Abstract
Ras/MEK/ERK pathway activation represents an important compensatory response of human multiple myeloma (MM) cells to checkpoint kinase 1 (Chk1) inhibitors. To investigate the functional roles of Src in this event and potential therapeutic significance, interactions between Src and Chk1 inhibitors (eg, UCN-01 or Chk1i) were examined in vitro and in vivo. The dual Src/Abl inhibitors BMS354825 and SKI-606 blocked Chk1-inhibitor–induced extracellular signal-regulated kinase 1/2 (ERK1/2) activation, markedly increasing apoptosis in association with BimEL up-regulation, p34cdc2 activation, and DNA damage in MM cell lines and primary CD138+ MM samples. Loss-of-function Src mutants (K297R, K296R/Y528F) or shRNA knock-down of Src prevented the ERK1/2 activation induced by Chk1 inhibitors and increased apoptosis. Conversely, constitutively active Ras or mitogen-activated protein kinase/ERK kinase 1 (MEK1) significantly diminished the ability of Src inhibitors to potentiate Chk1-inhibitor lethality. Moreover, Src/Chk1-inhibitor cotreatment attenuated MM-cell production of vascular endothelial growth factor and other angiogenic factors (eg, ANG [angiogenin], TIMP1/2 [tissue inhibitor of metalloproteinases 1/2], and RANTES [regulated on activation normal T-cell expressed and secreted]), and inhibited in vitro angiogenesis. Finally, coadministration of BMS354825 and UCN-01 suppressed human MM tumor growth in a murine xenograft model, increased apoptosis, and diminished angiogenesis. These findings suggest that Src kinase is required for Chk1-inhibitor–mediated Ras → ERK1/2 signaling activation, and that disruption of this event sharply potentiates the anti-MM activity of Chk1 inhi-bitors in vitro and in vivo.
Introduction
Multiple myeloma (MM) is a neoplastic disorder of mature, differentiated B lymphocytes. Whereas recent insights into MM molecular pathogenesis prompted the introduction of effective new agents, including the proteasome inhibitor bortezomib and the immunomodulatory agents thalidomide and lenalidomide, MM remains largely incurable1 and new strategies are clearly needed.
DNA-damage checkpoints halt cell-cycle progression after extrinsic DNA damage (eg, by genotoxic agents or radiation) or intrinsic DNA-replication stress during the undisturbed cell cycle, permitting DNA-repair machinery initiation or DNA-replication block circumvention.2 Checkpoint responses are initiated by ATM (Ataxia telangiectasia mutated) and ATR (Ataxia telangiectasia and Rad3-related), which induce checkpoint kinases (Chk1 and Chk2), thus disabling Cdk1/p34cdc2 or Cdk2 by preventing dephosphorylation at inhibitory sites (T14/Y15) via inhibition/degradation of Cdc25 phosphatases, resulting in cell-cycle arrest. Genomic instability and defective DNA-damage checkpoints are characteristic of diverse human cancers, including MM.3 Chk1 has a critical role in the DNA-damage–response network.2 Moreover, novel Chk1 functions in the DNA-replication checkpoint, the mitotic-spindle checkpoint, and DNA repair have been identified,2,4 stimulating clinical development of multiple Chk1 inhibitors, including UCN-01 (Kyowa), AZD7762 (AstraZeneca), LY2603618 (Lilly), SCH900776 (Schering-Plough), and PF-00477736 (Pfizer). Whereas these efforts have focused on chemotherapy or radiation sensitization,2,5,6 recent evidence implicating Chk1 in normal cell-cycle checkpoints (eg, the DNA replication checkpoint) suggests alternative therapeutic strategies.
