Abstract
Chronic lymphocytic leukemia (CLL) is characterized by a clonal accumulation of mature neoplastic B cells that are resistant to apoptosis. Aiolos, a member of the Ikaros family of zinc-finger transcription factors, plays an important role in the control of mature B lymphocyte differentiation and maturation. In this study, we showed that Aiolos expression is up-regulated in B-CLL cells. This overexpression does not implicate isoform imbalance or disturb Aiolos subcellular localization. The chromatin status at the Aiolos promoter in CLL is defined by the demethylation of DNA and an enrichment of euchromatin associated histone markers, such as the dimethylation of the lysine 4 on histone H3. These epigenetic modifications should allow its upstream effectors, such as nuclear factor-κB, constitutively activated in CLL, to gain access to promoter, resulting up-regulation of Aiolos. To determine the consequences of Aiolos deregulation in CLL, we analyzed the effects of Aiolos overexpression or down-regulation on apoptosis. Aiolos is involved in cell survival by regulating the expression of some Bcl-2 family members. Our results strongly suggest that Aiolos deregulation by epigenetic modifications may be a hallmark of CLL.
Introduction
The lymphocyte developmental is tightly controlled by a large number of transcription factors.1 Among these proteins, Aiolos has been identified as a homologue of the largest Ikaros isoform,2 with strong similarities in the DNA binding, activation, and dimerization domains.3 At least 16 isoforms of human Aiolos, generated by alternative splicing, have been described.4 These isoforms can be divided into 2 groups. One possesses at least 3 zinc fingers at the N-terminus and binds DNA, and the other possesses fewer than 3 N-terminal zinc fingers and is unable to bind DNA. Isoforms that cannot bind DNA but retain the capacity to dimerize are considered to act in a dominant-negative fashion.5,6 Aiolos is not expressed in mouse hematopoietic stem cells but is detectable in pro-B and double-negative CD4/CD8 thymocyte precursors. Expression of Aiolos is further up-regulated as these cells progress to the pre-B and double-positive CD4/CD8 stages of differentiation, respectively. Aiolos is highly expressed in mature murine peripheral B cells but not significantly in splenic T cells.7 In humans, B cells express the highest level of total Aiolos compared with T cells, natural killer cells, monocytes, and CD34+ hematopoietic progenitors. T and natural killer cells express comparable levels of Aiolos, and monocytes express almost undetectable level of Aiolos whereas CD34+ progenitors are negative for Aiolos expression.8 Aiolos plays an essential role during B-cell maturation and its inactivation in knockout mice results in an increase in B-cell precursors, breakdown in B-cell tolerance, and the development of B-cell lymphomas. In contrast, peritoneal, marginal, and recirculating B cells are severely depleted.7 Aiolos functions and the parameters involved in its transcriptional regulation are largely unknown in humans and need be better defined. Recently, we reported for the first time that Aiolos expression is deregulated in CLL,9 which was subsequently confirmed by the authors of other studies.10,11
Chronic lymphocytic leukemia (CLL)12 is characterized by the accumulation of monoclonal B lymphocytes in blood, bone marrow, and peripheral lymphoid tissues. CLL can be divided in 2 subsets on the basis of immunoglobulin heavy chain variable region (IgVH) gene mutation status. Patients whose CLL cells exhibit IgVH gene mutation have better a clinical prognosis than the patients whose CLL cells do not exhibit IgVH gene mutation.13 ZAP-70, which is involved in T-cell receptor signaling, is aberrantly expressed in some patients14 and shows partial overlap with overexpression of other risk factors such as the presence of CD38 or unmutated IgVH genes. CLL cells do not display a unique recurrent genomic alteration but have several chromosomal alterations, including 11q22-q23 (ATM, or ataxia telangiectasia mutated), 13q14, 17p13 (TP53), 6q21 deletions, and 12 trisomy. 11q and 17p deletions are associated with a poor outcome, whereas isolated 13q deletion is associated with a favorable prognosis.15
Aberrant gene function and altered gene expression are key features of cancer. Growing evidence shows that both acquired epigenetic abnormalities and genetic alterations contribute to cause deregulation of gene expression.16 Epigenetic is defined as heritable changes in gene expression that are not accompanied by changes in DNA sequence. Covalent modifications of histones but also of CpG sites on DNA participate in the establishment of local chromatin structures that influence permissivity of gene promoter for the subsequent assembly and/or activation of the transcriptional machinery.17 Among histones marks, covalent modifications of Lys 4, Lys 9, and Lys 27 of the histone H3 tail are known to play crucial roles. Histone H3 di/trimethylation on K4 and/or acetylation on K9 have been extensively associated with active regions of the genome (euchromatin) whereas histone H3 trimethylation on K9 and/or K27 and DNA methylation participate in the establishment and maintenance of silent domains (heterochromatin).18 In this study, we showed that Aiolos expression is up-regulated in a panel of 32 CLL patients. The overexpression does not implicate isoforms imbalance or disturbing Aiolos subcellular localization. To investigate the causes of this Aiolos deregulation, we analyzed the profiles of DNA methylation and histones modifications on Aiolos promoter. We show that enriched euchromatin-associated markers together with upstream Aiolos regulators such as nuclear factor κB (NF-κB) may generate Aiolos overexpression. Furthermore, we demonstrate that ectopic Aiolos overexpression decreases phorbol myristate acetate (PMA)/ionomycin-mediated apoptosis in CLL cells. These data suggest that Aiolos plays a role in apoptosis-mediated events and may be a hallmark of CLL.
Methods
Patients and B-cell isolation
In accordance with a protocol approved by the institutional ethics committee, peripheral blood mononuclear cell (PBMC) samples were collected upon informed consent obtained from patients with CLL at the Hematological Department of Pitié-Salpêtrière Hospital. Diagnosis was assessed according to the recent World Health Organization classification. In all cases, blood sample collection was performed at the time of diagnosis. Fresh blood from healthy donors (HDs) was collected by the Etablissement Français du Sang. Mononuclear cells isolated from peripheral blood were prepared by Ficoll gradient centrifugation, and B cells were isolated by negative selection with the use of Dynabeads untouched human B cells kit (Dynal).
Cell culture and inhibitors treatment
Daudi (human Burkitt lymphoma) and U937 (human histiocytic lymphoma) cell lines, purified normal and leukemic B cells, or PBMCs were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2mM l-glutamine, 1mM sodium pyruvate, 25mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 0.1mM nonessential amino-acids, and 100 U/mL penicillin/streptomycin. BAY 11-7082 was supplied by Calbiochem (Merck) in dimethyl sulfoxide (DMSO) and was used at 5μM final concentration. An equivalent volume of DMSO was used in all experiments as a control. The cell-permeable NEMO binding domain (NBD) peptide and its mutated control, mNBD, were a gift from Dr Fabrice Agou (Institut Pasteur) and used at 20μM final concentration. PMA and ionomycin (Sigma-Aldrich) was used for 8 hours at 10 ng/mL and 1 μg/mL, respectively.
