Abstract
Interleukin-1β (IL-1β) is critical for inflammation and control of infection. The production of IL-1β depends on expression of pro-IL-1β and inflammasome component induced by inflammatory stimuli, followed by assembly of inflammasome to generate caspase-1 for cleavage of pro-IL-1β. Here we show that tumor suppressor death-associated protein kinase (DAPK) deficiency impaired IL-1β production in macrophages. Generation of tumor necrosis factor-α in macrophages, in contrast, was not affected by DAPK knockout. Two tiers of defects in IL-1β generation were found in DAPK-deficient macrophages: decreased pro-IL-1β induction by some stimuli and reduced caspase-1 activation by all inflammatory stimuli examined. With a normal NLRP3 induction in DAPK-deficient macrophages, the diminished caspase-1 generation is attributed to impaired inflammasome assembly. There is a direct binding of DAPK to NLRP3, suggesting an involvement of DAPK in inflammasome formation. We further illustrated that the formation of NLRP3 inflammasome in situ induced by inflammatory signals was impaired by DAPK deficiency. Taken together, our results identify DAPK as a molecule required for full production of IL-1β and functional assembly of the NLRP3 inflammasome. In addition, DAPK knockout reduced uric acid crystal-triggered peritonitis, suggesting that DAPK may serve as a target in the treatment of IL-1β-associated autoinflammatory diseases.
Introduction
Interleukin-1β (IL-1β) plays an important role in infection and inflammation.1 The generation of IL-1β is divided into 2 stages. In the first stage, inflammatory stimuli activate nuclear factor-κB (NF-κB) to promote the synthesis of pro-IL-1β. In the second stage, pro-IL-1β protein is cleaved by caspase-1 to generate mature p17 IL-1β protein. The activation of caspase-1 is dependent on the formation of large multiprotein complexes inflammasomes in macrophages and monocytes.2-6 Assembly of inflammasomes is stimulated by infection, inflammation, or danger signals. The NACHT domain-, leucine-rich repeat-, and pyrin domain-containing protein 3 (NLRP3, also known as NALP3 or cryopyrin) inflammasome responds to a large array of stimulation, including microbial products, toxins, adenosine triphosphate (ATP), crystalline, aggregated particles.7-14 The NLRP3 protein consists of an N-terminal pyrin domain for protein-protein interaction, a central NACHT domain for nucleotide binding and self-oligomerization, and C-terminal leucine-rich repeat motifs for ligand sensing. Mutation of the NLRP3 gene is associated with several autoinflammatory disorders, and NLRP3 is also linked to gout and diabetes.5,15 Two signals are required for formation of NLRP3 inflammasome. NF-κB-containing inflammatory signals activate NLRP3 expression.16 A second inflammatory signal then stimulates the assembly of the NLRP3 inflammasome, through the binding of NLRP3 to apoptosis-associated speck-like protein containing a CARD domain (ASC) and recruitment of procaspase-1 molecule. Reactive oxygen species10,13,17 and lysosomal protease cathepsin B12,13 are potential activators of the NLRP3 inflammasome assembly. However, how inflammatory stimuli promote the formation of the NLRP3 inflammasome remains largely unclear.2-6 In contrast, NLRP1 inflammasome has been successfully reconstituted in vitro with the recombinant proteins.18 Part of the difficulties is the result of the spontaneous formation of inflammasome in vitro on cell membrane damage during the preparation of cell lysates.19
Death-associated protein kinase (DAP-kinase, DAPk, or DAPK) is calcium/calmodulin-regulated Ser/Thr kinase that acts as a tumor suppressor.20,21 Diminished DAPK expression is found in various types of cancer, including B lymphoma and chronic lymphocytic leukemia.22-24 DAPK also mediates pathologic damages, such as N-methyl-D-aspartate receptor-triggered neuronal cell death.25 DAPK is organized into multiple domains: an N-terminal kinase domain, followed by a calcium/calmodulin regulatory fragment, ankyrin repeats, a cytoskeleton binding region, and a C-terminal death domain. The multifunctional domains of DAPK are linked to diverse activities.20,21 DAPK is involved in apoptosis induced by different signals. DAPK phosphorylates myosin II regulatory light chain that participates in stress fiber formation, autophage vesicle formation, and membrane blebbing. Notably, DAPK often antagonizes activation signals, illustrated by the negative crosstalk between DAPK and Src/ERK,26-29 and specific inhibition of T cell receptor–induced activation by DAPK.30 DAPK is also part of a negative-feedback module in regulating the expression of inflammatory genes.31
Recent studies reveal an intriguing interplay between inflammasomes and autophagy. Caspase-1 activation suppresses Shigella-induced autophagy,32 whereas autophagy inhibits activation of inflammasome. Knockout of autophagy-associated genes Atg16L or Atg7 profoundly increases the activation of caspase-1.33 Because DAPK participates in autophagy formation,34,35 we investigated whether DAPK is a negative regulator of the inflammasome. Surprisingly, we observed that DAPK is required for the full IL-1β generation and NLRP3 inflammasome activation. DAPK deficiency repressed IL-1β production and caspase-1 activation. The generation of tumor necrosis factor-α (TNF-α), in contrast, was not affected in DAPK-null macrophages. In addition, through the use of proximity ligation assay,36 we were able to monitor the specific formation of NLRP3 inflammasome in situ on dual stimuli of lipopolysaccharide (LPS) and ATP. Our results reveal a novel function of DAPK by which DAPK regulates IL-1β production, and contributes to the formation of NLRP3 inflammasome. In addition, DAPK is a potential target in the treatment of IL-1β–associated autoinflammatory diseases.
Methods
Reagents
Crude LPS, Flag-M2 antibodies, Flag-M2 beads, and DAPK antibodies were purchased from Sigma-Aldrich. R837, R848, purified LPS, Pam3CSK4, nigericin, alum crystals, and monosodium urate (MSU) were obtained from InvivoGen. Antibodies specific for ASC (pAb, AL177), human NLRP3 (clone nalpy3-b), and mouse NLRP3 (clone cryo-2) were purchased from Axxora. Anti-ASC was obtained from MBL. Antihuman caspase-1 (A-19) and antimouse caspase-1 p10 (M-20) were purchased from Santa Cruz Biotechnology. Antiprocessed IL-1β p17 (Asp116) was obtained from Cell Signaling Technology. Polyclonal anti-NLRP3 was purchased from Genetex.
Cell cultures
Human monocyte cell line THP-1 was cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (Invitrogen), 10mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50μM 2-mercaptoethanol (complete RPMI medium). Mouse macrophage cell line J774A.1 was grown in Dulbecco modified Eagle medium with the same supplements (complete Dulbecco modified Eagle medium). Bone marrow cells were collected from tibias and femurs by flushing with cold RPMI medium through a 25-G needle and were cultured in complete Dulbecco modified Eagle medium with addition of 20% L929 cell-conditioned medium to generate bone marrow-derived macrophages. ASC was knocked down in THP-1 cells using siRNA purchased from Ambion, with sense sequence: GCA AGA UGC GGA AGC UCU UTT.