We previously reported that Chk1 inhibitors (eg, UCN-01 or more specific Chk1 inhibitors) activate extracellular signal-regulated kinase 1/2 (ERK1/2) in human MM and leukemia cells, while blockade of this event by MEK1/2 (mitogen-activated protein kinase [MAPK]/ERK kinase 1/2) inhibitor dramatically induces apoptosis.7,8 Furthermore, interruption of Ras function by farnesyltransferase inhibitors9,10 or statins11 acted similarly. Because Src plays an important role in Ras → ERK1/2 signaling activation,12 the possibility that Src may be involved in Chk1-inhibitor–mediated ERK1/2 activation arose. Src family kinases (SFKs) are up-regulated/activated in multiple human tumors.13 Src itself has been implicated in transformation, survival, proliferation, adhesion, migration, invasion,12,13 and angiogenesis.14 Src is generally activated by receptor tyrosine kinases or integrin-related kinases (eg, focal adhesion kinase [FAK]).13 Src signals downstream to multiple survival pathways, including Ras/Raf/MEK/ERK and PI3K/Akt.12 In MM, SFKs have been linked to growth factor (eg, interleukin-6 [IL-6])–mediated survival signaling,15 and selective SFK inhibitors (eg, PP2) inhibit MM-cell proliferation.16 Recently, Src inhibitors (eg, BMS354825) were shown to inhibit angiogenesis and the proliferative/survival effects of growth factors, including vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), in MM cells.17 Src is involved in angiogenesis through VEGF production regulation18 and transduction of VEGF-mediated signals in tumor-associated endothelial cells.17 MM cells produce VEGF, which contributes to MM progression directly by promoting tumor-cell survival (an autocrine mechanism) and by stimulating tumor-derived angiogenesis.19 Interestingly, the anti-MM activities of thalidomide, lenalidomide, and bortezomib have been attributed to antiangiogenic effects.20,21 All of these findings provide a rationale for developing Src inhibitors in MM.22
The relationship between Src and Ras → ERK signaling13 suggested that disruption of Src function might potentiate Chk1-inhibitor lethality. In this study, pharmacologic and genetic approaches demonstrated that clinically relevant Src/Abl inhibitors, including BMS354825 (dasatinib)23 and SKI-606 (bosutinib),24 abrogate Chk1-inhibitor–induced ERK1/2 activation in association with multiple downstream lethal events (eg, Bim up-regulation/activation, enhanced DNA damage, and p34cdc2 activation), culminating in pronounced MM-cell apoptosis in vitro and in vivo. The present results also suggest that inhibition of angiogenesis may contribute to the in vivo activity of Src/Chk1-inhibitor regimens.
Methods
Cells and reagents
Human MM cells (U266, RPMI 8226, H929, MM.1S, and MM.1R) were maintained as reported previously.8 All experiments used logarithmically growing cells (3-5 × 105cells/mL). UCN-01 was provided by the Cancer Therapy Evaluation Program (National Cancer Institute, Bethesda, MD). The dual Src/Abl inhibitor BMS354825 (dasatinib) was provided by Bristol-Myers Squibb. The Chk1 inhibitor Chk1i, the dual Src/Abl inhibitor SKI-606 (bosutinib), and the SFK inhibitor PP2 and its negative control PP3 were purchased from Calbiochem. Agents were dissolved in dimethylsulfoxide (DMSO) and stored at −20°C. Final DMSO concentrations did not exceed 0.1%. Recombinant human VEGF165 was from PeproTech.
Primary CD138+ MM cells
Bone-marrow samples were obtained with informed consent from 3 patients with MM undergoing routine diagnostic procedures. CD138+ cells were isolated using an immunomagnetic bead separation method, as described previously.8 These studies were approved by the institutional review board of Virginia Commonwealth University.
Plasmids and stable transfection
cDNAs for human wild-type (wt) c-Src and loss-of-function mutants (including kinase-inactive K297R and dominant-negative K296R/Y528F), constitutively active (CA)–H-Ras (Q61L) and -MEK1 (serine 218 and 222 mutated to aspartic acid) were obtained from Upstate Biotechnology. SureSilencing plasmids encoding shRNA targeting human c-Src were purchased from SA Biosciences. U266 cells were transfected using the Amaxa Nucleofector and Cell-line Specific Kit C (program X-005; Amaxa) per the manufacturer's instructions. Clones ectopically expressing Src (wt or mutants), CA-Ras or CA-MEK1, or down-regulated Src were selected by G418.