Immunofluorescence and confocal analysis
The subcellular localization of Aiolos protein was examined as previously described.19 In brief, B cells were fixed (4% paraformaldehyde for 20 minutes at room temperature), permeabilized (0.1% Triton X-100 for 5 minutes at room temperature), and then incubated with “in-house” rabbit polyclonal antiAiolos antibody overnight in phosphate-buffered saline/bovine serum albumin at 4°C. After several washing steps, samples were mounted and visualized with a Leica Leitz DMRB microscope fitted with a Leica DFC300FX camera.
Annexin V/propidium iodide staining
Annexin V (AV)/propidium iodide (PI) labeling was performed by use of the Vybrant Apoptosis Assay Kit #3 (Invitrogen) according to the manufacturer's instructions, followed by flow cytometry analysis. B cells CD19+ were scanned in FL1-H (fluorescein isothiocyanate) versus FL2-H (PI) channels with a FACSCalibur instrument equipped with CellQuest software (Becton Dickinson). Data analysis was performed with the quadrant statistics of WEASEL software.
Aiolos siRNA knockdown
The siRNA against human Aiolos, nontargeting negative control siRNA, and siRNA delivery media were from Thermo Scientific Dharmacon. The siRNA used were specially modified by Dharmacon's proprietary Accell technology. Cells were cultured at a 1 × 106 cells/mL density with siRNAs at a final concentration of 1μM in Accell siRNA delivery media for at least 72 hours. Modifications in gene expression were assessed by real-time polymerase chain reaction (PCR).
Nucleofection
The pcDNA3.1 vector (Invitrogen) was used to clone the cDNA encoding wild-type Aiolos (Aio-1) in the BamHI/HindIII cloning sites. Transient transfection of B cells was achieved by the Human B cell Nucleofactor Kit, following the Amaxa guidelines for B-cell transfection.
RNA preparation and reverse-transcriptase PCR
Total RNA extraction was performed with the QIAGEN RNeasy kit according to the manufacturer's instructions. RNA (500 ng) was reverse transcribed (RT) and amplified by the Titanium one-step RT-PCR (Clontech; for primers sequences, see supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The RT-PCR products were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining.
Quantitative RT-PCR
RNA was reverse transcribed by Superscript VILO cDNA synthesis kit (Invitrogen). Quantitative PCR (qPCR) was performed by use of the TaqMan gene expression assay (Applied Biosystems), and SYBR Green assays were performed with the SYBR Green PCR Master Mix (Applied Biosystems; for primers sequences, see supplemental Table 1).
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were performed as described.20 The primers sequences match the CpG island, as well as upstream and downstream of the CpG island (for primers sequences, see supplemental Table 1).
Methylated DNA immunoprecipitation
The methylated DNA immunoprecipitation (MeDIP) assay was performed as described.20 Commercial glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and testis/sperm-specific histone 2B (TSH2B) were used as a negative and positive control (Diagenode) for MeDIP, respectively.
Protein extraction and Western blotting
Cytosolic extracts were prepared with buffer A (10mM HEPES, pH 7.8; 2mM MgCl2; 10 mM KCl; 0.1mM ethylenediaminetetraacetic acid [EDTA]) and nuclear extracts with buffer B (500mM NaCl; 20mM HEPES, pH 7.8; 1.5mM MgCl2; 0.5mM EDTA; 25% glycerol), with both buffers containing 1mM phenylmethylsulfonyl fluoride, 5mM Na2VO4, 2mM dithiothreitol, and the Complete Protease Inhibitor Cocktail (Roche Applied Science). Total cellular lysates were prepared with the use of a high-salt lysis buffer (400mM NaCl; 25mM HEPES, pH 7.8; 1.5mM MgCl2; 0.2mM EDTA; 1% NP-40; 1mM phenylmethylsulfonyl fluoride; 5mM Na2VO4; 2mM dithiothreitol; and the Complete Protease Inhibitor Cocktail). Cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane (Bio-Rad), blocked in Odyssey Blocking Buffer (LI-COR), and incubated with a primary antibody. Blotted proteins were detected and quantified with the Odyssey infrared imaging system LI-COR. The following primary antibodies were used: Aiolos (“in-house” rabbit Ab), actin (rabbit Ab; Cell signaling), NF-κB RelA (rabbit Ab; Santa Cruz), and fibrillarin (mouse Ab; Abcam).
Electrophoretic mobility shift assays
Nuclear extracts were prepared and analyzed for DNA binding activity by use of the HIV-LTR tandem κB oligonucleotide as κB probe as previously described.21 For supershift assays, nuclear extracts were preincubated with antibodies specific for 30 minutes on ice before the addition of the labeled probe.
Statistical analysis
We used Prism 5.0c (GraphPad Software) for statistical analysis. Unpaired t test and Mann-Whitney U tests were realized, and statistical significance was set at P < .05 with the following degrees: *P < .05; **P < .01; and ***P < .001.
Results
Expression of Aiolos but not Ikaros is up-regulated in B cells from CLL patients
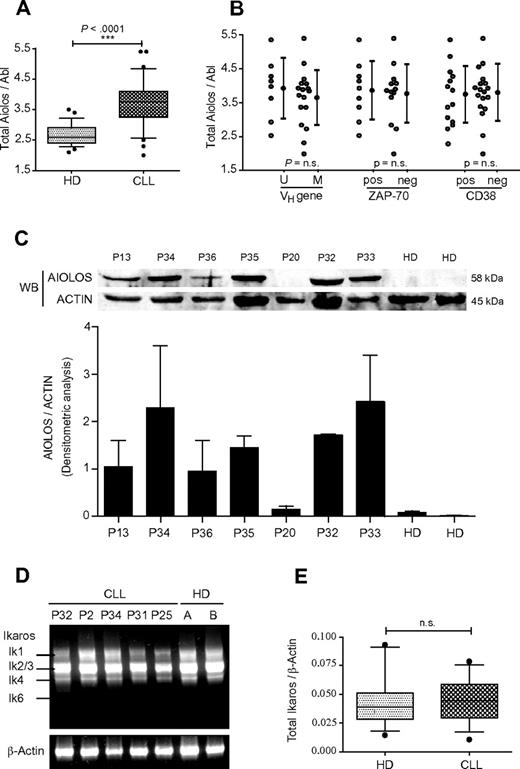
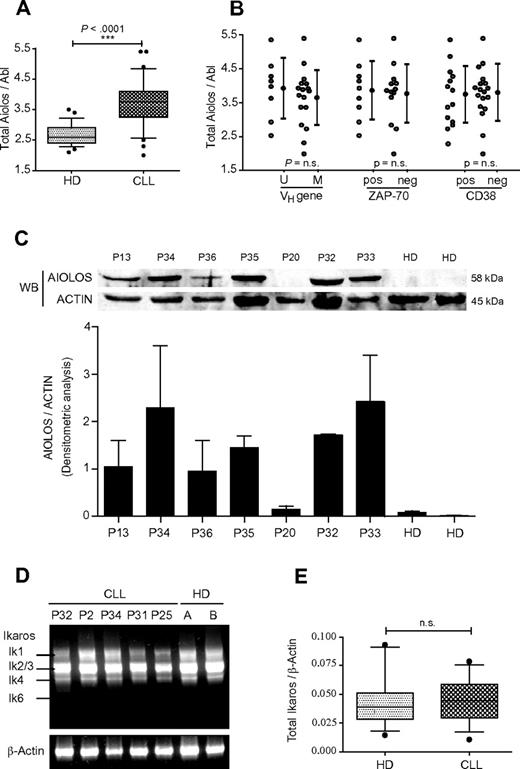
The level of Aiolos mRNA expression was determined by real-time PCR analysis. Aiolos transcripts were amplified with primers specific for the second and third exons, which amplify all Aiolos isoforms. We analyzed a total of 32 CLL samples and 28 HDs and normalized the amount of mRNA in each sample by using Abelson (Abl) RNA as internal control. Clinical and pathologic characteristics of patients used in this study and their Aiolos expression data are shown in Table 1. Total Aiolos mRNA was overexpressed in B cells from CLL patients compared with HDs (Table 1). A 1.5-fold increase of Aiolos transcripts in CLL samples compared with normal B cells was observed, which is statistically significant (P < .0001; Figure 1A). Given that unmutated IgVH gene status, high ZAP-70 expression, or high CD38 expression are associated with worse clinical outcome, we analyzed by qPCR the level of Aiolos expression in B cells from these CLL patient subgroups. No correlation of these clinical markers with Aiolos expression levels was observed (Figure 1B). We further analyzed Aiolos protein expression in PBMC from 2 HDs and 7 CLL patients. Aiolos protein was overexpressed in PBMCs isolated from all CLL patients except for P20, which shows a very weak expression as the HDs (Figure 1C). This new data set confirmed our previous observation that Aiolos expression is up-regulated in B cells from CLL patients.9 To investigate whether Ikaros, an homologue of Aiolos, is deregulated in B-CLL cells, we analyzed by RT-PCR the expression pattern of different Ikaros isoforms in PBMC from 5 CLL patients and 2 HDs. An analysis of HDs revealed the presence of 4 Ikaros variants: Ik-1, Ik-2, Ik-3, and Ik-4 but not Ik-6 (Figure 1D). No major differences in the distribution of Ikaros isoforms were detected between HDs and CLL patients.