DAPK overexpression, knockout, and knockdown
Overexpression of DAPK was performed by infection of THP-1 cells using pGC-YFP or pGC-DAPK-YFP.30 Forty-eight hours after infection, YFP-expressing THP-1 cells were isolated by sorting on a flow cytometer (FACSVantage SE, BD Biosciences). Production of Dapk−/− mice (in C57BL/6 background) was previously described.37 Mice were maintained in the SPF mice facility of the Institute of Molecular Biology, Academia Sinica. All mouse experiments were conducted with approval from the Institutional Animal Care and Use Committee, Academia Sinica. DAPK knockdown lentiviral construct was generated by subcloning human DAPK-specific shRNA sequence into pLentiLox vector (pLL3.7, gift of Dr I-Chen Ho, Harvard Medical School, Boston, MA). The sequence of the human DAPK-specific shRNA was: 5′ CAA GAA ACG TTA GCA AATG; the sequence of mouse Dapk shRNA was: CAC CAG TAC CCT TGC CAAA. Lentiviruses were harvested from culture supernatant of 293FT cells transfected with 20 μg pLL3.7 or pLL3.7DAPKshRNA, 15 μg psPAX2, and 6 μg VSVG. THP-1 or J774A.1 cells were infected with recombinant lentivirus, and green fluorescent protein-expressing cells were isolated by fluorescence sorting 48 hours later on a FACSVantage SE (BD Biosciences).
Measurement of IL-1β and caspase-1
THP-1 cells were treated with 0.5 μg/mL phorbol myristate acetate (PMA) for 3 hours for differentiation into macrophages and replaced with complete RPMI medium for overnight culture. THP-1 cells were then stimulated with crude LPS (15 μg/mL) or Pam3CSK4 (0.5 μg/mL) in serum-free RPMI medium for 5 hours. Culture supernatants from stimulated macrophages were harvested, aliquots of which were used for quantitation of IL-1β or TNF-α by enzyme-linked immunosorbent assay (ELISA). The remaining aliquots were precipitated with 2 volumes of acetone at −20°C for 1 hour, and precipitates were collected by centrifugation at 15 000g for 10 minutes. The pellets were resuspended with radio immunoprescripitation assay buffer (1% NP40, 0.1% sodium dodecyl sulfate [SDS], 50mM Tris HCl, pH 7.4, 150mM NaCl, 2mM ethylenediaminetetraacetic acid, and protease and phosphatase inhibitor) and resolved by 4% to 20% gradient SDS-polyacrylamide gel electrophoresis (PAGE). For J774A.1, cells were plated in 12-well plates for overnight culture, replaced with fresh medium, and primed with LPS (0.3 μg/mL), Pam3CSK4 (0.25 μg/mL), or R837 (10 μg/mL) for 4 hours, followed by stimulation with ATP (3mM) for 60 minutes. Bone marrow macrophages were cultured like J774A.1 cells, except the priming was 5 hours with LPS (0.5 μg/mL), Pam3CSK4 (1 μg/mL), R837 (2 μg/mL), or R848 (2 μg/mL), and the subsequent stimulation with ATP (5mM) was 60 minutes, with MSU, nigericin, or alum crystals was 6 hours. Notably, crude LPS (Sigma-Aldrich) was used to stimulate THP-1 cells, whereas purified LPS (InvivoGen) was used to prime J774A.1 and bone marrow macrophages.
Reconstitution of NLRP3 inflammasome in 293T cells
The 293 T cells were seeded onto 6-well plates at 5 × 105 per well for 1 day and transfected with 200 ng pcDNA4-proIL-1β, 25 ng pcDNA4-NLRP3-Myc, 20 ng pcDNA-ASC, and 10 ng pcDNA4-procaspase-1-Myc using Lipofectamine 2000 (Invitrogen). Cells were collected 48 hours after transfection and lysed in ristocetin-induced platelet aggregation buffer, and the lysates cleared by centrifugation (16 000g, 10 minutes). The protein concentration was determined by Bradford assay. Lysates (50 μg) were resolved by SDS-PAGE and analyzed by immunoblots.
In situ proximity ligation assay
In situ proximity ligation assay was performed according to Söderberg et al36 using the Duolink Proximity Ligation in situ reagent kit (Olink). The detailed procedures are included in the supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Monosodium urate crystal–mediated peritonitis
C57BL/6 mice received intraperitoneal injection of phosphate-buffered saline alone or 1 mg of monosodium urate crystal. The influx of neutrophil into peritoneal cavity was determined 6 hours later. The presence of IL-1β was quantitated by ELISA.
Results
Decreased IL-1β production and reduced caspase-1 activation in Dapk−/− macrophages
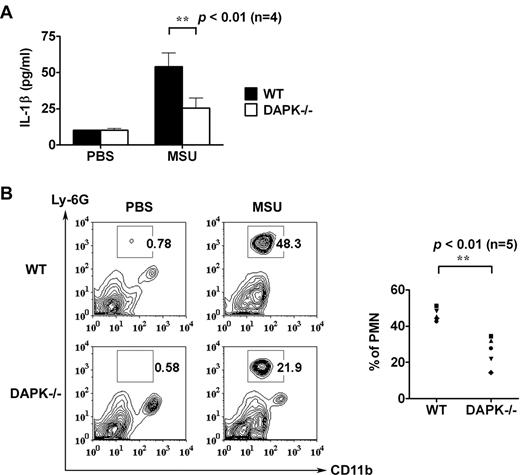
We used bone marrow–derived macrophages from Dapk−/− mice37 to determine the involvement of DAPK in IL-1β production. The expression of NLRP3, ASC, and procaspase-1 was comparable between normal littermate (WT) and Dapk−/− macrophages before stimulation (Figure 1A). TLR-mediated IL-1β production in macrophages involves 2 sequential processes: the activation of NF-κB, which contribute to the expression of pro-IL-1β and NLRP3, and, with a yet-to-be-characterized second signal, the assembly of inflammasome to generate active caspase-1 for processing pro-IL-1β. Figure 1B demonstrates that sequential treatment of bone marrow macrophages by LPS followed with ATP, nigericin, MSU, or alum led to secretion of IL-1β. IL-1β was similarly generated in macrophages primed with Pam3CSK4 or R848, followed by ATP stimulation (Figure 1C). The 2-step activation was illustrated by the induction of pro-IL-1β and NLRP3 in cell lysates after priming with LPS, Pam3CSK4, or R848, and the appearance of active caspase-1 and mature IL-1β (p17) in supernatants after the addition of MSU or ATP (Figure 1E-G). DAPK knockout significantly reduced the secretion of IL-1β in macrophages by all stimuli examined (Figure 1B-C). For pro-IL-1β expression, the involvement of DAPK was dependent on the type of TLR activated. LPS-stimulated pro-IL-1β expression was not apparently affected by DAPK deficiency, whereas pro-IL-1β production stimulated by Pam3CSK4 or R848 was decreased in Dapk−/− macrophages (Figure 1E-G; supplemental Figure 1). For caspase-1 activation, reduced generation of p10 caspase-1 was observed in Dapk−/− macrophages in all stimuli tested (Figure 1E-G; supplemental Figure 1). The diminished caspase-1 generation in DAPK-deficient macrophages was not the result of the availability of NLRP3, as the induction of NLRP3 by LPS, Pam3CSK4, or R848 was comparable between control and Dapk−/− macrophage (Figure 1E-G). Because DAPK knockdown did not affect the expression of NLRP3, ASC, and procaspase-1 after activation (Figure 1E-G), the decreased procaspase-1 processing suggests compromised inflammasome formation in DAPK-deficient macrophages. Therefore, DAPK was required for optimal IL-1β production and inflammasome formation in normal macrophages. In contrast, DAPK deficiency did not affect TNF-α production in the same macrophages stimulated by LPS, Pam3CSK4, or R848 (Figure 1D), illustrating the specific association of DAPK with IL-1β generation.
