Abstract
Talin functions both as a regulator of integrin affinity and as an important mechanical link between integrins and the cytoskeleton. Using genetic deletion of talin, we show for the first time that the capacity of talin to activate integrins is required for fibrin clot retraction by platelets. To further dissect which talin functions are required for this process, we tested clot retraction in platelets expressing a talin1(L325R) mutant that binds to integrins, but exhibits impaired integrin activation ascribable to disruption of the interaction between talin and the membrane-proximal region (MPR) in the β-integrin cytoplasmic domain. Talin-deficient and talin1(L325R) platelets were defective in retracting fibrin clots. However, the defect in clot retraction in talin1(L325R) platelets, but not talin-deficient platelets, was rescued by extrinsically activating integrins with manganese, thereby proving that integrin activation is required and showing that talin1(L325R) can form functional links to the actin cytoskeleton.
Introduction
Upon vascular injury, platelets encounter collagen and soluble factors that initiate intracellular signaling that up-regulates the integrin ligand-binding affinity (activation) required for platelet adhesion and aggregation, 2 key processes in thrombus initiation and growth.1 Subsequently, αIIbβ3 integrin-dependent retraction of clots by platelets promotes thrombus stability and wound healing.2 Thus, modulation of integrin activation and the mechanical linkage of integrins to the actin cytoskeleton are critical steps for primary hemostasis.
A necessary final step in activating integrins is the binding of talin to the β integrin cytoplasmic domain. Talin comprises an N-terminal FERM (4-point-one/ezrin/radixin/moesin) domain and a 200-kDa flexible-rod domain. The FERM domain binds to β-integrin cytoplasmic domains (tails), phosphatidylinositol phosphate kinase type 1γ-90 (PIPKIγ-90), and layilin. The rod domain has multiple binding sites for vinculin and actin.3 There are 2 talin isoforms, and global genetic deletion of talin1 in mice is early embryonic lethal, necessitating the use of tissue-specific knockout approaches.4 Previously, we have shown that platelet-specific deletion of talin1 by the use of a conditional talin1 knockout mouse (Tln1(fl/fl)) crossed with a mouse expressing a platelet-specific Cre under control of the platelet factor-4 promoter (PF4-Cre)5 results in impaired agonist-induced integrin activation in platelets.6 Furthermore, platelets from mice expressing a β3(L746A) mutation that disrupts talin binding to β3 integrin phenocopy the defects in αIIbβ3 activation observed in talin-deficient platelets.6-8 Together, these studies strongly suggest that binding of talin to integrins is required for integrin activation in platelets. However, deletion of talin or a β3(L746A) mutation ablates the talin-dependent link between integrins and the actin cytoskeleton.9,10
Recent structural and biochemical studies by Wegener et al suggest that talin interactions with a membrane-proximal region (MPR) of integrins is necessary for integrin activation.11 These investigators identified a talin1(L325R) mutation predicted to inhibit integrin activation by disrupting the talin-β3 integrin MPR. Furthermore, the talin1(L325R) mutation only slightly reduced the affinity of the talin FERM domain for a chimeric peptide containing the MPR of integrin β3.11 Together, their findings suggest that talin1(L325R) would be defective in integrin activation yet able to mediate other functions such as binding to PIPKIγ-90 and linking integrins to the actin cytoskeleton. To test the hypothesis that talin-integrin MPR interactions are required for integrin function in vivo, we generated mice expressing talin1(L325R) in platelets. Our results demonstrate that agonist-induced activation of platelet integrins is dependent on the talin-integrin MPR interaction and that this interaction is necessary for fibrin clot retraction by platelets.
Methods
Mice
Tln1(fl/fl),PF4-Cre mice have been described in detail.8 Conditional Tln1(L325R) mutant mice were generated by gene-targeting by the use of a targeting vector illustrated in supplemental Figure 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and characterized as described in supplemental Methods. Mice were housed in the animal facility of the University of California, San Diego, and experiments were approved by the University's Institutional Animal Care and Use Committee.
