating factor (2000 IU/mL; Novartis), and 1% pretested AB serum (c-c pro, Neustadt). On day 8, DCs were incubated with TNF-α (1100 IU/mL; R&D Systems), prostaglandin E2 (1 mg/mL; Pharmacia), IL-1β (1900 IU/mL; R&D Systems), and IL-6 (1000 IU/mL; R&D Systems) to induce maturation. For the second and third infusion of activated CD8+ T cells, immature DCs were cryopreserved and thawed later before the final maturation step was induced.
Human leukocyte Ag-restricted peptides derived from proteinase 3 (human leukocyte Ag-A2, VLQELNVTV23 ), WT1 (human leukocyte Ag-A2, RMFPNAPYL24 ; human leukocyte Ag-A24, RWPSCQKKF25 ), and B-cell receptor–ABL (human leukocyte Ag-A3, KQSSKALQR; human leukocyte Ag-A3/human leukocyte Ag-A11, HSATGFKQSSK; human leukocyte Ag-A3/human leukocyte Ag-A11, ATGFKQSSK; human leukocyte Ag-B8, GFKQSSKAL)13,26,27 synthesized to > 95% purity were purchased from Jerini. To generate peptide-specific CD8+ T cells, mature DCs were loaded with 20 μg/mL of leukemia antigen–derived peptides according to the patient human leukocyte Ag type. After washing, 2 × 105 peptide-loaded DCs were cocultured with 2 × 106 immunomagnetically isolated CD8+ T cells per well of a 24-well tissue culture plate (Greiner). T cells were cultured in 2 mL RPMI 1640 medium/well supplemented with AB-serum, 50 U/mL IL-2 (Proleukin; Chiron), and 100 U/mL IL-7 (R&D Systems). T cells were subsequently restimulated weekly 2 to 4 times with peptide-loaded DCs and evaluated for peptide-specific cytotoxic activity. Before infusion, CD8+ T cells were washed twice in phosphate-buffered saline and reconstituted in 0.9% physiologic NaCl solution for infusion into the recipient. The administration of in vitro–stimulated donor T cells was scheduled for days 28, 56, and 112 after transplantation (Figure 1). The target CD8+ T-cell dose per protocol was 0.5 × 106/kg recipient on day 28 and 1 × 106/kg on days 56 and 112, respectively. Release criteria for infusion of the cells were vitality of ≥ 90% determined by trypan blue exclusion, sterility, and negativity for mycoplasma by culture and polymerase chain reaction (PCR) in an aliquot derived from the last medium change. Only fresh cells were infused on day 28. For infusions on day 56 and day 112, previously cryopreserved CD8+ T cells were thawed and stimulated with peptide-loaded DCs as described in the preceding paragraph. After having demonstrated the safety of that dose level in the first 3 sequentially enrolled patients, the maximum CD8+ T-cell dose was amended to 1 × 107/kg. The protocol required the patient to have no signs of acute GVHD before the T-cell infusion could be scheduled.
Chromium release assay
Cytotoxic activity of in vitro–stimulated T cells was tested against (1) the human leukocyte Ag-A2-positive mutant cell line T2 or (2) autologous mature DCs loaded with peptides derived from leukemia antigens or an irrelevant human leukocyte Ag-A2–restricted peptide derived from HIV-1 reverse transcriptase as targets in a 4-hour standard 51Cr release assay.28 Briefly, T2 cells or DCs were incubated for 4 hours with individual peptides at a concentration of 50 μg/mL, labeled with 51Cr for 1 hour (sodium chromate; PerkinElmer Life and Analytical Sciences), and used as targets. Chromium-labeled target cells were washed 3 times and plated in round-bottomed 96-well plates at 5 × 103 cells/well. Effector cells were added as triplicates at different ratios. After 4 hours of incubation, 51Cr release was determined with a β-counter (PerkinElmer Life and Analytical Sciences).
FACS and tetramer/pentamer analyses
Standard flow cytometry (FACSCalibur; BD Biosciences) using fluorochrome-labeled antibodies was performed to measure reconstitution of CD3+/CD4+ T cells, CD3+/CD8+ T cells, CD3−/CD56+ NK cells, and CD19+ B cells. To confirm the quality of monocyte-derived DCs, the coexpression of CD83, human leukocyte Ag-DR, and the costimulatory molecules CD80 and CD86 was quantified for each DC preparation. Human leukocyte Ag/peptide tetramer or pentamer complexes were acquired from ProImmune Limited (www.proimmune.com). In the second phase of the trial, frequencies of peptide-specific CD8+ T cells were determined in donor-derived peripheral blood mononuclear cells before stimulation with peptide-loaded DCs and in the T-cell preparations, which were used for infusion. In addition, the frequency of peptide-specific CD8+ T cells after adoptive transfer was analyzed. Cells were incubated with R-phycoerythrin–labeled human leukocyte Ag/peptide tetramer or pentamer complex for 10 minutes at room temperature. Then, cells were washed and incubated with Ag-presenting cell–labeled anti-CD3 and FITC-labeled anti-CD8 (BD Biosciences) for 20 minutes at room temperature.