Attenuated caspase-1 activation and IL-1β secretion in Dapk−/− macrophages. (A) Normal expression of NLRP3, ASC, and procaspase-1 in DAPK-null macrophages. Bone marrow–derived macrophages (BMM) from normal littermate control (WT) and Dapk−/− mice were analyzed for the protein expression of DAPK, NLRP3, ASC, procaspase-1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (B-C) Reduced IL-1β secretion in DAPK-null macrophages. Bone marrow–derived macrophages from WT and Dapk−/− (−/−) were primed with LPS (0.5 μg/mL) (B), Pam3CSK4 (Pam3, 1 μg/mL), or R848 (2 μg/mL) (C) for 4 hours, followed by incubation with ATP (5mM) for 60 minutes (B-C), or nigericin (10μM), MSU (150 μg/mL), alum crystal (500 μg/mL) (B) for 6 hours. The secreted IL-1β in the supernatants was determined by ELISA. (D) Normal TNF-α secretion in DAPK-deficient macrophages. Bone marrow–derived macrophages from WT and Dapk−/− mice were stimulated with LPS, Pam3CSK4, and R848 for 6 hours, and TNF-α secreted measured by ELISA. (E-G) DAPK deficiency decreased caspase-1 activation. Macrophage and supernatants from LPS-primed and MSU-stimulated (E), Pam3CSK4-primed and ATP stimulated (F), and R848-primed and ATP stimulated (G) macrophages were collected before and after priming, and after MSU or ATP treatment. Cells were lysed to generate total cell lysates (TCL). Supernatants (SUP) were precipitated with cold (−20°C) acetone, and dissolved in ristocetin-induced platelet aggregation buffer. Both supernatant precipitates and cell lysates were resolved by SDS-PAGE. The amounts of pro-IL-1β, mature IL-1β (p17), NLRP3, ASC, procaspase-1, active caspase-1 (p10), and GAPDH in SUP and TCL were determined by immunoblotting. (B-D) Error bars represent SD of a specific experiment with triplicate samples. All experiments have been repeated at least 3 times with similar results. *P < .05, **P < .01, ***P < .001 for paired t test.
Attenuated caspase-1 activation and IL-1β secretion in Dapk−/− macrophages. (A) Normal expression of NLRP3, ASC, and procaspase-1 in DAPK-null macrophages. Bone marrow–derived macrophages (BMM) from normal littermate control (WT) and Dapk−/− mice were analyzed for the protein expression of DAPK, NLRP3, ASC, procaspase-1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (B-C) Reduced IL-1β secretion in DAPK-null macrophages. Bone marrow–derived macrophages from WT and Dapk−/− (−/−) were primed with LPS (0.5 μg/mL) (B), Pam3CSK4 (Pam3, 1 μg/mL), or R848 (2 μg/mL) (C) for 4 hours, followed by incubation with ATP (5mM) for 60 minutes (B-C), or nigericin (10μM), MSU (150 μg/mL), alum crystal (500 μg/mL) (B) for 6 hours. The secreted IL-1β in the supernatants was determined by ELISA. (D) Normal TNF-α secretion in DAPK-deficient macrophages. Bone marrow–derived macrophages from WT and Dapk−/− mice were stimulated with LPS, Pam3CSK4, and R848 for 6 hours, and TNF-α secreted measured by ELISA. (E-G) DAPK deficiency decreased caspase-1 activation. Macrophage and supernatants from LPS-primed and MSU-stimulated (E), Pam3CSK4-primed and ATP stimulated (F), and R848-primed and ATP stimulated (G) macrophages were collected before and after priming, and after MSU or ATP treatment. Cells were lysed to generate total cell lysates (TCL). Supernatants (SUP) were precipitated with cold (−20°C) acetone, and dissolved in ristocetin-induced platelet aggregation buffer. Both supernatant precipitates and cell lysates were resolved by SDS-PAGE. The amounts of pro-IL-1β, mature IL-1β (p17), NLRP3, ASC, procaspase-1, active caspase-1 (p10), and GAPDH in SUP and TCL were determined by immunoblotting. (B-D) Error bars represent SD of a specific experiment with triplicate samples. All experiments have been repeated at least 3 times with similar results. *P < .05, **P < .01, ***P < .001 for paired t test.
DAPK knockdown reduces IL-1β production and caspase-1 activation in THP-1 cells
The role of DAPK in IL-1β production was also examined in human monocyte cell line THP-1 cells by knockdown of DAPK.30 Down-regulation of DAPK did not affect the expression of NLRP3, procaspase-1, and ASC in THP-1 cells (Figure 2A). THP-1 cells are unique in that TLR engagement or danger signal alone is sufficient to produce IL-1β, shown by the secretion of mature p17 IL-1β in THP-1 cells stimulated with MSU or Pam3CSK4 (Figure 2B). IL-1β production could also be generated in THP-1 cells stimulated with crude LPS, but not purified LPS.38 DAPK knockdown significantly decreased MSU-, Pam3CSK4-, and crude LPS-stimulated IL-1β p17 generation in THP-1 cells (Figure 2B). As a control for IL-1β, TNF-α production triggered by crude LPS or Pam3CSK4 was identical between control and DAPK-knockdown THP-1 cells (Figure 2C). We further delineated the stage of IL-1β production that was affected by DAPK deficiency. The induction of pro-IL-1β was mostly unaffected in DAPK-knockdown THP-1 cells in response to crude LPS stimulation but was decreased when stimulated by MSU or Pam3CSK4 (Figure 2D-F, TCL). Reduced generation of p10 caspase-1 was found in DAPK-deficient THP-1 cells activated by MSU, Pam3CSK4, or crude LPS (Figure 2D-F, SUP). A stringent requirement for DAPK in the generation of mature p17 IL-1β, but not TNF-α, was also found in the murine macrophage cell line J774A.1 (supplemental Figure 2). Therefore, DAPK is required for optimal IL-1β generation and caspase-1 activation in macrophage cell lines, as well as in normal macrophages.