Analysis of Aiolos and Ikaros expression in CLL. (A) Quantification of total Aiolos transcripts in B cells from 32 CLL patients (P1-P18 and P20-P33) and 28 HDs obtained by RT-qPCR of B-cell RNA and normalized to the Abl gene. Absolute quantification of Aiolos and Abl was performed with the use of known quantities of pcDNA3.1-hAio1 construct and Daudi total RNA, respectively. Respective data are summarized and presented as the mean ± SEM. (B) Comparison of Aiolos levels in CLL subsets defined by VH gene mutation status, ZAP-70, and CD38 expression. Respective data are summarized and presented as the mean ± SEM. Differences between groups were tested using unpaired t test (Prism5.0c software; ns indicates non significant; *P < .05; **P < .01; ***P < .001). (C) Aiolos protein levels in PBMCs from 7 CLL patients and 2 HDs were detected by Western blot with a specific anti-Aiolos antibody. Actin was used as internal loading control (top). The histogram shows the densitometric analysis of 2 independent Western blot experiments with same protein samples. The error bars represent standard deviation (bottom). (D) Expression of Ikaros transcripts in PBMC from HD and CLL patients. Representative results of Ikaros isoforms expression in 2 HD and 5 CLL patients (P2, P25, P31, P32, and P34) after one-step RT-PCR amplification of PBMC RNA. β-actin was used as internal control. (E) Quantitative analysis of total Ikaros mRNA levels in B cells from 13 HD and 14 CLL patients (P17, P18, P20-P23, P25, P26, and P28-P33). Data are expressed as normalized expression by use of the 2−ΔCt calculation method (Ct indicates cycle threshold) and β-Actin as reference gene. n.s. indicates nonsignificant; *P < .05; **P < .01; ***P < .001.
Analysis of Aiolos and Ikaros expression in CLL. (A) Quantification of total Aiolos transcripts in B cells from 32 CLL patients (P1-P18 and P20-P33) and 28 HDs obtained by RT-qPCR of B-cell RNA and normalized to the Abl gene. Absolute quantification of Aiolos and Abl was performed with the use of known quantities of pcDNA3.1-hAio1 construct and Daudi total RNA, respectively. Respective data are summarized and presented as the mean ± SEM. (B) Comparison of Aiolos levels in CLL subsets defined by VH gene mutation status, ZAP-70, and CD38 expression. Respective data are summarized and presented as the mean ± SEM. Differences between groups were tested using unpaired t test (Prism5.0c software; ns indicates non significant; *P < .05; **P < .01; ***P < .001). (C) Aiolos protein levels in PBMCs from 7 CLL patients and 2 HDs were detected by Western blot with a specific anti-Aiolos antibody. Actin was used as internal loading control (top). The histogram shows the densitometric analysis of 2 independent Western blot experiments with same protein samples. The error bars represent standard deviation (bottom). (D) Expression of Ikaros transcripts in PBMC from HD and CLL patients. Representative results of Ikaros isoforms expression in 2 HD and 5 CLL patients (P2, P25, P31, P32, and P34) after one-step RT-PCR amplification of PBMC RNA. β-actin was used as internal control. (E) Quantitative analysis of total Ikaros mRNA levels in B cells from 13 HD and 14 CLL patients (P17, P18, P20-P23, P25, P26, and P28-P33). Data are expressed as normalized expression by use of the 2−ΔCt calculation method (Ct indicates cycle threshold) and β-Actin as reference gene. n.s. indicates nonsignificant; *P < .05; **P < .01; ***P < .001.
It is interesting to notice that, different from acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and chronic myelogenous leukemia (CML), CLL cells analyzed in this study do not express the dominant-negative isoform Ik-6. Nevertheless, this result should be verified on a larger group of CLL patients. In all cases, Ik-2/3 was the most abundant mRNA species, and the shorter variant Ik-4 was equally expressed in cells from HD and CLL patients. To investigate whether there was a change of total amounts of Ikaros transcripts, we performed quantitative analysis of total Ikaros expression on CD19-positive peripheral blood cells in a group of 14 CLL patients and 13 HDs. In contrast to Aiolos, the total Ikaros expression is similar between CLL patients and HDs (Figure 1E). Thus, in contrast to Aiolos, Ikaros expression is not deregulated in B cells from CLL. Therefore, B-CLL cells seem to exhibit a specific up-regulation of the Aiolos member of the Ikaros family.
B cells from CLL patients show a normal Aiolos subcellular distribution
The authors of previous studies revealed an isoform-specific cell distribution in the Aiolos isoforms. Aio-1, the full-length isoform, and Aio-Δ5, the isoform lacking one zinc finger, are both present in nuclei. By contrast, the Aio-Δ3,4,5,6 isoform, which lacks the DNA-binding domain, is predominantly localized in the cytoplasm,4 similar to the distribution described for the Ikaros dominant-negative isoforms. To investigate putative modifications in the subcellular localization of Aiolos in CLL cells, we analyzed by confocal microscopy the Aiolos cellular distribution by using the specific anti-Aiolos antibody. Daudi (B cells) and U937 (monocytes) cell lines were used as a positive and negative control for Aiolos expression, respectively. We observed that Aiolos protein has same localization in B cells from HD and from CLL patients, with intense punctuate green fluorescent staining in their nuclei (Figure 2).