Platelet function
Soluble fibrinogen and 9EG7 binding assays were performed on washed platelets exactly as previously described.8 Measurement of clot retraction was performed with the use of platelet-rich plasma (PRP) obtained from blood drawn into 1/10 volume of 3.8% sodium citrate and 1 volume of modified Tyrode buffer (140mM NaCl, 2.7mM KCl, 0.4M NaH2PO4.H2O, 10mM NaHCO3, 5mM dextrose, and 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [ie, HEPES]). Platelets were diluted to 3 × 108/mL with platelet-poor plasma obtained from wild-type mice. After the addition of 2mM CaCl2, PRP was added to siliconized glass cuvettes containing a paperclip, and clot retraction was initiated with the addition of 1 U/mL bovine thrombin (Sigma-Aldrich). In some samples, 0.5mM MgCl2, 10μM cytochalasin D, or 50μM eptifibatide was added to PRP 5 minutes before the addition of thrombin. Samples were incubated at 25°C for 2 hours and clot retraction analyzed by imaging the clot retraction and weighing the residual serum after removing the clot to calculate the percentage of serum extruded from the clot.
Affinity chromatography
Tln1(L325R/fl),Cre+ and Tln1(wt/fl),Cre+ platelets were solubilized as previously described.12 Affinity chromatography was performed by the use of recombinant integrin cytoplasmic tails bound to NeutrAvidin Resin (Thermo Scientific) as previously described.12,13 Bound proteins were fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and analyzed by Western blotting with anti-talin 8d4 (Sigma-Aldrich) antibody. Binding of recombinant integrin tails to the resin was verified by Coomassie Blue staining. Signal was detected and quantified using an Odyssey imaging system (LI-COR Biosciences).
Results and discussion
Talin(L325R) impairs integrin activation by selectively disrupting membrane-proximal interactions with integrins
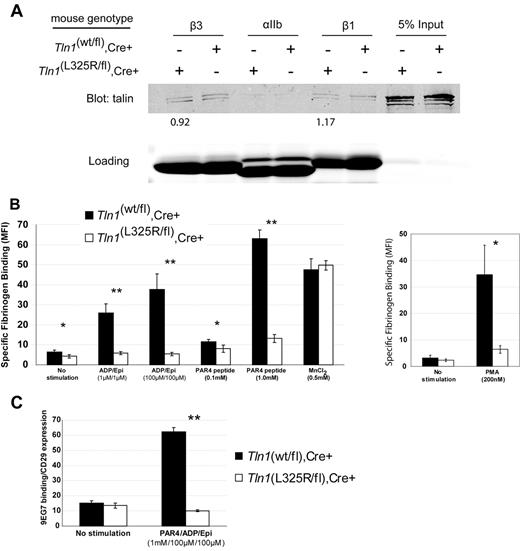
To test whether interaction of talin with the MPR of integrins is necessary for agonist-induced activation of integrins in platelets, we generated mice carrying a single amino acid substitution (L325R) in talin1 by gene-targeting. Considering global genetic deletion of talin1 is embryonic lethal and that a talin1(L325R) mutation is predicted to impart a strong loss-of-function effect, Tln1(L325R/wt) mice were crossed with Tln1(flox/flox),PF4-Cre+ mice so that talin1(L325R) was selectively expressed in platelets. Affinity chromatography showed that talin1(L325R) bound β1 and β3 integrin tails similar to wild-type talin1 (Figure 1A). In addition to integrins, talin also binds to PIPKIγ-90, a protein that contributes to anchoring the cytoskeleton to the plasma membrane.14 Of importance, the talin1(L325R) mutation does not affect talin binding to PIPKIγ-90, as shown by coimmunoprecipitation (supplemental Figure 1). Together, these data suggest that talin1(L325R) does not markedly affect the binding of talin to integrins or PIPKIγ-90.
Talin1(L325R) binds to integrins but is defective in agonist-induced platelet integrin activation. (A) Affinity chromatography with the use of recombinant β1, β3, and αIIb cytoplasmic domains and platelet lysates from Tln1(wt/fl),Cre+ (indicated as wt) or Tln1(L325R/fl),Cre+ (indicated as L325R) mice. Bound talin protein was visualized by Western blotting with anti-talin 8d4 antibody, and equal loading of integrins was verified by Coomassie stain. Densitometric quantitation of talin bound was normalized to integrin loading. The value shown indicates the amount of talin1(L325R) bound to the integrin relative to talin1 (wt). Data are representative of 2 independent experiments. (B) Specific binding of fluorescein isothiocyanate-fibrinogen to platelets was measured by the use of flow cytometry by subtracting the nonspecific fibrinogen binding that occurred in the presence of 5mM ethylenediaminetetraacetic acid in each condition. *P < .05, **P < .005. (C) Activation of β1 integrin was assessed by measuring the binding of the conformation-sensitive antibody 9EG7 relative to binding of the conformation-insensitive β1 integrin antibody HMβ1-1. **P < .005.