Analysis of donor chimerism
The methods used for DNA isolation and chimerism analysis have previously been described in detail.29 The HumanType Chimera kit (Biotype), which amplifies 12 STR loci and the amelogenin locus, was used. To assess the effects of CD8+ T-cell infusion on the development of T-cell chimerism, 30 to 40 mL heparinized blood was collected on days 56, 84, 112, and 180. As previously described, CD3+/CD4+ and CD3+/CD8+ T cells were sorted by FACS (FACSVantage or from 2009 FACSAria II, BD Biosciences) and were subjected to DNA isolation with subsequent chimerism analysis.30 Whenever possible, between 1500 and 10 000 cells were sorted for each population. Samples were also collected to assess the purity of the sorted cells. The median purity, as measured by repeated FACS analysis, was 98% (range, 92%-100%).
B-cell receptor–ABL and WT1 mRNA analysis
A quantitative B-cell receptor–ABL PCR assay was performed before conditioning, every 4 weeks during the first 12 months after transplantation, and every third month during the second to fifth year of follow-up. For all samples, real-time quantitative PCR was performed using the published EAC quantitative PCR primers and probes,31 with minor modifications and detection on an ABI7500 system (Applied Biosystems). In brief, white blood cells were obtained after erythrocyte lysis from peripheral blood and bone marrow samples, with approximately 5 × 105 to 1 × 107 cells being used for RNA extraction by the RNeasy Blood kit (QIAGEN). Complementary DNA synthesis was performed as described,32 and up to 5 μL of complementary DNA was used for real-time PCR. All reactions were performed in duplicate. Results were obtained using a second PCR reaction for a housekeeping gene (ABL) as an internal standard, which results in expression of B-cell receptor–ABL levels relative to ABL levels (ratio of copy number B-cell receptor–ABL: copy number ABL) as percentages. These results were adjusted to an international scale. Absolute quantification was performed using commercially available plasmid standards for B-cell receptor–ABL and ABL genes (Ipsogen). The assay detected 1 B-cell receptor–ABL-positive cell in 100 000 cells, as determined by dilution experiments with CML cells in normal controls. In the case of quantitative PCR negativity, nested PCR with a sensitivity of approximately 1 to 5 × 1:106 was performed as previously described.33 WT1 mRNA expression in the peripheral blood was assessed in 4 patients using real-time quantitative RT-PCR based on commercially available primer and probe sets (Applied Biosystems) and normalized to GAPDH using the ΔCt method. In normal peripheral blood, WT1 is not detectable in our hands (ΔCt > 25 cycles), in the 4 patients (nos. 11-14) analyzed, the ΔCt values were 8.9, 15.6, 17.9, and 22.6. However, only the first patient could be analyzed at diagnosis; the others were investigated during follow-up.
Statistical analysis
Means (± SE) or median with ranges were provided as descriptive parameters whenever applicable. The probability of overall and molecular relapse-free survival, including 95% confidence intervals, was calculated by the method of Kaplan and Meier using the SPSS software package 12.0 (SPSS Software GmbH).
Results
Patient characteristics
Fourteen patients 21 to 67 years of age (median, 45 years) were included between August 2003 and October 2008. Patient characteristics, the interval between diagnosis and transplantation, disease status at the time of transplantation, the European Group for Blood and Marrow risk score,34 and the details of pretreatment are summarized in Table 1. Given the lower frequency of referral of CML patients after approval of IM, these 14 patients represent 93% of patients with chronic or accelerated phase CML who underwent SCT at our center during the accrual period. Only 1 patient was not eligible because of a human leukocyte Ag type mismatch. The reasons for transplantation from human leukocyte Ag-A-, -B-, -C-, -DR-, and -DQ-matched related (n = 7) or unrelated (n = 7) donors were (1) loss of hematologic response/acceleration (n = 5), loss of cytogenetic remission (n = 2), loss of major molecular remission as warning signal (n = 3) or detection of a T315I mutation (n = 1); (2) intolerance to tyrosine kinase inhibitor therapy (n = 1, elevated liver enzymes); or (3) the patient's decision (n = 2). Only 7 patients (50%) were in the first chronic phase (CP) at the time of transplantation, whereas 7 patients were in second CP (n = 4) or presented with signs of acceleration (n = 3). Two patients had previously achieved a major cytogenetic response but preferred elective allogeneic SCT. Intensive chemotherapy with cytarabine and anthracyclines had become necessary to control blast transformation in 3 patients. In 1 patient (patient 10) with a disease history of > 10 years, autologous transplantation with peripheral blood stem cells collected after combination therapy was performed 5 years before allogeneic SCT. A second-line TKI (dasatinib) was prescribed in 3 patients after the occurrence of IM-resistant disease. A T315I mutation within the tyrosine kinase domain of B-cell receptor–ABL was identified as the reason for resistance against IM in 1 case. The high-risk characteristics of the included population is supported by the fact that the median European Group for Blood and Marrow risk score of the cohort was 3 (range, 2-6) with 11 patients having a score of > 2 predicting a transplantation-related mortality of 40% to 70%.