Flow cytometry
Apoptosis, viability, and mitochondrial membrane potential (ΔΨm) were evaluated by flow cytometry after staining with annexin V/fluorescein isothiocyanate/propidium iodide, 7-amino-actinomycin D, or DiOC6, respectively, as described previously.9
Western blot analysis
Samples from whole-cell pellets were prepared and 30μg of protein for each condition was subjected to Western blot analysis, as described previously.7 The stripped or parallel blots were probed with antibodies against β-actin (Transduction Laboratories) or α-tubulin (Calbiochem) to ensure equal loading and transfer. The following primary antibodies were used: phospho-Src (Y418), phospho-paxillin (Y118), phospho-p130Cas (Y410), phospho-FAK (Y576/577), FAK, phospho-PYK2 (Y402) PYK2, phospho-p44/42 MAPK (ERK1/2, T202/Y204), p44/42 MAPK, and cleaved poly(ADP-ribose) polymerase (PARP; Cell Signaling Technology); c-Src, Hck, Lyn, Fyn, H-Ras, MEK1, phospho-p34cdc2 (Y15), p34cdc2, VEGF, angiogenin 1 (ANG1), and tissue inhibitor of metalloproteinases 1 (TIMP-1; Santa Cruz Biotechnology); phospho-histone H2A.X (S139), phospho-Src (Y215), and phospho-tyrosine (4G10; Upstate Biotechnology); Bim (Calbiochem); Bim (ProSci); and PARP (BIOMOL Research Laboratories).
Angiogenic factor array
Angiogenic factor array was performed using RayBio Human Angiogenesis Antibody Array 1 Kit (RayBiotech) as per the manufacturer's instructions. One milliliter of cell culture medium per condition was analyzed using serum-containing medium as a control.
Analysis of VEGF expression by ELISA and quantitative PCR
VEGF levels in cell-culture media were determined using a human VEGF enzyme-linked immunosorbent assay (ELISA) kit (Signosis) as per the manufacturer's instructions. Quantitative polymerase chain reaction (qPCR) analysis using TaqMan gene expression assays and the 7900HT real-time PCR system (Applied Biosystems) were used to quantify human VEGF expression in MM cells (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Reference for quantitation was human β-actin.
In vitro angiogenesis assay
The endothelial tube formation assay kit and human umbilical vein endothelial cells (HUVECs; Invitrogen) were used to assess drug treatment effects on angiogenesis in vitro as per the manufacturer's instructions.
Animal studies
Animal studies were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee, and performed in accordance with current regulations and standards of the U.S. Department of Agriculture, the U.S. Department of Health and Human Services, and the National Institutes of Health. Female athymic NCr-nu/nu mice were purchased from The Jackson Laboratory, and inoculated subcutaneously with 107 MM.1S cells into the right rear flank. Treatment was administrated daily after tumors reached a volume of ∼100 mm.3 BMS354825 was freshly prepared in a 50:50 mixture of propylene glycol (Sigma-Aldrich) and H2O for a final dose of 50 mg/kg.25 UCN-01 in DMSO was diluted in 2% (wt/vol) Na citrate (pH 3.5) for a final dose of 0.5 mg/kg.26 Control animals were injected with equal volumes of vehicle. Tumors were monitored every 2 days by 3 operators. Tumor volumes were calculated from the formula (L × W2)/2, with L and W representing length and width, respectively.
TUNEL and immunohistochemistry
For animal studies, after 14 days of drug treatment, tumors were removed 30 minutes after the final dose. Tissue cryostat sections were prepared for immunohistochemical staining for the vascular endothelial marker CD3117 using CD31 antibody (Oncogene Research Products). To assess apoptosis, tumor sections were stained for terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL) using the In Situ Cell Death Detective Kit (fluorescein; Roche) as per the manufacturer's instructions.26 Images were captured using an Olympus BX40 fluorescence microscope at 20×/0.50 (Olympus) and a CE digital camera (Alpha Innotech) with RS Image software version 1.7.3 (Roper Scientific Photometrics).
