In this issue of Blood, Haling and colleagues demonstrate that in addition to talin-dependent integrin activation, talin is required for platelet fibrin clot retraction by physically linking integrins to the actin cytoskeleton.
Integrins are ubiquitous transmembrane α/β heterodimers that provide an essential link between the extracellular and intracellular environments, which is vital for both normal and pathophysiologic processes. The well-characterized platelet-specific integrin αIIbβ3 (like several other integrin receptors) is constitutively expressed on the cell surface in a low-affinity state. Agonist stimulation or exposure of platelets to extracellular matrix proteins generates intracellular signals that enhance integrin-binding affinity for ligands. Ligand binding to αIIbβ3, in turn, transduces signals from the extracellular environment into the cell leading to platelet adhesion and aggregation. Finally, integrin αIIbβ3-dependent clot retraction is necessary for normal thrombus stabilization and wound healing. In platelets, these bidirectional signaling events are tightly regulated processes; perturbation of this regulation can lead to pathologic conditions such as hemorrhage or occlusive platelet thrombi.
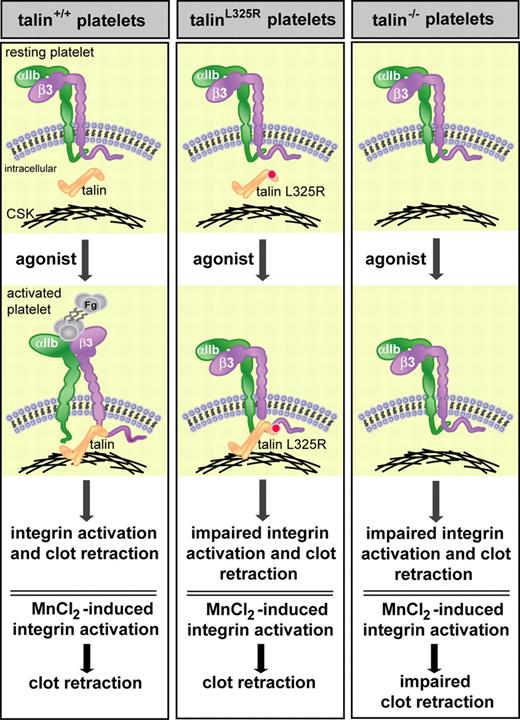
Talin-dependent integrin activation and association with the actin cytoskeleton is required for fibrin clot retraction. Haling and colleagues show that wild-type, but not talin-deficient (talin−/−), platelets undergo normal agonist-induced integrin activation and fibrin clot retraction. TalinL325R platelets also show impaired integrin activation and clot retraction despite the ability of talinL325R to interact with β-integrins and the actin cytoskeleton (CSK). The clot retraction defect in talin−/− platelets may result from loss of talin-dependent integrin activation or talin-dependent linkage of integrins to the cytoskeleton. To dissect these talin-dependent effects, artificial activation of integrins with manganese chloride (MnCl2) rescued clot retraction in wild-type and talinL325R, but not talin−/−, platelets, indicating that both talin-dependent integrin activation and linkage of integrins to the actin cytoskeleton are required for fibrin clot retraction. Fg indicates fibrinogen.
Talin-dependent integrin activation and association with the actin cytoskeleton is required for fibrin clot retraction. Haling and colleagues show that wild-type, but not talin-deficient (talin−/−), platelets undergo normal agonist-induced integrin activation and fibrin clot retraction. TalinL325R platelets also show impaired integrin activation and clot retraction despite the ability of talinL325R to interact with β-integrins and the actin cytoskeleton (CSK). The clot retraction defect in talin−/− platelets may result from loss of talin-dependent integrin activation or talin-dependent linkage of integrins to the cytoskeleton. To dissect these talin-dependent effects, artificial activation of integrins with manganese chloride (MnCl2) rescued clot retraction in wild-type and talinL325R, but not talin−/−, platelets, indicating that both talin-dependent integrin activation and linkage of integrins to the actin cytoskeleton are required for fibrin clot retraction. Fg indicates fibrinogen.