Engraftment- and transplant-related toxicity
After immunomagnetic CD34+ selection, the grafts infused on day 0 contained a median of 6.7 × 106/kg CD34+ cells (range, 4.0-10.8 × 106/kg) and only 0.5 × 104/kg CD3+ T cells (range, 0-9.8 × 104/kg). Prompt neutrophil (absolute neutrophil count > 0.5 × 109/L) and platelet engraftment (> 50 × 109/L) was achieved without growth factor administration after 13.5 days (range, 10-20 days) and 20 days (range, 12-28 days), respectively. Only patients receiving 1200 cGy TBI (n = 5) had grade 3 mucositis. Grade 3 to 4 gastrointestinal toxicity (according to National Cancer Institute Common Toxicity Criteria, Version 3.0) was observed in 7 patients (50%). In 4 patients, this was associated with neutropenic fever and positive blood cultures; the occurrence of neutropenic enterocolitis was most likely related to the combination of TBI and thiotepa and was reversible in all cases. Of 5 cytomegalovirus-seropositive patients, 2 experienced cytomegalovirus reactivation as measured by quantitative PCR, which resolved after preemptive therapy with ganciclovir. Human herpesvirus-6 viremia associated with exanthema occurred in 1 patient on day 40 after transplantation and remitted after 10 days of oral valganciclovir. Patient 10 with a 10-year disease history experienced pulmonary aspergillosis with central nervous manifestations, from which death occurred 4 months after transplantation despite escalation of antifungal therapy. Pulmonary tuberculosis was reactivated in patient 1 at 4 months after transplantation, and the patient required tuberculostatic therapy for 24 months. In 1 patient, toxoplasmosis of the retina occurred 6 months after transplantation; specific therapy was instituted, and all symptoms resolved without any functional impairment. No patient experienced acute GVHD, and 4 of 13 patients at risk (31%) had to be treated for mild or moderate chronic GVHD. Immunosuppressive therapy had been stopped in all these 4 cases at the time of reporting.
Donor chimerism and immune reconstitution
The number of total nucleated cells and CD8+ T cells per kilogram recipient infused on each occasion as well as the percentage of CD8+ T cells and the vitality of each cell preparation are provided for every patient in Table 2. A median dose of 1.85 × 106/kg CD8+ T cells (range, 0.06-4.1 × 106/kg CD8+ T cells), 1.7 × 106/kg CD8+ T cells (range, 0.06-5.7 × 106/kg CD8+ T cells), and 2.6 × 106/kg CD8+ T cells (range, 1.1-9.5 × 106/kg CD8+ T cells) could be administered on days 28, 56, and 112, respectively. In patient 2, only 2 infusions were possible because of the limited quantity and viability of cells obtained after culture at day 112. Patient 10 could only receive the first infusion of CD8+ T cells on day 28 because his clinical condition did not allow further participation in the trial. All other CTL infusions could be performed within 3 days of the scheduled dates. The infused preparations contained a median of 80% CD3+/CD8+ T cells (range, 50%-98%), 4.7% CD3+/CD4+ T cells (range, 0%-15.2%), and 0% CD3−/CD56+ natural killer cells (range, 0%-7.2%). The median vitality was 98% (range, 90%-100%). There was a considerable variability in the amplification of CD8+ T cells with a faster cell growth in the initial phase of stimulation and lower expansion factors during subsequent restimulation. The median expansion factor of CD8+ T cells after 3 to 4 weeks of cell culture was 1.6 (range, 0.3-10).