Localization of Aiolos protein in B cells fromHDsand CLL patients. Samples were stained with anti-Aiolos antibody (green) and DAPI (blue) and analyzed by confocal microscopy. Similar results were obtained in 8 CLL patients. Daudi and U937 cells were used as positive and negative control for Aiolos expression, respectively.
Localization of Aiolos protein in B cells fromHDsand CLL patients. Samples were stained with anti-Aiolos antibody (green) and DAPI (blue) and analyzed by confocal microscopy. Similar results were obtained in 8 CLL patients. Daudi and U937 cells were used as positive and negative control for Aiolos expression, respectively.
Aiolos promoter is associated with enriched active chromatin marks in B-CLL cells
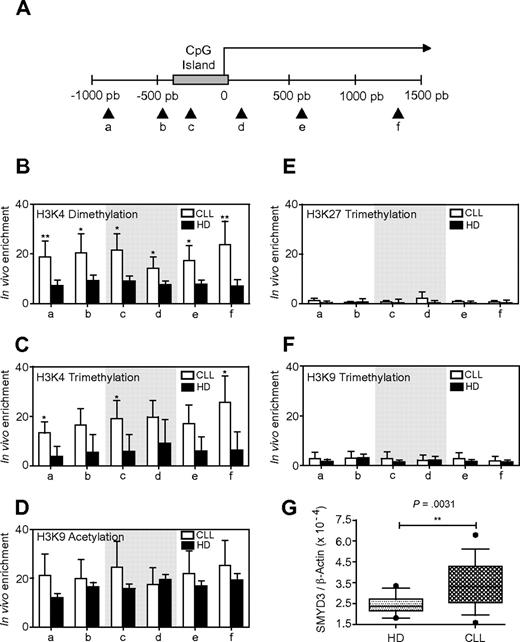
We have recently shown that Aiolos promoter activity is regulated by epigenetic mechanisms.22 To assess the involvement of this type of regulation in the Aiolos up-regulation, we first analyzed histone posttranslational modifications. We performed ChIP assays to investigate the chromatin status at the Aiolos promoter of B cells from CLL patients. Cross-linked chromatin from B cells of 8 HDs and 14 CLL patients was immunoprecipitated by the use of specific antibodies for histone H3 dimethylated on lysine 4 (H3K4me2), histone H3 trimethylated on lysine 4 (H3K4me3), 9 (H3K9me3) and 27 (H3K27me3), or histone H3 acetylated on lysine 9 (H3K9ac). PCR was performed by the use of primers spanning a 2-kb region upstream and downstream of the transcriptional initiation site (from −1 kb to + 1.5 kb; primers a-f, Figure 3A). The Aiolos promoter exhibited active chromatin marks (euchromatin), such as H3K4me2 (Figure 3B), H3K4me3 (Figure 3C), and H3K9ac (Figure 3D) from −1 to + 1.5-kb region spanning the Aiolos promoter in B cells from both CLL patients and HDs. However, the Aiolos promoter in CLL cells displays statistically significant enrichment for H3K4me2 and H3K4me3 (P < .05), but not for H3K9ac, compared with normal B cells. Of note we were not able to detect heterochromatin (inactive chromatin) markers such as H3K27me3 (Figure 3E) or H3K9me3 (Figure 3F) on Aiolos promoter, independently of the promoter region analyzed. This result suggests a more marked open chromatin structure of the Aiolos promoter in B-CLL cells compared with HDs, which highlights an increased transcriptional activity and correlates with an up-regulation of Aiolos expression in B-CLL cells.
ChIP analysis of histone modifications within the Aiolos promoter in CLL and normal B cells. (A) Schematic representation of the Aiolos locus. CpG island is represented by a gray box, and the arrows indicate the positions of the primer pairs used to analyze chromatin remodeling at Aiolos promoter. (B-F) ChIP analysis of H3K4 dimethylation (B), H3K4 trimethylation (C), H3K9 acetylation (D), H3K27 trimethylation (E), and H3K9 trimethylation (F) in B cells from HD (black bars) and CLL patients (white bars). qPCR was performed with primers a-f. The gray portions of the graphs correspond to the CpG island. The graph shows the average percent immunoprecipitation with SEM for 8 HD and 14 CLL patients (P1, P2, P17-P28) calculated for each position. (G) Quantitative analysis of SMYD3 mRNA levels in 14 HD and 16 CLL patients (P1, P2, P8, P17, P20-P23, P25, P26, and P28-P33). Data are expressed as normalized expression by use of the 2−ΔCT calculation method (Ct indicates cycle threshold) and β-actin as reference gene. Differences between groups were tested by use of Mann-Whitney U test (Prism5.0c software; *P < .05; **P < .01; ***P < .001).
ChIP analysis of histone modifications within the Aiolos promoter in CLL and normal B cells. (A) Schematic representation of the Aiolos locus. CpG island is represented by a gray box, and the arrows indicate the positions of the primer pairs used to analyze chromatin remodeling at Aiolos promoter. (B-F) ChIP analysis of H3K4 dimethylation (B), H3K4 trimethylation (C), H3K9 acetylation (D), H3K27 trimethylation (E), and H3K9 trimethylation (F) in B cells from HD (black bars) and CLL patients (white bars). qPCR was performed with primers a-f. The gray portions of the graphs correspond to the CpG island. The graph shows the average percent immunoprecipitation with SEM for 8 HD and 14 CLL patients (P1, P2, P17-P28) calculated for each position. (G) Quantitative analysis of SMYD3 mRNA levels in 14 HD and 16 CLL patients (P1, P2, P8, P17, P20-P23, P25, P26, and P28-P33). Data are expressed as normalized expression by use of the 2−ΔCT calculation method (Ct indicates cycle threshold) and β-actin as reference gene. Differences between groups were tested by use of Mann-Whitney U test (Prism5.0c software; *P < .05; **P < .01; ***P < .001).
We then determined whether modifications in chromatin structure at the Aiolos promoter were associated with changes in histone-modifying enzymes expression. Because H3K4me2 and H3K4me3 were enriched significantly in CLL patients, we analyzed the SMYD3 (SET and MYND domain-containing protein 3) expression, which has a histone H3-lysine 4–specific methyltransferase activity and is frequently up-regulated in some human carcinomas. As shown in Figure 3G, SMYD3 mRNA was significantly overexpressed in B cells from CLL patients, compared with HDs (P = .0031). This result shows for the first time a SMYD3 up-regulation in CLL.