Down-regulation of DAPK in THP-1 decreases caspase-1 activation and p17 IL-1β generation, but not TNF-α production. (A) DAPK knockdown did not affect the expression of NLRP3, ASC, and procaspase-1. THP-1 cells were infected with lentivirus (pLL3.7) containing DAPK-specific shRNA (shDAPK) or control shRNA (shCtrl), the infected cells were sorted, and the contents of DAPK, NLRP3, ASC, and procaspase-1 determined by immunoblots. (B-C) Down-regulation of DAPK decreased secretion of IL-1β but not TNF-α. Control and DAPK-knockdown THP-1 cells, pretreated with PMA (500 ng/mL) for 3 hours and cultured overnight, were stimulated with or Pam3CSK (Pam3, 0.5 μg/mL) or crude LPS (15 μg/mL) for 5 hours, MSU (150 μg/mL) for 6 hours, after which cells and supernatants were collected. The levels of IL-1β (B) and TNF-α (C) in supernatants were determined by ELISA. (D-F) Reduced generation of p17 IL-1β and p10 caspase-1 in DAPK-knockdown THP-1 cells. Cell supernatants (SUP) from panel B were precipitated by cold acetone and analyzed, together with total cell lysates (TCL) from panel B, by SDS-PAGE. The contents of pro-IL-1β, active IL-1β, procaspase-1, active caspase-1, and GAPDH in THP-1 stimulated with MSU (D), Pam3CSK (E), or crude LPS (F) were determined by blotting with specific antibodies. Error bar represents SD of a specific experiment of triplicate samples. All experiments have been repeated 3 times with similar results. **P < .01, ***P < .001 for paired t test.
Down-regulation of DAPK in THP-1 decreases caspase-1 activation and p17 IL-1β generation, but not TNF-α production. (A) DAPK knockdown did not affect the expression of NLRP3, ASC, and procaspase-1. THP-1 cells were infected with lentivirus (pLL3.7) containing DAPK-specific shRNA (shDAPK) or control shRNA (shCtrl), the infected cells were sorted, and the contents of DAPK, NLRP3, ASC, and procaspase-1 determined by immunoblots. (B-C) Down-regulation of DAPK decreased secretion of IL-1β but not TNF-α. Control and DAPK-knockdown THP-1 cells, pretreated with PMA (500 ng/mL) for 3 hours and cultured overnight, were stimulated with or Pam3CSK (Pam3, 0.5 μg/mL) or crude LPS (15 μg/mL) for 5 hours, MSU (150 μg/mL) for 6 hours, after which cells and supernatants were collected. The levels of IL-1β (B) and TNF-α (C) in supernatants were determined by ELISA. (D-F) Reduced generation of p17 IL-1β and p10 caspase-1 in DAPK-knockdown THP-1 cells. Cell supernatants (SUP) from panel B were precipitated by cold acetone and analyzed, together with total cell lysates (TCL) from panel B, by SDS-PAGE. The contents of pro-IL-1β, active IL-1β, procaspase-1, active caspase-1, and GAPDH in THP-1 stimulated with MSU (D), Pam3CSK (E), or crude LPS (F) were determined by blotting with specific antibodies. Error bar represents SD of a specific experiment of triplicate samples. All experiments have been repeated 3 times with similar results. **P < .01, ***P < .001 for paired t test.
To probe DAPK-dependent pro-IL-1β expression, we found that DAPK deficiency did not affect MAPK activation (supplemental Figure 3). A moderate decrease in NF-κB activation was observed in DAPK-null macrophage but not in THP-1 or J774.1 cell lines (supplemental Figures 4-5). Because DAPK deficiency affected pro-IL-1β expression by selected stimuli but inhibited active caspase-1 generation by all stimuli examined, we investigated the potential involvement of DAPK in inflammasome activation in the following studies.
DAPK is required for reconstitution of NLRP3 inflammasome in 293T cells
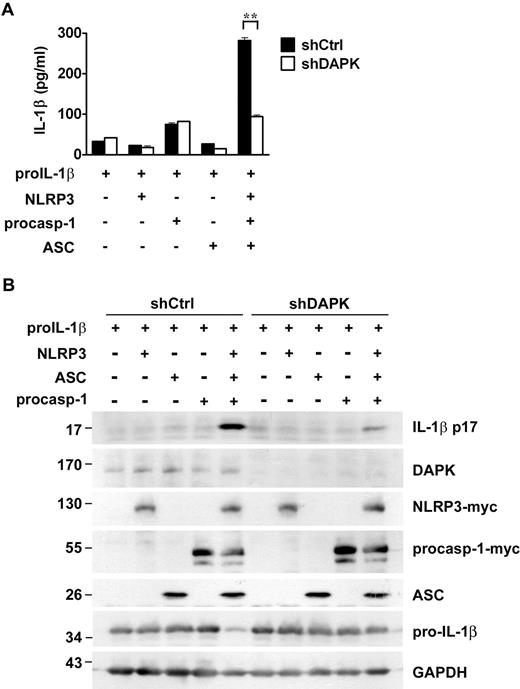
To minimize the variation in the pro-IL-1β level, we examined the effect of DAPK in the reconstitution of NLRP3 inflammasome with the exogenously expressed pro-IL-1β. To this end, 293T cells were transfected with pro-IL-1β, procaspase-1, NLRP3, and ASC. The expression of each component and/or substrate of NLRP3 inflammasome was confirmed by immunoblotting (Figure 3). Simultaneous expression of IL-1β, pro-caspase-1, NLRP3, and ASC led to secretion of mature IL-1β by 293T cells (Figure 3A). This was confirmed by the detection of p17 IL-1β in total cell lysates of 293T cells (Figure 3B). Knockdown of DAPK did not affect the expression of pro-IL-1β but profoundly reduced the secretion of IL-1β (Figure 3A) as well as the generation of p17 IL-1β in cell lysates (Figure 3B). Therefore, under constant expression of pro-IL-1β, DAPK is required for the full functional operation of the reconstituted NLRP3 inflammasome.
Requirement of DAPK in functional reconstitution of NLRP3 inflammasome. Procession of mature IL-1β by reconstituted inflammasome was impaired in DAPK-knockdown HEK293T cells. The 293T cells were transduced by pLL3.7 with shDAPK or shCtrl. Control or 293T cells with DAPK knockdown were transfected with 200 ng pro-IL-1β, 25 ng NLRP3-myc, 10 ng procaspase-1, or 20 ng ASC-green fluorescent protein, as indicated. Culture supernatant and total cell lysates were prepared 48 hours after transfection. (A) The levels of IL-1β in supernatant were quantitated by ELISA. (B). The expression of DAPK, NLRP3, procaspase-1, and IL-1β in TCL and the protein levels of p17 IL-1β in the lysates were determined as described in Figure 1. **P < .01 for paired t test.