Talin1(L325R) binds to integrins but is defective in agonist-induced platelet integrin activation. (A) Affinity chromatography with the use of recombinant β1, β3, and αIIb cytoplasmic domains and platelet lysates from Tln1(wt/fl),Cre+ (indicated as wt) or Tln1(L325R/fl),Cre+ (indicated as L325R) mice. Bound talin protein was visualized by Western blotting with anti-talin 8d4 antibody, and equal loading of integrins was verified by Coomassie stain. Densitometric quantitation of talin bound was normalized to integrin loading. The value shown indicates the amount of talin1(L325R) bound to the integrin relative to talin1 (wt). Data are representative of 2 independent experiments. (B) Specific binding of fluorescein isothiocyanate-fibrinogen to platelets was measured by the use of flow cytometry by subtracting the nonspecific fibrinogen binding that occurred in the presence of 5mM ethylenediaminetetraacetic acid in each condition. *P < .05, **P < .005. (C) Activation of β1 integrin was assessed by measuring the binding of the conformation-sensitive antibody 9EG7 relative to binding of the conformation-insensitive β1 integrin antibody HMβ1-1. **P < .005.
To assess activation of integrin αIIbβ3 in platelets from Tln1(wt/fl),Cre+ or Tln1(L325R/fl),Cre+ mice, we measured agonist-induced soluble fibrinogen binding. Tln1(L325R/fl),Cre+ platelets demonstrated a striking reduction in fibrinogen binding despite similar amounts of talin and integrin expression as platelets from Tln1(wt/fl),Cre+ mice (Figure 1B; supplemental Figure 3). However, in the presence of manganese, which activates αIIbβ3 extrinsically, Tln1(L325R/fl),Cre+ and Tln1(wt/fl),Cre+ platelets bound similar amounts of fibrinogen, indicating that, upon activation, the αIIbβ3 expressed on Tln1(L325R/fl),Cre+ platelets is capable of binding fibrinogen (Figure 1B). The defect in agonist-induced fibrinogen binding was not caused by impaired agonist stimulation because agonist-induced P-selectin expression was similar in Tln1(wt/fl),Cre+ and Tln1(L325R/fl),Cre+ platelets (supplemental Figure 3).
Because there is evidence that β1 and β3 integrin activation may be regulated differently,15 we measured β1 integrin activation in Tln1(L325R/fl),Cre+ and Tln1(wt/fl),Cre+ platelets. Binding of 9EG7, an antibody that selectively binds to the active β1 integrin conformation, was completely impaired in Tln1(L325R/fl),Cre+ platelets (Figure 1C). The reduction in β1 and β3 integrin activation measured in Tln1(L325R/fl),Cre+ platelets was similar to that previously reported in talin-deficient platelets.6 Altogether, our results show that the talin1(L325R) mutation disrupts agonist-induced activation of both β1 and β3 integrins in platelets without markedly affecting the binding of talin to integrins or PIPKIγ-90.
Talin-dependent integrin activation is required for fibrin clot retraction
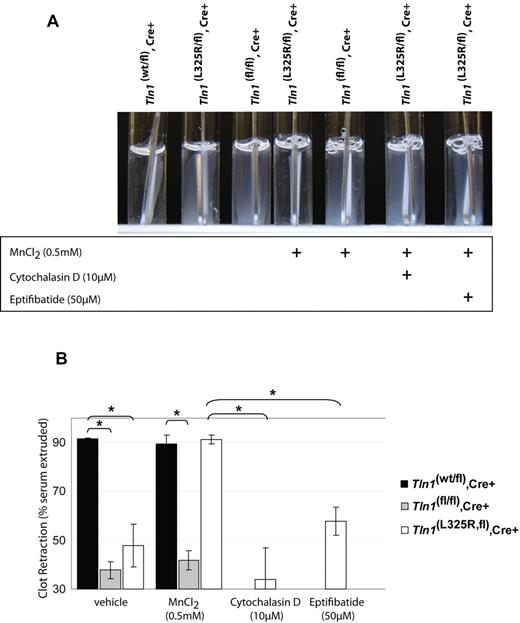
Talin links integrins to the actin cytoskeleton and is important for transmitting mechanical force across the plasma membrane via integrins.16 Because platelet retraction of fibrin clots requires integrins to be connected to the actin cytoskeleton,17,18 we tested the role of talin in platelet-mediated fibrin clot retraction. Indeed, talin-deficient platelets are defective in retracting a thrombin-induced clot, demonstrating that talin is required to link the actin cytoskeleton to integrins during platelet clot retraction (Figure 2). The clot retraction defect observed in talin-deficient platelets could be ascribable to impaired integrin activation, disruption of a talin-dependent mechanical linkage to integrins, or a disruption of the function of talin-binding proteins such as PIPKIγ-90.