Demethylated Aiolos promoter CpG island in malignant and normal B cells
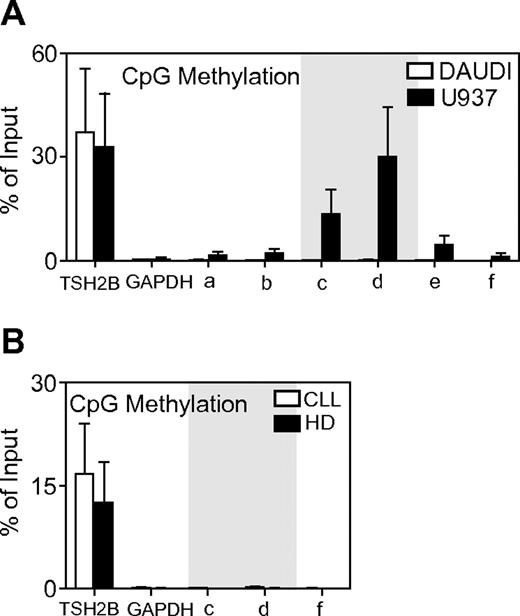
In addition to modification of the N-terminal tail of histones, DNA methylation of CpG islands plays a crucial role in chromatin organization and function. The main CpG island on Aiolos promoter was detected between −344 bp and the transcription initiation site (Figure 3A) by Methprimer software analysis. We have previously shown that this CpG island exhibits differential methylation between Aiolos expressing and nonexpressing cell lines. We analyzed by the use of MeDIP assays the methylation patterns of a 2-kb region spanning the upstream and downstream transcriptional initiation site that contains the CpG-rich region of the Aiolos promoter. Sonicated genomic DNA from 3 HDs and 3 CLL patients' B cells was immunoprecipited with antibody specific for 5-methylcytidine. PCR was performed by use of the previously described primers for ChIP assays (Figure 3A). The primer pairs c and d amplified the CpG-rich region on Aiolos promoter. Promoters of actively transcribed GAPDH gene and silent TSH2B gene in B cells were used as negative and positive controls for MeDIP, respectively. Figure 4A shows that the Daudi cell line has demethylated promoter, which correlates with Aiolos expression. In contrast, Aiolos promoter is densely methylated on the CpG-rich region in U937 cell line, which correlates with Aiolos silencing. These results are in agreement with our previous results obtained by bisulfite sequencing. The Aiolos promoter is densely demethylated in B cells from CLL and HD patients (Figure 4B). This result strongly suggests that Aiolos up-regulation in B cells from CLL patients is not caused by an abnormal promoter demethylation but rather by an increase of euchromatin markers, notably H3K4.
MeDIP analysis of DNA methylation within the Aiolos promoter containing a CpG rich region. (A) Aiolos promoter methylation status was analyzed in Daudi and U937 human cell lines, positive and negative for Aiolos expression, respectively. CpG island is marked in gray. (B) Aiolos promoter methylation status was analyzed in CLL patients and HD. The graph shows the average percent immunoprecipitation with SEM for HD (n = 3) and CLL patients (n = 3) calculated for each position. GAPDH and TSH2B loci were used as negative and positive control for MeDIP, respectively.
MeDIP analysis of DNA methylation within the Aiolos promoter containing a CpG rich region. (A) Aiolos promoter methylation status was analyzed in Daudi and U937 human cell lines, positive and negative for Aiolos expression, respectively. CpG island is marked in gray. (B) Aiolos promoter methylation status was analyzed in CLL patients and HD. The graph shows the average percent immunoprecipitation with SEM for HD (n = 3) and CLL patients (n = 3) calculated for each position. GAPDH and TSH2B loci were used as negative and positive control for MeDIP, respectively.
Inhibition of NF-κB activation induces down-regulation of Aiolos expression
CLL cells show increased NF-κB–binding activity compared with normal B cells23 and we have previously shown functional NF-κB binding sites in the Aiolos promoter.24 The NF-κB proteins are key regulators of differentiation and survival in B cells. In mammals, this protein family includes NF-κB1 (p50 and its precursor p105), NF-κB2 (p52 and its precursor p100), RelA (p65), Rel (cRel), and RelB. In the inactive state, NF-κB proteins occur as homodimeric or heterodimeric complexes in the cytoplasm bound to IκB proteins. After appropriate stimulation, IκB are phosphorylated, ubiquinated, and degraded, allowing the translocation of NF-κB dimers to the nucleus and subsequent transcription of NF-κB target genes.
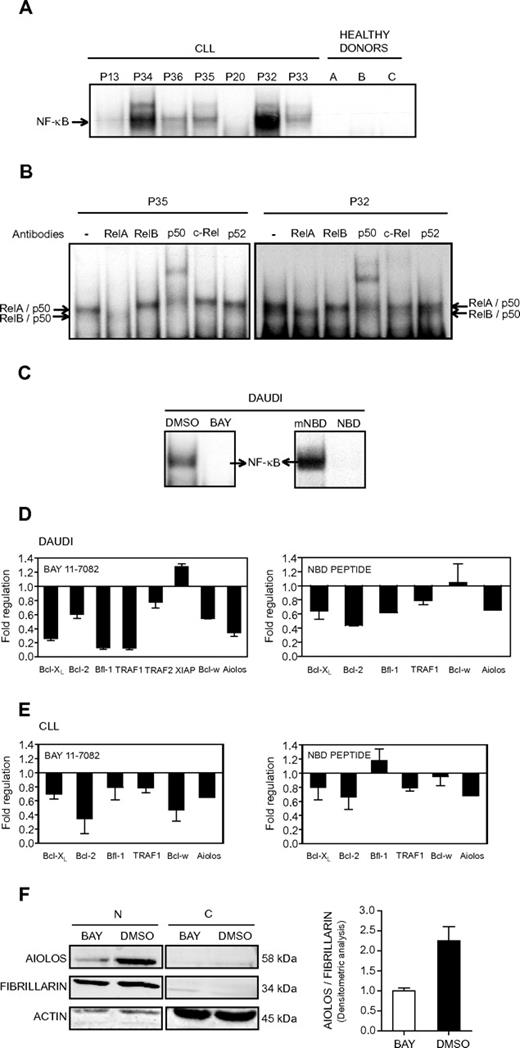
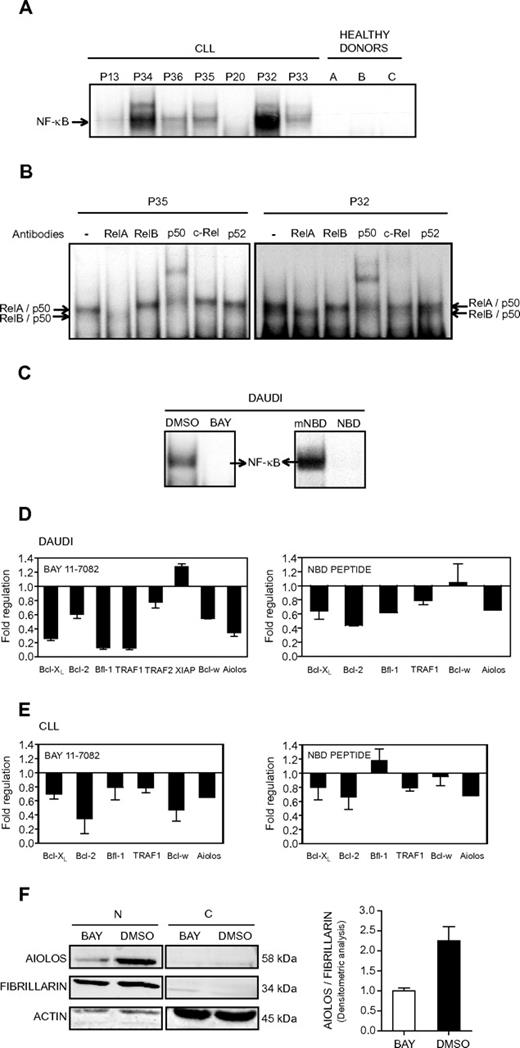
We decided to analyze the contribution of NF-κB to the control of Aiolos expression in CLL cells. We first analyzed NF-κB DNA-binding activity in whole cell extracts from the PBMCs of 7 CLL patients and 3 HDs. All CLL patients, except P20, presented a strong constitutive NF-κB DNA binding activity that was not observed in the HDs (Figure 5A). Supershift analysis demonstrated that DNA-bound NF-κB complexes mainly comprised RelA, p50, and RelB (Figure 5B). Interestingly, P20 exhibited weak Aiolos expression levels, whereas all others CLL patients tested presented a high level of Aiolos protein expression (Figure 1C), thus suggesting a correlation between NF-κB activity and Aiolos expression. To further investigate the influence of NF-κB on Aiolos expression, we analyzed the effect of NF-κB inhibition.