The integrin cytoplasmic domains are key regulatory sites for integrin bidirectional signaling. Multiple proteins have been identified that bind to integrin cytoplasmic domains and, as such, are likely to play a role in regulating integrin function.1 One of these proteins, talin, binds to β-integrin cytoplasmic tails and is an important regulator of integrin activation (reviewed in Shattil et al2 ). Talin is an abundant (∼ 3%-8% of total platelet protein3 ) cytoskeletal protein composed of a 220-kDa C-terminal rod domain and a 50-kDa N-terminal FERM (4-point-one/erzrin/radixin/moesin) head domain. This N-terminal FERM domain binds to β-integrin cytoplasmic domains and the C-terminal rod domain interacts with F-actin, thus providing a physical link between the actin cytoskeleton, integrins, and the extracellular matrix. Several elegant biochemical, mutational, and structural studies identified sites in both talin and the β-integrin cytoplasmic domains that mediate the interaction between these 2 binding partners.2 Talin binds to a conserved membrane distal NPxY motif, which is hypothesized to be important for talin recruitment to β-integrin tails.4,5 Key studies, however, revealed a second critical site of interaction between the β-integrin membrane proximal region and the talin head FERM domain that is necessary for talin-dependent integrin activation.2 Of particular relevance to the study by Haling and colleagues in this issue6 is a talin L325R (talinL325R) point mutation that blocks talin binding to the β-integrin membrane proximal domain but retains talin binding to the more membrane distal region of the β-integrin tail.4,5
The first direct evidence that talin binding to β-integrins is the final step required for integrin activation was demonstrated by depletion of talin from murine stem cell–derived megakaryocytes.7 A definitive role for talin in integrin activation was later revealed by ex vivo and in vivo studies of mice expressing conditional talin-deficient platelets (Tln−/−, Cre-loxP).8,9 Tln−/− platelets show a complete loss of both β1 and β3 integrin activation and platelet aggregation in response to a wide variety of platelet agonists. Furthermore, mice expressing Tln−/− platelets show severe pathologic bleeding resulting from a loss of platelet adhesion and clot formation. Thus, these studies underscore the importance of the talin–β-integrin interaction in regulating platelet hemostatic functions.
Here, Haling and colleagues dissect the role of talin in integrin activation and linkage of integrins to the actin cytoskeleton in the process of platelet clot retraction. Taking into consideration that talinL325R fails to activate integrin αIIbβ3 in vitro, while retaining the ability to bind to the β-integrin tail and other binding partners,5 the authors generated mice conditionally expressing talinL325R in platelets. Consistent with its predicted function, talinL325R platelets showed a marked reduction in both integrin αIIbβ3 and β1-integrin activation without affecting talinL325R interaction with integrins or cytoskeletal proteins. These results provide intriguing evidence for a specific integrin activation site within talin that does not affect other talin-binding functions. In addition, wild-type platelets, but not talin−/− or talinL325R platelets, show defective agonist-induced integrin activation and clot retraction (see figure), suggesting that integrin activation is required for fibrin clot retraction. It is not clear from these results, however, whether the clot retraction defect in talin−/− platelets is due to impaired integrin activation and/or loss of talin-dependent integrin linkage to the cytoskeleton. To discern between these possibilities, the authors treated platelets with manganese (MnCl2) which bypasses intracellular signals and extrinsically activates integrin αIIbβ3. Clot retraction was rescued in MnCl2-treated talinL325R, but not talin−/− platelets. The rescue in talinL325R platelets could be blocked by disrupting the cytoskeleton with cytochalasin D or inhibiting ligand binding to αIIbβ3. The finding that clot retraction is rescued by integrin activation in talinL325R, but not talin−/− platelets indicates that talin also serves as a functional link between the extracellular matrix and the cytoskeleton and ascribes a second important role for talin in integrin function.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■