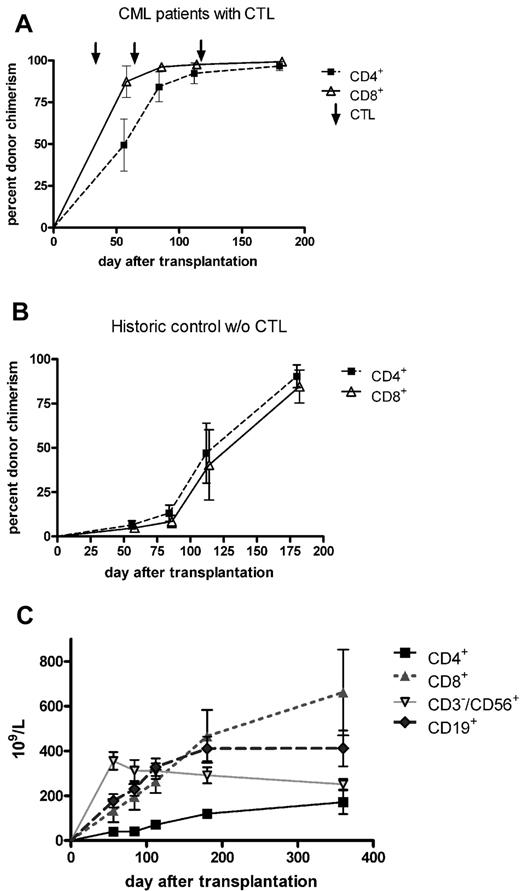
As shown in Figure 2A, the infusion of activated donor CD8+ T cells on day 28 was associated with a prompt increase in the percentage of donor CD4+ and CD8+ T cells in the patient's peripheral blood. At the time of the third infusion (day 112), complete T-cell chimerism could be documented in most patients. Figure 2B summarizes the course of CD4+ and CD8+ donor chimerism in a historical control cohort that was transplanted with T cell–depleted grafts from matched sibling donors without receiving prophylactic DLI.21 In these patients, the mean percentage of donor chimerism in T-cell subsets remained < 90% until 6 months after transplantation. The absolute count of lymphocyte subsets is shown in Figure 2C. CD4+ T cells were the only subset not to return to normal levels, remaining < 200/μL within the first 12 months after transplantation. CD8+ T cells dominated during that period, CD19+ B cells reached normal levels as soon as 3 months after transplantation, whereas CD3−/CD56+ NK cells peaked 2 months after transplantation and slowly declined for another 10 months.
T-cell chimerism and immune reconstitution. (A) Infusion of ex vivo-activated donor T cells on days 28, 56, and 112 was associated with a prompt increase in the percentage of donor CD4+ and CD8+ T cells in the peripheral blood of recipients. Six months after transplantation, all 13 patients who could be evaluated showed complete donor T-cell chimerism. Compared with a historical cohort of patients who did not receive prophylactic donor T-cell infusion after T cell–depleted SCT (B), the increase in donor T-cell chimerism to levels > 50% had occurred > 3 months earlier. (C) The absolute count of lymphocyte subsets. CD4+ T-cell numbers remained less than 200/μL within the first 12 months after transplantation, whereas CD8+ T cells dominated during that period. CD19+ B cells reached normal levels as soon as 3 months after transplantation, whereas CD3−/CD56+ NK cells peaked 2 months after transplantation and slowly declined for further 10 months.
T-cell chimerism and immune reconstitution. (A) Infusion of ex vivo-activated donor T cells on days 28, 56, and 112 was associated with a prompt increase in the percentage of donor CD4+ and CD8+ T cells in the peripheral blood of recipients. Six months after transplantation, all 13 patients who could be evaluated showed complete donor T-cell chimerism. Compared with a historical cohort of patients who did not receive prophylactic donor T-cell infusion after T cell–depleted SCT (B), the increase in donor T-cell chimerism to levels > 50% had occurred > 3 months earlier. (C) The absolute count of lymphocyte subsets. CD4+ T-cell numbers remained less than 200/μL within the first 12 months after transplantation, whereas CD8+ T cells dominated during that period. CD19+ B cells reached normal levels as soon as 3 months after transplantation, whereas CD3−/CD56+ NK cells peaked 2 months after transplantation and slowly declined for further 10 months.
Induction of peptide-reactive CD8+ T cells in vitro and detection of peptide-specific CD8+ T cells in vivo after adoptive transfer
Immunomagnetic selection of CD14+ monocytes from an aliquot of the leukapheresis product resulted in a median purity of 98% (range, 94%-100%) with a median recovery rate of 95% (range, 60%-100%). FACS analysis of human leukocyte Ag-DR, CD80, CD83, and CD86 was used to evaluate DC maturation. The median percentage of mature DCs coexpressing CD83/human leukocyte Ag-DR and CD80/CD86 after maturation was 70% (range, 22%-93%) and 80% (range, 24%-89%), respectively. To obtain sufficient amounts of peptide-specific CD8+ T cells for adoptive transfer, DCs were loaded with peptides derived from proteinase 3, WT1, and/or B-cell receptor–ABL and cocultured with CD8+ T cells.
In the second phase of the clinical trial, we systemically analyzed the frequency of peptide-specific CD8+ T cells in donor-derived peripheral blood mononuclear cells before stimulation with peptide-loaded DCs and in the T-cell preparations, which were adoptively transferred. As demonstrated in Table 3, the percentage of preexisting peptide-specific CD8+ T cells in the blood of 8 evaluated donors for patients 7 to 14 was low or not detectable. After stimulation with peptide-loaded DCs, the frequency of peptide-reactive CD8+ T cells was markedly increased in cell preparations for 3 patients (patients 11, 12, and 14).
In further experiments, we evaluated the cytotoxic potential of the generated CD8+ T cells. As shown in Table 4, peptide-specific CD8+ T cells exhibiting marked cytotoxic activity could be induced in 7 of 14 donors. The frequency of peptide-specific CD8+ T cells in the peripheral blood was monitored for at least 1 year after transplantation. Maximum values related to the day of analysis are included in Table 4. Before the first infusion on day 28, the level of CD3+ T cells in the peripheral blood was, in general, too low for subset chimerism and multimer FACS analyses, as is expected after T-depleted SCT.