Requirement of DAPK in functional reconstitution of NLRP3 inflammasome. Procession of mature IL-1β by reconstituted inflammasome was impaired in DAPK-knockdown HEK293T cells. The 293T cells were transduced by pLL3.7 with shDAPK or shCtrl. Control or 293T cells with DAPK knockdown were transfected with 200 ng pro-IL-1β, 25 ng NLRP3-myc, 10 ng procaspase-1, or 20 ng ASC-green fluorescent protein, as indicated. Culture supernatant and total cell lysates were prepared 48 hours after transfection. (A) The levels of IL-1β in supernatant were quantitated by ELISA. (B). The expression of DAPK, NLRP3, procaspase-1, and IL-1β in TCL and the protein levels of p17 IL-1β in the lysates were determined as described in Figure 1. **P < .01 for paired t test.
Overexpression of DAPK increases caspase-1 activation and IL-1β generation
Next, we examined whether DAPK overexpression enhanced IL-1β generation in THP-1 cells. DAPK was transduced into THP-1 cells by retroviral infection, and overexpression was confirmed by anti-DAPK (Figure 4A-B). In THP-1 cells expressing DAPK, the IL-1β secretion stimulated by R848 or MSU was significantly higher than in the YFP control cells (Figure 4C). The increased generation of mature IL-1β was correlated with an enhanced generation of p10 caspase-1 (Figure 4A-B). We further determined the requirement of the kinase activity of DAPK in the activation of NLRP3 inflammasome using kinase-inactive [K42A]DAPK. DAPK-expressing THP-1 cells produced higher levels of pro-IL-1β than [K42A]DAPK-expressing cells (Figure 4A-B). Despite the difference in pro-IL-1β levels, expression of [K42A]DAPK was as effective as DAPK in enhancing the caspase-1 processing and IL-1β p17 generation in THP-1 cells (Figure 4A-C), suggesting that the kinase activity of DAPK is not essential for NLRP3 inflammasome activation.
Overexpression of DAPK and [K42A]DAPK increases caspase-1 activation and mature IL-1β generation. THP-1 cells were infected with pGC-YFP, pGC-YFP-DAPK, or pGC-YFP-[K42A]DAPK, and YFP-expressing cells isolated by sorting. Control (YFP) and DAPK-expressing THP-1 cells were treated with PMA for 16 hours and then stimulated with MSU (A), or R848 (B) for 6 hours. The IL-1β contents in supernatant were determined by ELISA (C). The amounts of the cleaved IL-1β, procaspase-1, caspase-1 (p10), DAPK, pro-IL-1β, and GAPDH in total cell lysates were determined by immunoblots (A-B). All experiments have been repeated 3 times with similar results. **P < .01, ***P < .001 for paired t test.
Overexpression of DAPK and [K42A]DAPK increases caspase-1 activation and mature IL-1β generation. THP-1 cells were infected with pGC-YFP, pGC-YFP-DAPK, or pGC-YFP-[K42A]DAPK, and YFP-expressing cells isolated by sorting. Control (YFP) and DAPK-expressing THP-1 cells were treated with PMA for 16 hours and then stimulated with MSU (A), or R848 (B) for 6 hours. The IL-1β contents in supernatant were determined by ELISA (C). The amounts of the cleaved IL-1β, procaspase-1, caspase-1 (p10), DAPK, pro-IL-1β, and GAPDH in total cell lysates were determined by immunoblots (A-B). All experiments have been repeated 3 times with similar results. **P < .01, ***P < .001 for paired t test.
DAPK deficiency decreases MSU-triggered peritonitis
In assay for in vivo activation of NLRP3 inflammasome, control and Dapk−/− mice received intraperitoneal injection of phosphate-buffered saline alone or MSU crystals. Subsequently, the influx of neutrophils into the peritoneal cavity and the presence of IL-1β were determined. As shown in Figure 5A, significantly lower quantities of IL-1β were generated in Dapk−/− mice receiving MSU compared with those measured for the control group. In addition, peritoneal infiltration of polymorphonuclear neutrophils was reduced by 50% in Dapk−/− mice (Figure 5B). Together, these results support a pivotal function of DAPK in inflammasome activation in vivo.
Reduced MSU-triggered peritonitis in DAPK-deficient macrophages. Decreased IL-1β production and neutrophil influx in MSU-treated Dapk−/− mice. Phosphate-buffered saline (0.5 mL) with or without MSU crystals (1 mg) was intraperitoneally administered to control and Dapk−/− mice. (A) Mice were killed 6 hours later, and levels of IL-1β in peritoneal lavage were determined. (B) The population of neutrophils in peritoneal lavage was assessed by staining with anti–Ly-6G and anti-CD11b. Numbers indicate percentage of polymorphonuclear neutrophils (PMN).
Reduced MSU-triggered peritonitis in DAPK-deficient macrophages. Decreased IL-1β production and neutrophil influx in MSU-treated Dapk−/− mice. Phosphate-buffered saline (0.5 mL) with or without MSU crystals (1 mg) was intraperitoneally administered to control and Dapk−/− mice. (A) Mice were killed 6 hours later, and levels of IL-1β in peritoneal lavage were determined. (B) The population of neutrophils in peritoneal lavage was assessed by staining with anti–Ly-6G and anti-CD11b. Numbers indicate percentage of polymorphonuclear neutrophils (PMN).
Interaction of DAPK with NLRP3
The requirement of DAPK for full inflammasome activation led us to examine the potential interaction between DAPK and the NLRP3 inflammasome. We detected an association of DAPK with NLRP3 when both were expressed in 293T cells. DAPK-FLAG was pulled down together with NLRP3-Myc by immunoprecipitation using either anti-Flag or anti-Myc (Figure 6A). We then delineated the domain of DAPK that accounts for the association with NLRP3. Fragments containing different domains of DAPK were expressed in 293 T cells (Figure 6B). Regions containing ankyrin repeats or death domain of DAPK were immunoprecipitated by NLRP3-Myc (Figure 6B). A weaker binding of NLRP3 to DAPK cytoskeleton binding region was also detected. DAPK may thus interact with NLRP3 through different regions. We also identified the leucine-rich repeat motif as the DAPK-interacting region on NLRP3 (supplemental Figure 6).
Interaction of DAPK with NLRP3. (A) Interaction between DAPK and NLRP3 in HEK293T cells. The 293T cells were transfected with DAPK-Flag and/or NLRP3-Myc, as indicated, and cell lysates were prepared 48 hours later. Cell lysates were precipitated with anti-Flag or anti-Myc, and the amount of NLRP3-myc and DAPK-Flag in the precipitates determined by immunoblots. (B) Multiple domains of DAPK mediate interaction with NLRP3. Flag-tagged DAPK fragments containing kinase (K), calcium/calmodulin (CaM) regulatory domain, ankyrin repeats (AR), cytoskeleton binding (Cy), linking (Li), and death domain (DD), as indicated, were transfected into 293T cells together with NLRP3-Myc. Cell lysates were prepared 48 hours after transfection. The expression of each DAPK mutant in lysates (Input) was determined by immunoblots. DAPK mutant in lysates were pulled down by anti-Flag (M2) beads, and the associated NLRP3 detected by anti-Myc.