Statistical analysis
Values represent the means ± SD, and significance of differences between experimental variables was determined using the Student t test. Analysis of synergism was performed according to median dose–effect analysis using the software program Calcusyn Version 1.1 (Biosoft).9
Results
Src inhibitors block Chk1-inhibitor–induced ERK1/2 activation
Exposure (24 hours) of U226, RPMI 8226, MM.1S, and MM.1R cells to the Chk1 inhibitor UCN-01 (100-150nM) resulted in ERK1/2 phosphorylation/activation (Figure 1A), which is consistent with previous findings.8 Notably, coadministration of the dual Src/Abl inhibitor BMS354825 substantially attenuated UCN-01–induced ERK1/2 phosphorylation in a dose-dependent manner (0.5-2.5μM [Figure 1A] and 50-200nM [supplemental Figure 1A]). Similar results were observed with another Src/Abl inhibitor, SKI-606 (Figure 1B) or the selective Chk1 inhibitor Chk1i26 (supplemental Figure 1B).
Src inhibitors block ERK1/2 activation in human MM cells exposed to UCN-01, in association with synergistic induction of apoptosis. (A-B) Human MM cells were exposed (24 hours) to UCN-01 (U266, 150nM; RPMI 8226, MM.1S, and MM.1R, 100nM) ± BMS354825 (0.5-2.5μM [A]) or SKI606 (U266 and RPMI 8226, 2μM; MM.1S and MM.1R, 1μM [B]), after which Western blot analysis was performed to detect expression of total and/or phosphorylated c-Src (Y418) and ERK1/2. (C) RPMI 8226 and U266 cells were treated as described in panel A for 24 hours and 48 hours, respectively. (D) MM.1S and MM.1R cells were exposed to 100nM UCN-01 ± 1μM BMS354825 for 24 hours. (E) MM cell lines were treated as described in (B) for 24 hours (RPMI 8226, MM.1S, and MM.1R) or 48 hours (U266). For C-E, after drug treatment, the percentage of apoptotic (annexin V+) cells was determined by flow cytometry (means ± SD, *P < .01 and **P < .001 vs UCN-01 alone). (C) Inset: RPMI 8226 cells were exposed to a range of BMS354825 (0.5-1.25μM) and UCN-01 (50-125nM) concentrations alone and in combination at fixed ratio (10:1) for 24 hours. At the end of this period, the percentage of annexin V+ cells was determined for each condition. Fractional effect values were obtained by comparing results with those of untreated controls, and median dose-effect analysis was used to characterize the nature of the interaction between these agents. Combination index values less than 1.0 denote a synergistic interaction. The results of a representative experiment are shown; 2 additional studies yielded equivalent results. (F) CD138+ cells isolated from the bone marrow of a patient (patient #2) with MM were exposed (24 hours) to 100nM UCN-01 ± 0.5 or 1.0μM BMS354825, after which the extent of apoptosis was determined by annexin V/propidium iodide (PI) staining and flow cytometry. Values shown represent the percentage of annexin V+ cells including early (annexin V+/PI−, bottom right quadrants) and late apoptosis (annexin V+/PI+, top right quadrants). Two additional experiments yielded equivalent results.