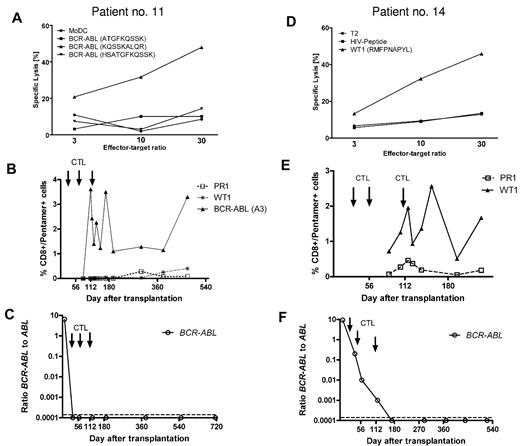
Table 5 categorizes patients according to the infusion of CD8+ T cells with cytotoxic activity in vitro and the occurrence of peptide-specific CD8+ T cells in vivo, as determined by tetramer or pentamer staining. Interestingly, 4 of 5 patients in whom the infusion of donor T cells with peptide-specific cytotoxicity was followed by measurable pentamer+/CD8+ T cells in vivo (patients 3, 5, 11, and 14) have remained B-cell receptor–ABL negative between 31 and 55 months after transplantation. None of these patients has shown clinical signs of acute or chronic GVHD during follow-up. In addition, Table 5 provides B-cell receptor–ABL mRNA levels measured by PCR 4 weeks after conditioning therapy/transplantation before the first infusion of activated CD8+ T cells and by the time of last follow-up. Figure 3 shows the cytotoxic potential of peptide-reactive CD8+ T cells and the course of tetramer-positive CD8+ T cells after adoptive transfer in 2 patients (patients 11 and 14) from this group in conjunction with the level of minimal residual disease after transplantation until final follow-up. Only patient 1, who had undergone transplantation in the advanced phase of CML and had received a low dose of donor T cells, experienced cytogenetic relapse and was successfully treated with IM.
In vitro cytotoxicity of CD8+ T cells, the frequency of peptide-specific CD8+ T cells in vivo, and B-cell receptor–ABL levels. In vitro cytotoxicity of infused CTLs was tested with peptide-loaded antigen-presenting cells (DCs or T2 cells) as targets. Panels A and D confirm antigen-specific cytotoxicity against a human leukocyte Ag-A3–restricted B-cell receptor–ABL-derived peptide (A) and an human leukocyte Ag-A2-binding WT1 peptide (D) in patients 11 and 14, respectively. (B,E) The corresponding frequency of human leukocyte Ag/peptide tetramer-positive CD8+ T cells after transplantation and CD8+ T-cell infusion in each case. (C,F) B-cell receptor–ABL mRNA became undetectable in both patients, and PCR negativity was maintained until the last follow-up.
In vitro cytotoxicity of CD8+ T cells, the frequency of peptide-specific CD8+ T cells in vivo, and B-cell receptor–ABL levels. In vitro cytotoxicity of infused CTLs was tested with peptide-loaded antigen-presenting cells (DCs or T2 cells) as targets. Panels A and D confirm antigen-specific cytotoxicity against a human leukocyte Ag-A3–restricted B-cell receptor–ABL-derived peptide (A) and an human leukocyte Ag-A2-binding WT1 peptide (D) in patients 11 and 14, respectively. (B,E) The corresponding frequency of human leukocyte Ag/peptide tetramer-positive CD8+ T cells after transplantation and CD8+ T-cell infusion in each case. (C,F) B-cell receptor–ABL mRNA became undetectable in both patients, and PCR negativity was maintained until the last follow-up.
In vitro-generated CD8+ T cells displaying marked cytotoxic potential without detectable increase in the frequency of CD8+ T-cell responses in vivo occurred in patients 1 and 6, who experienced cytogenetic and molecular relapse, respectively. In both cases, a major molecular response could be achieved by salvage therapy with IM and unmodified DLI. In the case of patient 2, neither in vitro cytotoxicity of the T-cell preparation nor pentamer+ CD8+ T cells in the recipient were detectable. Molecular relapse in this patient occurred 11 months after transplantation and was successfully treated with IM and DLI.