Effect of NF-κB inhibition on Aiolos expression. (A) Analysis of NF-κB DNA-binding activity in B cells from CLL. Electrophoretic mobility shift assays were performed with total extracts from PBMC of 7 CLL patients (P13, P20, and P32-P36) and 3 HD using a 32P-labeled human immunodeficiency virus-long terminal repeat tandem κB oligonucleotide as a probe. (B) For supershift analysis, total extracts from 2 CLL patients (P35 and P32) were incubated with the indicated antibodies before incubation with the labeled probe. (C) Analysis of NF-κB DNA-binding activity in Daudi cells. Electrophoretic mobility shift assays were performed with nuclear extracts from Daudi cells in 3 independent experiments. Daudi cells were treated for 8 hours with the NF-κB inhibitor BAY 11-7082 at 5μM (referred as BAY, left) or for 2 hours with the wild-type NEMO binding domain peptide at 20μM (referred as NBD, right). DMSO or mNBD was used as a control. (D-E) Expression of Aiolos and antiapoptotic molecules in NF-κB inhibitor-treated Daudi and CLL cells. The expression of different genes was analyzed by qRT-PCR and normalized by use of the 2−ΔCt calculation method (Ct indicates cycle threshold) and β-actin as reference gene. Daudi and CLL cells were treated with BAY 11-7082 (5μM, 8 hours; panels D-E left) or NBD peptide (20μM, 2 hours; panels D-E right). CLL cells from 2 (P1 and P37) and 4 (P1 and P38-40) patients were treated with BAY and NBD, respectively. DMSO or mNBD was used as controls. Results are expressed as fold down- or up-regulation of inhibitor-treated cells compared with control cells. Data are mean ± SEM of at least 3 independent experiments. (F) Aiolos expression at the protein level was analyzed by Western blotting in nuclear (N) or cytosolic (C) extracts isolated from Daudi cells treated or not with BAY (5μM, 8 hours). Fibrillarin and β-actin expression were used as internal control of the nuclear and cytosolic fraction purity, respectively. Densitometric analysis of nuclear proteins and molecular weight of the proteins are shown. Similar results were obtained in 2 independent experiments.
Effect of NF-κB inhibition on Aiolos expression. (A) Analysis of NF-κB DNA-binding activity in B cells from CLL. Electrophoretic mobility shift assays were performed with total extracts from PBMC of 7 CLL patients (P13, P20, and P32-P36) and 3 HD using a 32P-labeled human immunodeficiency virus-long terminal repeat tandem κB oligonucleotide as a probe. (B) For supershift analysis, total extracts from 2 CLL patients (P35 and P32) were incubated with the indicated antibodies before incubation with the labeled probe. (C) Analysis of NF-κB DNA-binding activity in Daudi cells. Electrophoretic mobility shift assays were performed with nuclear extracts from Daudi cells in 3 independent experiments. Daudi cells were treated for 8 hours with the NF-κB inhibitor BAY 11-7082 at 5μM (referred as BAY, left) or for 2 hours with the wild-type NEMO binding domain peptide at 20μM (referred as NBD, right). DMSO or mNBD was used as a control. (D-E) Expression of Aiolos and antiapoptotic molecules in NF-κB inhibitor-treated Daudi and CLL cells. The expression of different genes was analyzed by qRT-PCR and normalized by use of the 2−ΔCt calculation method (Ct indicates cycle threshold) and β-actin as reference gene. Daudi and CLL cells were treated with BAY 11-7082 (5μM, 8 hours; panels D-E left) or NBD peptide (20μM, 2 hours; panels D-E right). CLL cells from 2 (P1 and P37) and 4 (P1 and P38-40) patients were treated with BAY and NBD, respectively. DMSO or mNBD was used as controls. Results are expressed as fold down- or up-regulation of inhibitor-treated cells compared with control cells. Data are mean ± SEM of at least 3 independent experiments. (F) Aiolos expression at the protein level was analyzed by Western blotting in nuclear (N) or cytosolic (C) extracts isolated from Daudi cells treated or not with BAY (5μM, 8 hours). Fibrillarin and β-actin expression were used as internal control of the nuclear and cytosolic fraction purity, respectively. Densitometric analysis of nuclear proteins and molecular weight of the proteins are shown. Similar results were obtained in 2 independent experiments.
All pharmacologic NF-κB inhibitors potentially suffer from a lack of selectivity and off-target effects, and we therefore used 2 distinct NF-κB pathway inhibitors, BAY 11-7082 (referred as BAY) and NBD peptide. The pharmacologic inhibitor BAY inhibits the tumor necrosis factor-α–inductible phosphorylation of IκBα, whereas the NEMO binding domain peptide disrupts the association of NEMO (or IKKγ) with IKKβ and therefore blocks tumor necrosis factor-α–induced NF-κB activation. The inhibitors efficiency was tested on the human Burkitt lymphoma Daudi cells, which present a strong NF-κB DNA binding activity and a high level of Aiolos expression. Figure 5C shows the specific inhibition of NF-κB activation in Daudi cells upon treatment with BAY or NBD peptide. As expected, treatment with DMSO and the mNBD peptide had no significant effect on NF-κB activation. We then analyzed the expression of Aiolos and several antiapoptotic molecules upon treatment of the Daudi cell line (Figure 5D) and B-CLL cells (Figure 5E) with BAY (Figure 5D-E, left) or NBD peptide (Figure 5D-E, right). All the analyzed genes, Bcl-XL, Bcl-2, Bfl-1, TRAF1, TRAF2, XIAP, and Bcl-w, have been reported to be NF-κB target genes. Upon treatment with BAY, a down-regulation of Aiolos, Bcl-w, Bcl-XL, Bcl-2, Bfl-1, TRAF1, and TRAF2 expression was observed in the Daudi cells (Figure 5C, left). Similarly, Aiolos, Bcl-2, Bfl-1, TRAF1, and Bcl-XL expression was down-regulated upon treatment with NBD (Figure 5D, right). Treatment of B cells from 2 CLL patients by the inhibitor BAY strongly inhibits expression of Aiolos, Bcl-2, Bfl-1, Bcl-w, TRAF1, and Bcl-xL (Figure 5E, left). Similarly, expression of Aiolos, Bcl-2, TRAF1, and Bcl-XL was down-regulated upon NBD treatment of cells from 3 CLL patients (Figure 5E, right). The inhibition of Aiolos expression upon BAY treatment was also confirmed at the protein level in nuclear extracts (N) of Daudi cells (Figure 5F). Taken together, these results strongly suggest the implication of NF-κB in the control of Aiolos expression in CLL cells.