Interaction of DAPK with NLRP3. (A) Interaction between DAPK and NLRP3 in HEK293T cells. The 293T cells were transfected with DAPK-Flag and/or NLRP3-Myc, as indicated, and cell lysates were prepared 48 hours later. Cell lysates were precipitated with anti-Flag or anti-Myc, and the amount of NLRP3-myc and DAPK-Flag in the precipitates determined by immunoblots. (B) Multiple domains of DAPK mediate interaction with NLRP3. Flag-tagged DAPK fragments containing kinase (K), calcium/calmodulin (CaM) regulatory domain, ankyrin repeats (AR), cytoskeleton binding (Cy), linking (Li), and death domain (DD), as indicated, were transfected into 293T cells together with NLRP3-Myc. Cell lysates were prepared 48 hours after transfection. The expression of each DAPK mutant in lysates (Input) was determined by immunoblots. DAPK mutant in lysates were pulled down by anti-Flag (M2) beads, and the associated NLRP3 detected by anti-Myc.
DAPK is required for optimal interaction between ASC and NLRP3 during inflammasome formation
Cell membrane damage promotes inflammasome formation in vitro regardless of the activation status of THP-1 cells,19 precluding the possibility to elucidate TLR-dependent NLRP3 inflammasome assembly on cell extracts. Thus, we proceeded to monitor the formation of inflammasome in situ, using the proximity ligation assay.36 Oligonucleotides linked to 2 different antibodies are brought to close proximity by the interaction between 2 target proteins, allowing formation of circular DNA strands for amplification and detection. In PMA-primed THP-1 cells, inflammasome was activated by crude LPS stimulation alone (Figure 2). In untreated THP-1 cells, the proximity ligation assay detected no interaction between NLRP3 and ASC (supplemental Figure 8A). Stimulation with crude LPS promoted the close association of NLRP3 and ASC in THP-1 cells. The specificity of the proximity ligation assay was confirmed by ASC knockdown in THP-1 cells (supplemental Figure 7A), completely abrogated crude LPS-activated IL-1β generation (supplemental Figure 7B), and abolished the association between NLRP3 and ASC or between NLRP3 and procaspase-1 in THP-1 cells (supplemental Figure 8A). In normal macrophages, LPS stimulation alone failed to activate NLRP3 inflammasome and did not promote the association of NLRP3 and ASC (Figure 7A). Only after the addition of ATP to LPS-primed macrophages did NLRP3 become situated in close proximity to ASC, confirming the dual-signals requirement of NLRP3 inflammasome activation in normal macrophages. A similar association was found with NLRP3 and procaspase-1. Whereas NLRP3 was not located in proximity to procaspase-1 in untreated macrophages or LPS-treated macrophages, nearby localization of NLRP3 and procaspase-1 was observed after addition of ATP to LPS-treated macrophages (Figure 7A). Therefore, the close association between NLRP3 and either ASC or procaspase-1, as detected by proximity ligation assay, is indicative of inflammasome formation in bone marrow–derived macrophages. We were then able to determine the involvement of DAPK in the assembly of inflammasome using proximity ligation assay. LPS/ATP-stimulated close association of NLRP3-ASC or NLRP3-procaspase-1 was largely diminished in DAPK-null macrophages (Figure 7A), supporting the critical role of DAPK in the assembly of inflammasome. We further used proximity ligation assay to measure the association of DAPK with NLRP3 and procaspase-1 after inflammatory stimulation. The close association between NLRP3 and DAPK, or procaspase-1 and DAPK, was detected only in normal macrophages stimulated with both LPS and ATP, resembling the condition for NLRP3-ASC and NLRP3-procaspase-1 interaction (Figure 7B). Analogous to NLRP3-ASC association and NLRP3-procaspase-1 interaction in macrophages, crude LPS-mediated interaction of NLRP3-ASC and NLRP3-procaspase-1 was profoundly reduced in DAPK-knockdown THP-1 cells (supplemental Figure 8A). Crude LPS stimulation in THP-1 cells brought DAPK to close proximity of NLRP3 or procaspase-1 (supplemental Figure 8B).
LPS/ATP-induced association of NLRP3, ASC, and procaspase-1 requires DAPK in macrophages. (A) Formation of inflammasome measured by the association of NLRP3 and ASC in LPS/ATP-treated macrophages. Bone marrow-derived macrophages (2 × 105) were seeded overnight, treated without or with LPS, followed with or without addition of ATP. Macrophages were stained with anti-NLRP3 and anti-ASC antibody pair, or anti-NLRP3 and anti-caspase-1 antibody pair overnight at 4°C. Macrophages were then incubated with oligonucleotide-conjugated antimouse antibodies and antirabbit antibodies. The primers were then annealed, circularized, and amplified via rolling-circle amplification. Images were obtained on a Zeiss Axioplan 2 imaging microscope with objective lens of plan-Apochromat 63×/1.4 oil DIC at room temperature. Samples (fixed macrophages) were mounted on Duolink mounting medium. Texas Red–conjugated probe was detected using filter set of 598 nm (Ex) and 613 nm (Em), whereas DAPI-bound DNA was detected using filter set of 360 nm (Ex) and 460 nm (Em). The pinholes are Ch3-1:106 (red), Ch2-2:82 (blue), and ChD-3.0. Images were acquired by LSM 510 META (Zeiss) digital camera using Zeiss LSM Image Browser Version 4.2.0.121 software. For quantitation, individual amplicons were counted in 5 different fields and divided by the number of cells to obtain spots/nucleus. (B) Association of DAPK and NLRP3 in LPS/ATP-stimulated macrophages. Bone marrow-derived macrophages were treated as in panel A, incubated with anti-DAPK and anti-NLRP3, or anti-DAPK and anti–caspase-1, and the proximity of DAPK to NLRP3 detected as in panel A. ***P < .001 for paired t test.
LPS/ATP-induced association of NLRP3, ASC, and procaspase-1 requires DAPK in macrophages. (A) Formation of inflammasome measured by the association of NLRP3 and ASC in LPS/ATP-treated macrophages. Bone marrow-derived macrophages (2 × 105) were seeded overnight, treated without or with LPS, followed with or without addition of ATP. Macrophages were stained with anti-NLRP3 and anti-ASC antibody pair, or anti-NLRP3 and anti-caspase-1 antibody pair overnight at 4°C. Macrophages were then incubated with oligonucleotide-conjugated antimouse antibodies and antirabbit antibodies. The primers were then annealed, circularized, and amplified via rolling-circle amplification. Images were obtained on a Zeiss Axioplan 2 imaging microscope with objective lens of plan-Apochromat 63×/1.4 oil DIC at room temperature. Samples (fixed macrophages) were mounted on Duolink mounting medium. Texas Red–conjugated probe was detected using filter set of 598 nm (Ex) and 613 nm (Em), whereas DAPI-bound DNA was detected using filter set of 360 nm (Ex) and 460 nm (Em). The pinholes are Ch3-1:106 (red), Ch2-2:82 (blue), and ChD-3.0. Images were acquired by LSM 510 META (Zeiss) digital camera using Zeiss LSM Image Browser Version 4.2.0.121 software. For quantitation, individual amplicons were counted in 5 different fields and divided by the number of cells to obtain spots/nucleus. (B) Association of DAPK and NLRP3 in LPS/ATP-stimulated macrophages. Bone marrow-derived macrophages were treated as in panel A, incubated with anti-DAPK and anti-NLRP3, or anti-DAPK and anti–caspase-1, and the proximity of DAPK to NLRP3 detected as in panel A. ***P < .001 for paired t test.