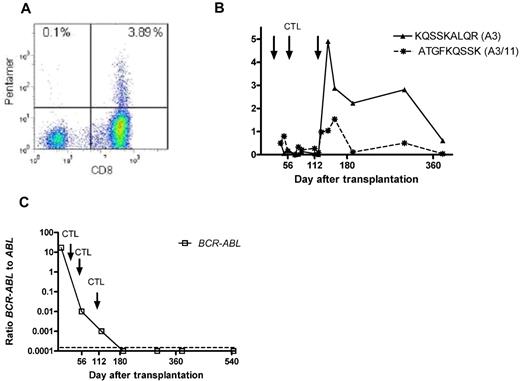
Pentamer+ CD8+ T cells have been identified after transplantation in 6 recipients in whom donor CD8+ T cells with either no detectable in vitro cytotoxicity (patients 4, 7, 8, 9, and 13) or a cytotoxic response against another peptide (patient 12) were infused. Three of these patients (patients 4, 7, and 8) experienced molecular or hematologic relapse and had to be treated with IM and/or DLI. Patient 12, who had received PR1-reactive CD8+ cells but showed WT1/pentamer+ cells during follow-up, has not achieved complete molecular remission. Sustained negativity for B-cell receptor–ABL has been maintained in patients 9 and 13 for 42 and 39 months, respectively. Figure 4 summarizes the course of patient 9, in whom a considerable proportion of B-cell receptor–ABL-specific CD8+ T cells could be detected during follow-up. Figure 4A shows the representative heat map of KQSSKALQR/human leukocyte Ag-A3 pentamer+ CD8+ T cells detectable in peripheral blood of patient 9 at 3 weeks after the third infusion of the in vitro-generated CD8+ donor T-cell preparation. As shown in Figure 4B, this was accompanied by the detection of 1.5% ATGFKQSSK/human leukocyte Ag-A11/-A3 pentamer+ CD8+ T cells. The patient has remained in molecular remission > 3 years after transplantation (panel 4C).
Peptide+/human leukocyte Ag+ CD8+ T cells and B-cell receptor–ABL levels. (A) FACS heat map of KQSSKALQR/human leukocyte Ag-A3 pentamer+ CD8+ T cells detectable in the peripheral blood of patient 9 at 3 weeks after the third infusion of the in vitro-generated CTL preparation. As demonstrated in panel B, this was accompanied by the detection of 1.5% ATGFKQSSK/human leukocyte Ag-A11/-A3 pentamer+ CD8+ T cells. The patient has remained in molecular remission throughout follow-up, with no signs of acute or chronic GVHD (C).
Peptide+/human leukocyte Ag+ CD8+ T cells and B-cell receptor–ABL levels. (A) FACS heat map of KQSSKALQR/human leukocyte Ag-A3 pentamer+ CD8+ T cells detectable in the peripheral blood of patient 9 at 3 weeks after the third infusion of the in vitro-generated CTL preparation. As demonstrated in panel B, this was accompanied by the detection of 1.5% ATGFKQSSK/human leukocyte Ag-A11/-A3 pentamer+ CD8+ T cells. The patient has remained in molecular remission throughout follow-up, with no signs of acute or chronic GVHD (C).
Survival
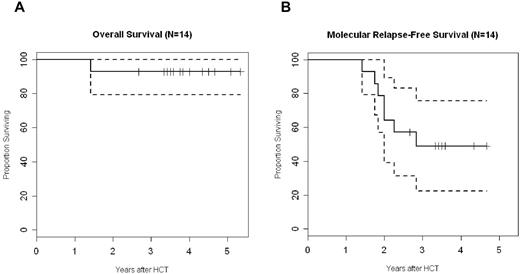
With a median follow-up of 45 months, the probability of overall and molecular relapse-free survival is 93% (confidence interval, 79%-100%) and 49% (confidence interval, 22%-76%), respectively (Figure 5). Although the lack of a control arm precludes drawing any conclusions on the association between the specific intervention and clinical outcome, the favorable survival and the limited rate of molecular relapse are encouraging, in particular in the light of the older patient cohort included with high European Group for Blood and Marrow pretransplantion risk scores. As mentioned in “Engraftment- and transplant-related toxicity,” only 1 patient died of cerebral aspergillosis 3 months after transplantation. Six patients experienced molecular (n = 4), cytogenetic (n = 1), or hematologic relapse (n = 1). Five of them were successfully treated with IM, which was combined with unmodified DLIs in 4 cases. Two of these patients have subsequently achieved molecular remission. A major molecular response has been reached in 3 cases, whereas 1 patient with molecular relapse has maintained a stable Bcr-Abl/Abl ratio of 0.12 without further intervention until 3 years after transplantation.
Overall and molecular relapse-free survival. Graphs represent Kaplan-Meier estimates of overall survival (A) and molecular relapse-free survival (B) for all 14 patients. The dashed line represents the respective 95% confidence interval.
Overall and molecular relapse-free survival. Graphs represent Kaplan-Meier estimates of overall survival (A) and molecular relapse-free survival (B) for all 14 patients. The dashed line represents the respective 95% confidence interval.