Fibrin clot retraction is impaired in talin1-deficient and talin1(L325R) mutant platelets. (A) Clot retraction of PRP from Tln1(wt/fl),Cre+ or Tln1(L325R/fl),Cre+, or Tln1(fl/fl),Cre+ mice was initiated by 1.0 U/mL thrombin and photographed after 2 hours. As indicated, 0.5mM MnCl2, 10μM cytochalasin D, or 50μM eptifibatide was added to PRP 5 minutes before the addition of thrombin. Efficient clot retraction, visible as a consolidated clot and transparent clot liquor, was observed in PRP from Tln1(wt/fl),Cre+ mice and Tln1(L325R/fl),Cre+ mice in the presence of manganese. (B) Fibrin clot retraction was quantified by calculating the weight of the serum extruded from the clot relative to the initial clot weight and expressed as a percentage. n = 3. *P < .005.
Fibrin clot retraction is impaired in talin1-deficient and talin1(L325R) mutant platelets. (A) Clot retraction of PRP from Tln1(wt/fl),Cre+ or Tln1(L325R/fl),Cre+, or Tln1(fl/fl),Cre+ mice was initiated by 1.0 U/mL thrombin and photographed after 2 hours. As indicated, 0.5mM MnCl2, 10μM cytochalasin D, or 50μM eptifibatide was added to PRP 5 minutes before the addition of thrombin. Efficient clot retraction, visible as a consolidated clot and transparent clot liquor, was observed in PRP from Tln1(wt/fl),Cre+ mice and Tln1(L325R/fl),Cre+ mice in the presence of manganese. (B) Fibrin clot retraction was quantified by calculating the weight of the serum extruded from the clot relative to the initial clot weight and expressed as a percentage. n = 3. *P < .005.
To investigate these possibilities we examined clot retraction in Tln1(L325R/fl),Cre+ platelets in which αIIbβ3 activation is impaired without disrupting talin binding to integrins or PIPKIγ-90. Tln1(L325R/fl),Cre+ platelets also showed defects in clot retraction, similar to talin-deficient platelets (Figure 2). However, clot retraction by Tln1(L325R/fl),Cre+ platelets, but not by talin-deficient platelets, could be rescued by 0.5mM MnCl2, indicating that integrin activation, in addition to talin expression, is necessary for platelets to retract fibrin clots (Figure 2). The rescue of clot retraction by manganese was blocked by cytochalasin D or eptifibatide, showing that it was dependent on actin polymerization and αIIbβ3 ligand binding, respectively. Together these results show for the first time that both talin-dependent αIIbβ3 activation and talin-dependent linkage of the fibrin clot to the actin cytoskeleton via integrins are required for fibrin clot reaction by platelets.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Ralph Tiedt and Dr Radek Skoda for the gift of PF4-Cre mice. We gratefully acknowledge Dr Mark Ginsberg, Dr Sanford Shattil, and Dr Feng Ye for their careful reading of the manuscript.
This work was supported by grants from the National Institutes of Health (HL078784 and ARA027214) and the American Heart Association (0830213N).
National Institutes of Health
Authorship
Contribution: J.R.H. performed experiments, analyzed data, and edited the manuscript; S.J.M. and D.R.C. provided vital reagents and edited the manuscript; and B.G.P. designed and performed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brian G. Petrich, Department of Medicine, University of California, San Diego, Leichtag Bldg, Rm 111, 9500 Gilman Dr, Dept 0726, La Jolla, CA 92093-0726; e-mail: bpetrich@ucsd.edu.