Src inhibitors block ERK1/2 activation in human MM cells exposed to UCN-01, in association with synergistic induction of apoptosis. (A-B) Human MM cells were exposed (24 hours) to UCN-01 (U266, 150nM; RPMI 8226, MM.1S, and MM.1R, 100nM) ± BMS354825 (0.5-2.5μM [A]) or SKI606 (U266 and RPMI 8226, 2μM; MM.1S and MM.1R, 1μM [B]), after which Western blot analysis was performed to detect expression of total and/or phosphorylated c-Src (Y418) and ERK1/2. (C) RPMI 8226 and U266 cells were treated as described in panel A for 24 hours and 48 hours, respectively. (D) MM.1S and MM.1R cells were exposed to 100nM UCN-01 ± 1μM BMS354825 for 24 hours. (E) MM cell lines were treated as described in (B) for 24 hours (RPMI 8226, MM.1S, and MM.1R) or 48 hours (U266). For C-E, after drug treatment, the percentage of apoptotic (annexin V+) cells was determined by flow cytometry (means ± SD, *P < .01 and **P < .001 vs UCN-01 alone). (C) Inset: RPMI 8226 cells were exposed to a range of BMS354825 (0.5-1.25μM) and UCN-01 (50-125nM) concentrations alone and in combination at fixed ratio (10:1) for 24 hours. At the end of this period, the percentage of annexin V+ cells was determined for each condition. Fractional effect values were obtained by comparing results with those of untreated controls, and median dose-effect analysis was used to characterize the nature of the interaction between these agents. Combination index values less than 1.0 denote a synergistic interaction. The results of a representative experiment are shown; 2 additional studies yielded equivalent results. (F) CD138+ cells isolated from the bone marrow of a patient (patient #2) with MM were exposed (24 hours) to 100nM UCN-01 ± 0.5 or 1.0μM BMS354825, after which the extent of apoptosis was determined by annexin V/propidium iodide (PI) staining and flow cytometry. Values shown represent the percentage of annexin V+ cells including early (annexin V+/PI−, bottom right quadrants) and late apoptosis (annexin V+/PI+, top right quadrants). Two additional experiments yielded equivalent results.
Western blot analysis was performed to examine the effects of exposure to Chk1 and Src inhibitors alone or in combination on the phosphorylation of Src and its substrates (eg, paxillin, FAK, and p130Cas).27 Whereas Western blot analysis revealed variable protein expression of SFKs, including Src, Lyn, Fyn, and Hck, in the MM cell lines evaluated (eg, U266, RPMI 8226, MM.1S, and MM.1R) (supplemental Figure 2A), most lines displayed relatively low basal SFK tyrosine phosphorylation (supplemental Figure 2B-C) and Src phosphorylation at the active site Y418 (Figure 1A and supplemental Figure 2D), that reflects both basal activity because of autophosphorylation24 and inducible activation.17 Phosphorylation of the downstream targets paxillin (Y118; supplemental Figure 2D), FAK (Y576/577), and p130Cas (Y410; data not shown) were also low. UCN-01 (Figure 1A and supplemental Figure 2D) or Chk1i (data not shown) induced little or modest increases in SFK and Src (Y418) tyrosine phosphorylation. Moreover, UCN-01 failed to induce Src phosphorylation at other active sites such as Y21528 (supplemental Figure 2E). Nevertheless, both BMS354825 and SKI-606 clearly diminished SFK tyrosine phosphorylation and Src autophosphorylation (Y418), as well as phosphorylation of the downstream targets paxillin (Y118), FAK (Y576/577), and p130Cas (Y410) in various MM cell lines (Figure 1A and supplemental Figure 2D), including U266 cells ectopically expressing wt c-Src (supplemental Figure 2F and Figure 4D). These findings suggest that the clinically relevant Src/Abl inhibitors BMS354825 and SKI-606 block Chk1-inhibitor–induced ERK1/2 activation in MM cells in association with SFK kinase inhibition.
Src inhibitors synergistically potentiate lethality of Chk1 inhibitors
To assess the consequences of blockade of Chk1-inhibitor–induced ERK1/2 activation by Src inhibitors, apoptosis was monitored by annexin V/propidium iodide staining and flow cytometry. Coexposure of U266 (48 hours) or RPMI 8226 (24 hours) to marginally toxic concentrations of UCN-01 (U266, 150nM; RPMI 8226, 100nM) and BMS354825 (0.5-2.5μM) induced significant dose-dependent increases in apoptosis (Figure 1C; P < .01 or P < .001 vs UCN-01 alone). Similar interactions occurred in other MM cells, including MM.1S and dexamethasone-resistant MM.1R cells (Figure 1D, P < .001 vs UCN-01 alone), as well as H929 cells (supplemental Figure 3A). Analogously, minimally toxic SKI-606 concentrations (1-2μM) dramatically increased UCN-01 lethality in various MM cell lines (Figure 1E, P < .001 vs UCN-01 alone). BMS354825 and SKI-606 also interacted with the selective Chk1 inhibitor Chk1i to induce MM-cell apoptosis (supplemental Figure 3B). Moreover, the selective SFK inhibitor PP2, but not the inactive PP3, potentiated UCN-01 lethality in various MM cells (supplemental Figure 3C). Finally, median dose-effect analysis yielded combination index values < 1.0, denoting synergistic interactions between Src and Chk1 inhibitors (Figure 1C inset). Coadministration of BMS354825 and UCN-01 also markedly induced cell death in primary CD138+ MM cells (Figure 1F and supplemental Figure 3D, P < .05 vs UCN-01 alone), findings that were confirmed by morphologic assessment (supplemental Figure 3E), but not in CD138− bone marrow cells (supplemental Figure 3D, P > .05 vs UCN-01 alone).