Aiolos is involved in the survival of cells from CLL patients
NF-κB is responsible for up-regulating gene products that control cell survival. Furthermore, we have previously shown that Aiolos is involved in the control of Bcl-2 expression in a lymphokine-dependent murine T-cell line.25 To analyze the implication of Aiolos in the control of apoptosis in CLL cells, we inhibited Aiolos expression in PBMCs from CLL patients by specific Aiolos siRNA followed by analysis of the expression of antiapoptotic genes. Aiolos siRNA did not completely abolish Aiolos expression, but attenuated Aiolos message levels by approximately 50%. Interestingly, Aiolos knockdown also resulted in decreased levels of Bcl-XL, Bcl-2, Bfl-1, TRAF1, and Bcl-w transcripts in PBMCs from CLL patients (Figure 6A left). We then analyzed the effect of Aiolos overexpression on these antiapoptotic genes. Ectopic overexpression of Aio-1 wild-type isoform in PBMCs from CLL patients induces a strong increase of Bcl-2 expression and a more modest but significant induction of Bcl-w and Bcl-XL mRNA expression. The effect on Bfl-1 and TRAF1 expression was not significant (Figure 6A right). We further analyzed the effect of Aiolos overexpression on apoptosis by PI and AV/PI staining. Transfection per se with pcDNA-Flag vector (control) or pcDNA-Flag-Aio-1 vector (Aiolos expression) in PBMC cells from CLL patients induced approximately 16% and 14% of apoptosis, respectively. When transfected cells were stimulated with PMA/ionomycin, the average level of apoptosis reached approximately 26% in cells with pcDNA3.1-Flag vector and 14% in cells with pcDNA3.1-Flag-Aio-1 vector. At same time, the proportion of viable cells was more elevated in cells with pcDNA3.1-Flag-Aio-1 vector (41% vs 54%; Figure 6B). These results should be verified on a larger group of CLL patients. They demonstrate that Aiolos overexpression confers CLL cells resistance to PMA/ionomycin-induced apoptosis. These results were confirmed by measurement of caspase activity (data not shown). Taken together, our data suggest that Aiolos may be involved in apoptosis regulation of CLL cells.
Effect of Aiolos on apoptosis in B cells from CLL patients. (A) Influence of Aiolos knockdown and Aiolos overexpression on antiapoptotic gene expression analyzed by qRT-PCR and normalized by use of the 2−ΔCt calculation method (Ct indicates cycle threshold) and β-actin as reference gene. Results are expressed as fold down- or up-regulation of transfected cells by pcDNA3.1-Flag-Aio-1 vector or Aiolos siRNA compared with transfected cells by pcDNA3.1-Flag vector or nontargeting negative control siRNA, respectively. The histograms represent the mean ± SD of genes down-regulation (n = 3, left) or up-regulation (n = 2, right). (B) PMA/ionomycin-induced cell death assayed by AV/PI double staining and flow cytometry. B cells from CLL patients transfected with pcDNA-Flag-Aio-1 or pcDNA-Flag vector. Eighteen hours after transfection, cells were stimulated by PMA and ionomycin (10 ng/mL and 1 μg/mL) for 8 hours before apoptosis analysis. Representative dot plots show AV/PI staining on B cells from patient P44. Percentages of viable (AV−/PI−), apoptotic (AV+/PI−), secondary apoptotic/necrotic (AV+/PI+), and necrotic (AV−/PI+) populations are indicated (left). The histograms represent the mean proportions of AV−/PI−, AV+/PI−, AV+/PI+, and AV−/PI+ cells obtained with cells from 5 patients (P37-39, P43, P44; right).
Effect of Aiolos on apoptosis in B cells from CLL patients. (A) Influence of Aiolos knockdown and Aiolos overexpression on antiapoptotic gene expression analyzed by qRT-PCR and normalized by use of the 2−ΔCt calculation method (Ct indicates cycle threshold) and β-actin as reference gene. Results are expressed as fold down- or up-regulation of transfected cells by pcDNA3.1-Flag-Aio-1 vector or Aiolos siRNA compared with transfected cells by pcDNA3.1-Flag vector or nontargeting negative control siRNA, respectively. The histograms represent the mean ± SD of genes down-regulation (n = 3, left) or up-regulation (n = 2, right). (B) PMA/ionomycin-induced cell death assayed by AV/PI double staining and flow cytometry. B cells from CLL patients transfected with pcDNA-Flag-Aio-1 or pcDNA-Flag vector. Eighteen hours after transfection, cells were stimulated by PMA and ionomycin (10 ng/mL and 1 μg/mL) for 8 hours before apoptosis analysis. Representative dot plots show AV/PI staining on B cells from patient P44. Percentages of viable (AV−/PI−), apoptotic (AV+/PI−), secondary apoptotic/necrotic (AV+/PI+), and necrotic (AV−/PI+) populations are indicated (left). The histograms represent the mean proportions of AV−/PI−, AV+/PI−, AV+/PI+, and AV−/PI+ cells obtained with cells from 5 patients (P37-39, P43, P44; right).
Discussion
Aiolos is a transcription factor mainly expressed in mature B cells. Given its expression pattern, we decided to study the contribution of this transcription factor to CLL pathogenesis. This disease is characterized by the monoclonal expansion of B lymphocytes in the peripheral blood, bone marrow, and lymphoid organs with an indolent course that can become aggressive or even fatal.12 Current knowledge of the pathogenesis is limited because no specific genetic alteration has yet been associated with this disease.26 In this study, we showed an overexpression of total Aiolos transcripts and proteins in cells from CLL patients compared with HDs. The increase in Aiolos expression in CLL does not seem to be related to patients' age. Aiolos expression levels do not correlate with the well-known CLL clinical markers such as IgVH gene status, high ZAP-70 expression, or high CD38 expression. It is known that Aiolos proteins exhibit an isoform-specific cell distribution, the dominant-negative isoform being mainly localized in the cytoplasm.4 We observed by confocal microscopy that the Aiolos protein show a nuclear localization both in normal and B cells from CLL patients. Taken together, these data show that Aiolos deregulation in CLL, in contrast to Ikaros in ALL,27 CML,28 and AML,29 might be caused by an overexpression of the all Aiolos isoforms and not to an overexpression of a dominant-negative isoform, which would preferentially localize in the cytoplasm.