Discussion
In this study, we found a requirement of DAPK in IL-1β production from macrophages. DAPK is involved in 2 different stages of IL-1β generation: the induction of pro-IL-1β and the generation of p10 caspase-1. There is selectivity on the requirement of DAPK in pro-IL-1β expression from different stimuli. TLR2- and TLR7-activated pro-IL-1β expression, but not TLR4-mediated pro-IL-1β induction, was decreased by DAPK deficiency (Figure 1E-G; supplemental Figure 1). We found that NF-κB activation in DAPK-null macrophages was moderately reduced when stimulated by Pam3CSK4 and R837 (supplemental Figure 5). This suggests that DAPK is involved in TLR2- and TLR7-stimulated NF-κB activation, which may partly account for the decrease of pro-IL-1β expression in primary macrophages. It may be noted that NF-κB activation alone cannot explain the reduced pro-IL-1β expression. In Pam3CSK-treated THP-1 cells, normal NF-κB activation was accompanied with decreased pro-IL-1β expression (Figure 2; supplemental Figure 4). In addition, the production of TNF-α, another NF-κB–dependent event, was not affected in Dapk−/− macrophages, in accordance with the mild attenuation of NF-κB (supplemental Figure 5). The small reduction in NF-κB activation may also explain why NLRP3 induction16 was not affected in Dapk−/− macrophages stimulated by LPS, Pam3CSK, or R848 (Figure 1E-G). Our results suggest that DAPK promotes pro-IL-1β expression also through NF-κB-independent processes. In THP-1 cells expressing DAPK, we found that pro-IL-1β expression was increased by the expression of wild-type DAPK but not by kinase-dead [K42A]DAPK (Figure 4A). The involvement of DAPK in pro-IL-1β expression therefore requires the kinase activity of DAPK. DAPK phosphorylates ribosome S6 protein and promotes protein translation,39,40 suggesting that DAPK may contribute to pro-IL-1β expression through DAPK-mediated protein translation. This and other possibilities are now explored by us.
A second defect in DAPK-deficient macrophages in IL-1β production is the impaired procaspase-1 processing. The absence of DAPK decreased caspase-1 activation in macrophages in response to all inflammatory stimuli examined in this study. The decreased caspase-1 activation was independent of NF-κB, as NF-κB activation was normal in DAPK-knockdown THP-1 and J774A.1 cells (supplemental Figure 4). The decreased caspase-1 processing was also dissociated from the attenuated pro-IL-1β expression in DAPK-deficient macrophages because caspase-1 activation was attenuated in LPS-primed Dapk−/− macrophages with normal pro-IL-1β expression (Figure 1E; supplemental Figure 1). The impaired caspase-1 processing was not caused by incomplete induction of NLRP3 inflammasome components in DAPK-deficient cells. The expression of NLRP3, ASC, and procaspase-1 was comparable between control and DAPK-null macrophages before and after stimulation (Figure 1E-G). The reduced caspase-1 activation is thus attributed to defective inflammasome formation in DAPK-deficient macrophages. We found that DAPK is associated with the NLRP3 inflammasome through binding to NLRP3. We further demonstrated that NLRP3 inflammasome assembly was defective in the absence of DAPK, suggesting that DAPK is required for full functional operation of NLRP3 inflammasome.
Autophagy suppresses inflammasome activation and regulates inflammatory immune response, as evidenced by inhibition of LPS-induced IL-1β production by autophagic machinery components, including Atg16L, Atg7, and Atg4.33 DAPK is involved in autophagosome formation.34,35 In the present study, we found that DAPK does not suppress inflammasome formation but instead is required for full caspase-1 activation. DAPK differs from Atg16L and Atg7 in the modulation of IL-1β production by the type of TLR that is triggered. Atg16L and Atg7 inhibit IL-1β production mediated by TLR4 ligands but exhibit no effect on the inflammasome activated by TLR2 ligands.33 By contrast, DAPK is required for IL-1β generation via activation through both a TLR2 ligand (Pam3CSK4) and a TLR4 ligand (LPS), as found in the present study (Figure 1). Therefore, DAPK participates in inflammasome activation in processes that are clearly distinct from its involvement in autophagy.
How NLRP3 inflammasome is assembled in response to inflammatory stimuli remains unclear.2-6 Our study points out the possibility that DAPK represents a molecular component upstream of NLRP3 inflammasome formation. It is well known that DAPK phosphorylates numerous substrates41 and that its kinase activity is required for its various physiologic functions.20 However, the observation that [K42A]DAPK was as effective as wild-type DAPK in promoting IL-1β processing (Figure 4) suggests that, for the formation of the NLRP3 inflammasome, the kinase activity of DAPK is not essential. Several DAPK-interacting proteins are not substrates of DAPK,29 and some of the biologic effects of DAPK are likely mediated through protein recruitment without phosphoryation of DAPK-interacting proteins. The activation of the NLRP3 inflammasome is therefore dependent on the interaction between DAPK and NLRP3 (Figure 6), whereas DAPK may serve a structural role in the assembly of the NLRP3 inflammasome.
Inflammasomes are essential for the processing of procaspase-1 and pro-IL-1β, yet the formation of an inflammasome remains difficult to probe with the available biochemical methods. In this study, we used the proximity ligation assay36 to illustrate the feasibility on monitoring NRLP3 inflammasome formation in situ. The specificity of the assay was confirmed in ASC-knockdown THP-1 cells, where the inability to activate inflammasome (supplemental Figure 7) was correlated with failure of crude LPS to promote NLRP3-ASC or NLRP3–procaspase-1 interaction (supplemental Figure 8A). The proximity ligation assay also confirmed the dual signal requirement for inflammasome activation in normal macrophages. The association between NLRP3 and ASC was not detectable in resting macrophages and LPS-primed macrophages (Figure 7A). Only in macrophages treated with both LPS and ATP, the dual signals required to activate the NLRP3 inflammasome, the NLRP3-ASC interaction became visible (Figure 7A). A similar observation was made on NLRP3–caspase-1 association. To our knowledge, this represents the first demonstration that the formation of NLRP3 inflammasome could be accurately monitored. Using the proximity ligation assay, we also illustrated that DAPK became associated with NLRP3 and caspase-1 on inflammasome activation (Figure 7B; supplemental Figure 8B). Furthermore, we found that NLRP3-ASC or NLRP3-caspase-1 association was profoundly repressed in Dapk−/− deficient macrophages (Figure 7A), indicating a critical role of DAPK in the formation of NLRP3 inflammasome.