Src inhibitors attenuate Chk1-inhibitor–mediated Bim inactivation/degradation while promoting p34cdc2 activation and DNA damage
Given evidence implicating BimEL up-regulation, p34cdc2 activation, and DNA damage in potentiation of Chk1-inhibitor lethality by agents disrupting Ras → ERK1/2 signaling,29 parallel studies were performed with Src inhibitors. Exposure of MM cells to UCN-01 (Figure 2A) or Chk1i (supplemental Figure 4A) induced phosphorylation-related BimEL degradation, manifested by diminished expression of the slower migrating phosphorylated form. This was substantially attenuated by BMS354825 (Figure 2A) or SKI-606 (supplemental Figure 4A), resulting in accumulation of the fast-migrating, unphosphorylated active BimEL form.30 SKI-606 (Figure 2B and supplemental Figure 4A) or BMS354825 (supplemental Figure 4B) markedly enhanced dephosphorylation (activation) of p34cdc2 at inhibitory sites induced by marginally toxic concentrations of UCN-01 or Chk1i. Furthermore, coexposure of MM cells to UCN-01 and BMS354825 (Figure 2C) or Chk1i and SKI-606 (supplemental Figure 4A) markedly increased γH2A.X expression, a DNA damage marker,26 and sharply increased PARP degradation (Figure 2D and supplemental Figure 4C). Similar events occurred in primary bone-marrow CD138+ MM cells, but not in their CD138− counterparts (Figure 2E).
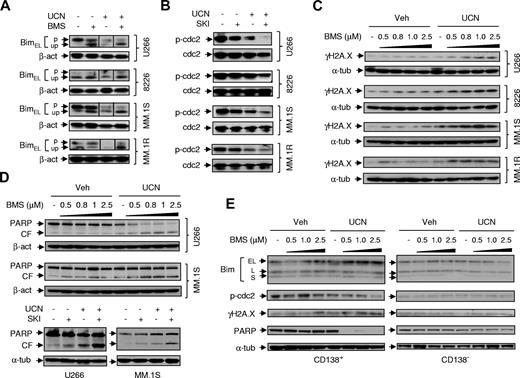
Coexposure to Src inhibitors and UCN-01 leads to unphosphorylated BimEL accumulation, enhanced p34cdc2activation, and increased γH2A.X expression. (A) MM cell lines were exposed (24 hours) to UCN-01 (U266, 150nM; RPMI 8226, MM.1S, and MM.1R, 100nM) ± 1μM BMS354825, after which phosphorylation and expression of BimEL were assessed by Western blot analysis using a Bim antibody (Calbiochem) that is able to distinguish both the phosphorylated (p, slowly migrating) form from the unphosphorylated (up, fast-migrating) form. Vertical lines have been inserted to indicate repositioned gel lanes. (B) After being treated with UCN-01 (U266, 150nM; RPMI 8226, MM.1S, and MM.1R, 100nM) ± SKI606 (U266 and RPMI 8226, 2μM; MM.1S and MM.1R, 1μM), Western blot analysis was performed to examine dephosphorylation/activation of p34cdc2 at the inhibitory site Y15. In parallel, total p34cdc2 was monitored for comparison. (C) Cells were exposed (24 hours) to UCN-01 (U266, 150nM; RPMI 8226, MM.1S, and MM.1R, 100nM) ± the indicated concentrations of BMS354825, after which phosphorylation of the atypical histone H2A.X at S139 (designated γH2A.X) was monitored by Western blot analysis. (D) MM.1S and U266 cells were treated as described in (B) and (C) for 24 or 48 hours, respectively, after which Western blot analysis was performed to monitor PARP cleavage. (E) Primary CD138+ MM cells (Pt2) and their CD138− counterparts were exposed (24 hours) to 100nM UCN-01 ± the indicated concentration of BMS354825, after which expression of Bim using an antibody (ProSci) that recognizes total protein levels of 3 isoforms (EL, L, and S), T14/Y15 phosphorylated p34cdc2, γH2A.X, and PARP were assessed by Western blot analysis. CF indicates cleavage fragment.