We have recently proposed that epigenetic mechanisms, such as DNA methylation and histone modifications, could control the activity of its promoter.22 Our previous study showed that different mechanisms trigger Aiolos repression in tumor cell lines and hematopoietic primary cells. DNA methylation seems to be an important epigenetic modification controlling Aiolos expression and chromatin modifications in tumor cell lines whereas histone modifications are the main epigenetic modification in hematopoietic primary cells. In a preliminary work, on the basis of 4 CLL patients and 1 HD,9 we observed no significant modifications of the H3K4me3 and H3K9ac throughout the Aiolos promoter between CLL patients and HDs. In this study, realized on a new larger group of CLL patients, we were able to correlate Aiolos expression in CLL cells with an increase of enriched euchromatin associated markers, such as H3K4me2, H3K4me3, and H3K9ac. The statistically significant enrichment in H3K4me2 and H3K4me3 confers an open chromatin status at the Aiolos promoter, resulting in an increase of its transcriptional activity in CLL cells compared with HDs. Furthermore, we observed for the first time an up-regulation of the SMYD3 transcripts in B cells from CLL patients compared with HDs. SMYD3 is a H3K4 specific dimethyltransferase and trimethyltransferase and plays an important role in oncogenesis.30-32
The epigenetic mechanisms are very important in the Aiolos activity, not only at the level of its transcriptional regulation,22 but also as means to exercise its own function.33 Recently, it was shown that Aio-1 wild-type isoform interacts with the deacetylation and chromatin-remodeling complex Mi2/NuRD, colocalizing either with the HDAC1 or with the MTA2 subfraction. However, Aio-1 associates specifically with the promoter of SIRT1, a class III nicotinamide adenine dinucleotide–dependent histone deacetylase that is thought to participate in the formation of facultative heterochromatin.4 Furthermore, induction of Aio-1 expression in Aiolos negative cell line results in a global acetylation decrease of histones H3 and H4 and of specific lysine residues, such as K8 and K16 of histone H4 and K9 of histone H3.4 The Aiolos overexpression could therefore have a global impact on acetylation levels and contribute to epigenetic alterations in CLL cells. Global and gene-specific aberrant DNA methylation34 has been described for genes that are specifically deregulated in CLL such as those encoded for Bcl-2,35 TCL1 (T cell leukemia/lymphoma1),36 ZAP70,37 and DAPK1 (ie, Death-associated protein kinase 1).38 However, it is difficult to determine whether the epigenetic deregulations in CLL are the cause or the consequence of the tumoral transformation.
The open chromatin status at the Aiolos promoter in CLL might allow its upstream effectors to gain access to promoter (Figure 7). The increased activity or amount of the factors controlling, directly or indirectly, Aiolos expression in CLL patients may be the origin of Aiolos up-regulation. In a previous study, we showed the role of Ikaros and NF-κB transcription factors, which have binding sites on the Aiolos promoter, in the control of Aiolos expression.24 Contrary to the data observed in ALL,27 CML28 and AML,29 Ikaros does not seem deregulated at expression level regarding its total transcripts. Modifications in its cellular localization or in its association with chromatin-remodeling complexes could however be involved in controlling Aiolos expression.
Hypothetical model of the mechanisms involved in regulation of Aiolos transcription and consequence of its overexpression on the cell survival in leukemogenesis of CLL. The chromatin status at the Aiolos promoter in CLL is defined by the demethylation of DNA and an enrichment of euchromatin associated histone markers, compared with normal B cells. These epigenetic modifications should allow its upstream effectors, such as NF-κB, constitutively activated in CLL, to gain access to promoter, resulting overexpression of Aiolos. Ikaros does not seem deregulated at its expression level in CLL. This Aiolos deregulation could participate in the survival of cells from CLL patients.
Hypothetical model of the mechanisms involved in regulation of Aiolos transcription and consequence of its overexpression on the cell survival in leukemogenesis of CLL. The chromatin status at the Aiolos promoter in CLL is defined by the demethylation of DNA and an enrichment of euchromatin associated histone markers, compared with normal B cells. These epigenetic modifications should allow its upstream effectors, such as NF-κB, constitutively activated in CLL, to gain access to promoter, resulting overexpression of Aiolos. Ikaros does not seem deregulated at its expression level in CLL. This Aiolos deregulation could participate in the survival of cells from CLL patients.
Aiolos promoter possesses also binding sites for NF-κB transcription factor that is constitutively activated in B-CLL cells,23,39-41 and the binding activity of p65 was found to be a prognostic marker in CLL.42 We confirmed the constitutive activation of p65/p50 in 6 of 7 CLL patients studied. The strong NF-κB activity was associated with a high expression of the Aiolos protein. The inhibition of NF-κB activity resulted in a down-regulation of the Aiolos expression. These results suggest that Aiolos may be a direct or indirect target of NF-κB transcription factor and acts in survival/proliferation mechanisms.
Several evidence strongly support the view that Aiolos is involved in regulation of B-cell apoptosis. Aiolos overexpression rescues the number of viable cells, decreases the number of apoptotic cells and induces overexpression of some Bcl-2 family members. Aiolos knockdown down-regulates expression of some Bcl-2 family members and Aiolos has been implicated in the direct control of the Bcl-2 gene promoter activity in murine T cells.25 Furthermore, the absence of Aiolos accelerates avian premature B-cell apoptosis mediated by B-cell antigen receptor (BCR) signaling.43,44 CLL is considered to be a disease in which apoptosis was deregulated, suggesting that Aiolos deregulation could contribute to leukemogenesis by suppressing B-cells apoptosis, through up-regulation of the Bcl-2 antiapoptotic proteins. The antiapoptotic effect of Aiolos protein in CLL cells is in contradiction to the data observed in Aiolos-deficient mice that develop B lymphoma7 and to the confirmed function of the wild-type isoform Ik-1 as a tumor suppressor gene.45 Activity of the wild-type isoform Aio-1 in CLL is more similar to that of the dominant-negative Ik-6.
Induction of apoptosis in tumoral cells is an efficient antitumoral strategy. Several preclinical studies indicate that NF-κB is a relevant target in CLL, because inhibition of its activation, leads to apoptosis of CLL cells.46,47 Although the exact mechanism of the aberrant NF-κB activity in CLL remains unresolved, it is well known that NF-κB is activated downstream of BCR activation. The BCR components are weakly expressed on the surface of CLL cells, one of the main characteristics of this malignant hemopathy.48,49 Aiolos is required for the pre-BCR control by regulating λ5 expression at the transition from the pre-BI to pre-BII stage50 and for the inhibition of the threshold of BCR activation in part by modulating tyrosine kinase Btk in murine model.7 Unlocking the mechanism by which Aiolos affects gene expression could be critical for improving antitumoral therapy. Moreover, a better understanding of the molecular consequences of Aiolos overexpression can provide insight into the network of deregulated gene expression and signaling pathways that contribute to CLL pathogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Inserm and Spanish Ministry of Science. K.B. and F.C. are supported by a doctoral fellowship from the French Minister of Education. J.S. and M.-E.H. are supported by CNRS and Institut Curie. V.B. is supported by Agence Nationale pour la Recherche, Association pour la recherche sur le Cancer, Belgian InterUniversity Attraction Pole, Cancéropole Ile-de-France, Institut National du Cancer, and Université Paris Descartes.
Authorship
Contribution: K.B. designed the study, performed experiments, analyzed data, and wrote the original draft of the manuscript; J.S. performed the functional study on apoptosis by flow cytometry; F.C. performed EMSA; I.A. contributed to the discussion; H.M.-B. provided the clinical samples of CLL patients; H.M.B., M.-E.H., D.M., and V.B. contributed to scientific discussion and improving the manuscript; and A.R. initiated the study, analyzed the results, participated in the discussion, and improved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angelita Rebollo, Hôpital Pitié Salpêtrière, Bâtiment CERVI, Inserm UMR-S 945, 83, bd de l'hôpital 75013 Paris, France; e-mail: angelita.rebollo@upmc.fr.