The activation of caspase-1 leads to cell death, characterized by membrane pore formation and osmotic lysis,42-44 which is different from classic apoptosis and necrosis. Caspase-1–induced cell death, also termed pyroptosis, is often triggered by toxin, myocardial infarction, or infection of Salmonella, Shigella, or Listeria.42-44 DAPK has been shown to mediate cell death induced by a wide variety of stimuli, such as IFN-γ, TNF-α, Fas, transforming growth factor-β, DNA damage, c-Myc, E2F-1, endoplasmic reticulum stress, ceramide, matrix detachment, autophage, mitochondrial toxin, netrin-1 receptor UNC5H2, or cerebral ischemia.20,21,25,29,37,45-48 Our observations that DAPK promotes inflammasome assembly and caspase-1 activation reveal a potential role of DAPK in caspase-1–induced cell death. Caspase-1–mediated cell death is expected to be attenuated in DAPK-deficient cells because of diminished activation of caspase-1 (Figures 1,Figure 2–3), whereas increased caspase-1 activation in DAPK-overexpressing cell (Figure 4) shall result in enhanced cell death. Therefore, in accordance with the proapoptotic nature of DAPK, pyroptosis is probably another form of death that is regulated by DAPK. Notably, the processes that DAPK is involved in caspase-1–induced death are distinct from the mechanisms DAPK modulates other types of cell death. The kinase activity of DAPK, essential for the regulation of apoptosis and autophagic death,20 is dispensable for caspase-1 activation (Figure 4). Our results also suggest that DAPK may be a target for the control of the pathogenic cell death triggered by caspase-1.
We found that DAPK deficiency led to a substantial decrease in caspase-1 activation, but residual procaspase-1 cleavage was detected. The abolition of DAPK, in contrast to ASC knockdown, does not completely abrogate the activation of the NLRP3 inflammasome (supplemental Figure 7B). Therefore, the role of DAPK in the NLRP3 inflammasome formation could be similar to that of ASC in NLRP1 inflammasome assembly. ASC is not absolutely required for the formation of NLRP1 inflammasome in vitro, yet it is a potent enhancer.18 The assembly of the NLRP3 inflammasome, which is activated by many different microbial products and danger signals, is probably initiated through a number of pathways. We propose that additional enhancers may be required for the optimum operation of the NLRP3 inflammasome. A few different proteins have been found to bind NLRP3, including SGT-1, HSP90, and thioredoxin-interacting protein.17,49 These enhancers may serve to couple the upstream triggering signaling to NLRP3. For example, the NLRP3-binding protein HSP90 could act as a sensor for NLRP3 as it interacts with more than 200 proteins and recognizes denatured proteins. Similarly, DAPK is an attractive candidate for an enhancer of inflammasome because it senses a diverse array of signals. DAPK responds to calcium, a key factor in innate immune signaling,50 and is connected to DNA damage through transcription activation by p53 or binding to p53.45,46 Furthermore, DAPK is activated by ischemia47 and constitutes a sensor for the mitochondrial membrane potential.48 DAPK may also participate in the activation of NLRP3 inflammasome by bridging NLRP3 with DAPK-interacting proteins. Consistent with this proposed role of DAPK, we found that DAPK is required for activation of NLRP3 inflammasome by all stimuli we have examined. Whether DAPK is also linked to processes associated with reactive oxygen species and cathepsin B is being investigated. In addition, it will be interesting to determine whether DAPK is involved in the activation of other types of inflammasomes.
Active mutants of the NLRP3 gene are linked to autoinflammatory disorders, including familial cold autoinflammatory syndrome, Muckle-Wells syndrome, and chronic infantile neurologic cutaneous and articular syndrome.15 NLRP3 inflammasome-regulated IL-1β production is also involved in gout and type II diabetes.1,5,9 The present study reveals a notable role of DAPK for its involvement in both inflammasome activation and specific TLR-induced pro-IL-1β expression, suggesting the possibility that inhibition of DAPK may interfere with pro-IL-1β expression and caspase-1 activation. Inhibition of the binding of DAPK to N-methyl-D-aspartate receptor is recently shown to protect neurons from cerebral ischemic insults.25 Whether blocking DAPK-NLRP3 interaction could be used in the treatment of NLRP3-associated autoinflammatory diseases deserves full exploration.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs I-Chen Ho, Gina Costa, and Garry Nolan for critical reagents, Yamin Lin and FACS Core, Sue-Ping Lee and Confocal Core of Institute of Molecular Biology, Academia Sinica for cell sorting and confocal microscopy, and Dr Heiko Kuhn for editing the manuscript.
This work was supported by the National Science Council (grant NSC98-2321-B001-015) and Academia Sinica, Taiwan, Republic of China (Academia Sinica Investigator Award).
Authorship
Contribution: M.-Z.L. and Y.-T.C. designed the experiments; Y.-T.C., Y.-C.L., K.-H.L., and T.-F.C. performed the experiments; W.-C.K., K.-T.Y., P.-R.W., and R.-H.C. generated critical constructs of NLRP3, caspase-1, ASC, and DAPK; A.K. produced Dapk−/− mouse; and M.-Z.L. prepared the manuscript (in consultation with R.-H.C.).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ming-Zong Lai, Institute of Molecular Biology, Academia Sinica, Taipei 11529, Taiwan, Republic of China; e-mail: mblai@imb.sinica.edu.tw.



![Figure 4. Overexpression of DAPK and [K42A]DAPK increases caspase-1 activation and mature IL-1β generation. THP-1 cells were infected with pGC-YFP, pGC-YFP-DAPK, or pGC-YFP-[K42A]DAPK, and YFP-expressing cells isolated by sorting. Control (YFP) and DAPK-expressing THP-1 cells were treated with PMA for 16 hours and then stimulated with MSU (A), or R848 (B) for 6 hours. The IL-1β contents in supernatant were determined by ELISA (C). The amounts of the cleaved IL-1β, procaspase-1, caspase-1 (p10), DAPK, pro-IL-1β, and GAPDH in total cell lysates were determined by immunoblots (A-B). All experiments have been repeated 3 times with similar results. **P < .01, ***P < .001 for paired t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/3/10.1182_blood-2010-08-303115/4/m_zh89991064580004.jpeg?Expires=1765947909&Signature=hu9NqfETAFEe2nUMok9auLz6koyHufyB2LvP-vWR9skmPmKjTON1w2D-rBekVzCiJ8ZPymYsjBtJpL870C4VAOwCW1itS0bccMCU8LKlrkFzsHqqJBGdRKfZ4SsCwIfBHinKt6BOeJUOtr~TIE-2fsWk4LB6LhU5d62u93eApEzPfz8DnC9GwLhdisLWVf-S8h4Oxreprhh0TxxEs9E0JNO8L~t4d0844aKs9zoAq6iV7nzHbMKtrmSwQMh8ozaQfeE0qelkCBHxQsXAdl93ljvVJo4if-5c5sBBIYVfQvub4CHyNwmrefOG9PTzu1iGVDiwkBzhSupJ2g998npwgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)