Discussion
Establishing graft-versus-leukemia effects without inducing GVHD remains a major challenge after allogeneic SCT. This is especially true for patients with CML who are less frequently referred for transplantation because of the success of TKI therapy with IM or second-line compounds. Although scoring systems have been developed to estimate the individual risk of therapy-related death,34 most referring physicians are hesitant to recommend a procedure associated with considerable morbidity and mortality. Obviously, the aforementioned scoring systems were developed before the era of IM in a retrospective fashion and are therefore difficult to extrapolate into current therapeutic algorithms. All efforts to avoid these risks using in vitro or in vivo T-cell depletion have been offset by a significant increase in the incidence of relapse.35 Infusion of unselected donor T cells to treat relapse in leukemia is effective in a significant proportion of CML patients but also causes significant morbidity because of GVHD even when incremental doses are infused.2 The encouraging clinical results of our study are in line with the data reported by Elmaagacli and coworkers.4 They transplanted CD34+ purified granulocyte colony-stimulating factor-mobilized allogeneic stem cells from matched sibling donors and infused unmodified donor T cells in increasing doses starting on day 120, to preemptively encounter molecular or cytogenetic relapse.4 The high probability of survival and limited incidence of acute and chronic GVHD in this series prompted us to use this platform to prophylactically infuse ex vivo-activated donor T cells. Most importantly, this strategy allowed avoidance of the use of pharmacologic immunosuppression, which would suppress the activity of adoptively transferred T cells and reduce the antileukemic efficacy of the overall approach. Stable hematopoietic engraftment could be achieved even in recipients of transplants from unrelated donors. This, of course, requires an immunosuppressive conditioning regimen. Besides TBI, thiotepa, and ATG, the use of cyclosporine before transplantation may have ensured rapid and stable hematopoietic engraftment in all patients. The omission of postgrafting cyclosporine and methotrexate contributed to a major reduction in extramedullary toxicity compared with transplantation with T-cell-replete grafts. Further reduction in the intensity of our protocol will have to be developed with caution because less intensive conditioning therapy followed by the infusion of T cell–depleted donor cells has been associated with a high incidence of relapse in CML patients.36
The successful infusion of leukemia-reactive T-cell lines generated against leukemic targets was described > 10 years ago in a patient with CML relapsing after allogeneic SCT.18 Protocols to generate CD8+ T cells specifically targeted against hematopoiesis-restricted mHAg ex vivo have subsequently been developed.37 Although considered to be leukemia specific, a recent report has demonstrated that the adoptive transfer of ex vivo-expanded mHAg-specific CTL can lead to significant pulmonary toxicity because of cross-reactivity with antigens expressed in lung tissue.20 Again, clinical responses were observed, but only a minority of T-cell lines or clones that had been prospectively isolated could be infused at the time of hematologic relapse. Interestingly, peptide-based vaccination therapy inducing T-cell responses against potential autoantigens, such as proteinase 3 or WT1, has so far not been associated with clinically relevant toxicity or autoimmunity.8,11,12,38
Our protocol allowed the induction of peptide-specific CD8+ T cells exhibiting marked cytotoxic activity in 50% (n = 7) of healthy donors. The frequency of detectable peptide-specific CD8+ T-cell responses against various epitopes may vary significantly. Whereas B-cell receptor–ABL-specific CD8+ T cells have rarely been detected or induced in healthy volunteers, this may be possible for up to 90% with regard to WT1.8,–10,16,39 Because we did not infuse T-cell clones or purified antigen-specific T-cell populations, we cannot exclude the possibility that a proportion of the antileukemic efficacy may be linked to alloreactivity of T cells within bulk populations.
To decrease the risk of immune escape by target deletion, we combined several antigens whenever possible. In human leukocyte Ag-A2-positive donors, PR1 and WT1 were used concomitantly, but the responses observed were always stronger for WT1. This also correlated with a higher frequency of CD8+ T cells binding with the WT1/A2 pentamer during follow-up, whereas no significant increase in PR1-reactive CD8+ T cells was detectable even after adoptive transfer of in vitro-activated CTL. PR1-specific T-cell frequencies have been correlated with clinical response to interferon or after allogeneic SCT.6 Results of a recent vaccination trial using both WT1 and PR1 peptides together with adjuvant suggest that robust cellular immune responses can be induced against both epitopes in vivo and that these responses correlate with eradication of residual disease.11 This may be different when CD8+ T cells with a minimal precursor frequency are primed with peptide-loaded antigen-presenting cells in vitro. In addition, the avidity of PR1-specific T cells induced in vitro may be closely related to the peptide concentration chosen, thereby suggesting that activation-induced cell death may contribute to the rapid deletion of these cells after infusion.40 Another reason could be the low level of target antigen in the first 6 to 8 weeks after conditioning therapy, which may hamper proliferation of infused CTL.
Besides leukemia-associated antigens, B-cell receptor–ABL represents a disease-specific target, which has been established as the ideal candidate for cellular immune therapies. Both major histocompatibility complex class I–restricted and 17-mer peptides have been described, which are able to induce a CD8+ and CD4+ T-cell response after vaccination.17 In our series, the highest frequency of B-cell receptor–ABL-specific CD8+ T cells was observed in response to treatment with human leukocyte Ag-A3/-A11-specific peptides. The human leukocyte Ag-B8–restricted peptide was used in 4 patients, and peptide-specific CD8+ T cells could be found in 2 of these patients.
Our data confirm a recent report in which B-cell receptor–ABL-specific CD8+ T cells could be detected in CML patients as well as in healthy donors, albeit in much lower frequencies.41 Interestingly, in this report, an in vitro induction of higher frequencies of peptide-specific CD8+ responses was barely possible in CML patients but could be achieved in healthy donors, thus arguing for the presence of a tolerance mechanism in CML patients. This finding argues in favor of our approach using human leukocyte Ag-matched CD8+ T cells from healthy donors and allogeneic SCT as a platform for effective adoptive immunotherapy.
A recent report from Warren et al suggests that the half-life of adoptively transferred CTL clones is approximately 7 days, and homing to the bone marrow may occur.20 Of course, the dynamics may be different in patients with minimal levels of residual disease at the time of infusion of polyclonal T-cell preparations. Although we can only speculate on the fate of infused T-cell preparations, we have indirect evidence of their biologic activity. Lineage-specific chimerism analyses revealed a steep increase in donor signals for CD8+ and CD4+ T cells after the first and second infusion of ex vivo-generated CTL. This increase in donor T-cell chimerism occurred 3 to 4 months earlier compared with a historical cohort of patients receiving T cell–depleted SCT with no planned adoptive T-cell transfer.21 Therefore, we hypothesize that lymphodepletion induced by the conditioning regimen and the infusion of CD34+ donor cells allowed for a rapid peripheral proliferation of transferred T-cell preparations without any competition against existing T-cell populations, and the lack of calcineurin inhibitors as pharmacologic immunosuppression did not jeopardize the proliferation of donor lymphocytes infused early after transplantation. Although the dose of ATG used in this trial is at the lower end of current dosing schedules, there might have been residual levels of ATG in the plasma of our patients potentially suppressing the activity of the first CTL dose on day 28. Pharmacokinetic data obtained in recipients of T-depleted transplants suggest that the levels of active ATG decrease below therapeutic levels between 8 and 38 days after transplantation.42 Therefore, no relevant interference with the second and third CTL infusion can be assumed.
The current approach can be improved to obtain increased numbers of T cells reactive against leukemia antigen–derived peptides and to include a higher proportion of patients with CML requiring transplantation after failure of conventional pharmacologic therapy. Thus, loading of DCs with synthetic overlapping long peptides composing various epitopes for CD8+ T cells as well as CD4+ T cells can induce and expand both T-cell subsets simultaneously, which can subsequently be administered to patients. This immunotherapeutic strategy combines the antitumor effects mediated by both T-cell subsets and may induce more effective tumor-directed responses.43 Furthermore, peptides derived from additional leukemia antigens, such as elastase, prame, and hyaluronic acid receptor,44,–46 can be used to generate specific T cells. Purifying antigen-specific T cells by their secretion of interferon-γ or their binding to peptide/human leukocyte Ag complexes may increase the specificity of adoptive cellular therapy and thereby decrease the risk for acute and chronic GVHD.47,48 Another attractive approach would be the combination of vaccination and adoptive cellular therapy to maximize efficacy in the lymphopenic host after T-depleted allogeneic SCT.49
In conclusion, this is the first study to demonstrate that the prophylactic infusion of B-cell receptor–ABL-, WT1-, and PR1-reactive CD8+ donor T cells after allogeneic SCT from matched sibling and unrelated donors is safe and feasible. In cases with detectable in vitro cytotoxicity and measureable peptide-specific CD8+ T cells in the recipient, molecular remission of Ph+ CML could be maintained for > 4 years after transplantation, with no occurrence of acute or chronic GVHD. Given the minimal treatment-related morbidity and mortality observed so far in this cohort with predominantly high pretransplantation risk scores, this strategy may represent a promising therapeutic option for CML patients with IM resistance or intolerance to TKI therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Bärbel Löbel, Diana Döhler, Anja Maiwald, Anja Zenkel, and Claudia Klotsche for their excellent technical assistance.
This work was supported in part by Deutsche Krebshilfe (grant 70-2755, M.B., C.T.; grant 70-2980, M.B., M.S.) and the Deutsche Forschungsgemeinschaft (SFB655, project B2 to M.B.).
Authorship
Contribution: M.B., M.S., E.P.R., and G.E. designed the study protocol; C.T., U.O., J.B., R.W., H.J., M.B., and M.S. performed the monitoring of immune monitoring and of minimal residual disease; A.K., U.P., S.T., J.R., M.W., and J.S. were responsible for clinical care and out-patient follow-up; M.B., M.P.B., and M.S. analyzed data; M.B. and M.S. wrote the paper; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin Bornhäuser, Medizinische Klinik und Poliklinik I, Universitätsklinikum Carl Gustav Carus, Fetscherstrasse 74, 01307 Dresden, Germany; e-mail: martin.bornhaeuser@uniklinikum-dresden.de.