Coexposure to Src inhibitors and UCN-01 leads to unphosphorylated BimEL accumulation, enhanced p34cdc2activation, and increased γH2A.X expression. (A) MM cell lines were exposed (24 hours) to UCN-01 (U266, 150nM; RPMI 8226, MM.1S, and MM.1R, 100nM) ± 1μM BMS354825, after which phosphorylation and expression of BimEL were assessed by Western blot analysis using a Bim antibody (Calbiochem) that is able to distinguish both the phosphorylated (p, slowly migrating) form from the unphosphorylated (up, fast-migrating) form. Vertical lines have been inserted to indicate repositioned gel lanes. (B) After being treated with UCN-01 (U266, 150nM; RPMI 8226, MM.1S, and MM.1R, 100nM) ± SKI606 (U266 and RPMI 8226, 2μM; MM.1S and MM.1R, 1μM), Western blot analysis was performed to examine dephosphorylation/activation of p34cdc2 at the inhibitory site Y15. In parallel, total p34cdc2 was monitored for comparison. (C) Cells were exposed (24 hours) to UCN-01 (U266, 150nM; RPMI 8226, MM.1S, and MM.1R, 100nM) ± the indicated concentrations of BMS354825, after which phosphorylation of the atypical histone H2A.X at S139 (designated γH2A.X) was monitored by Western blot analysis. (D) MM.1S and U266 cells were treated as described in (B) and (C) for 24 or 48 hours, respectively, after which Western blot analysis was performed to monitor PARP cleavage. (E) Primary CD138+ MM cells (Pt2) and their CD138− counterparts were exposed (24 hours) to 100nM UCN-01 ± the indicated concentration of BMS354825, after which expression of Bim using an antibody (ProSci) that recognizes total protein levels of 3 isoforms (EL, L, and S), T14/Y15 phosphorylated p34cdc2, γH2A.X, and PARP were assessed by Western blot analysis. CF indicates cleavage fragment.
Loss-of-function Src mutants or Src shRNA knock-down prevents Chk1-inhibitor–induced ERK1/2 activation
To elucidate the functional role of Src kinase in response to Chk1 inhibitors, U266 cells were stably transfected with wt or loss-of-function mutant (kinase-inactive/K297R31 or dominant-negative/K297R/Y528F32 ) forms of human c-Src (Figure 3A). In contrast to wt Src, K297R and K297R/Y528F mutants exhibited neither autophosphorylation of Src (eg, Y418 and Y215) nor phosphorylation of downstream targets (ie, paxillin [Y118], Figure 3B), indicating loss of Src tyrosine kinase function. Both mutants displayed lower basal ERK1/2 phosphorylation compared with Src/wt and empty-vector controls (Figure 3A). Notably, UCN-01 failed to induce ERK1/2 activation in cells expressing either loss-of-function Src mutant (Figure 3C). This was associated with diminished degradation of phosphorylated BimEL, in-creased γH2A.X expression (Figure 3D), and enhanced p34cdc2 dephosphorylation/activation (supplemental Figure 4D), accompanied by increased PARP cleavage (Figure 3C). Similar results occurred in U266 cells with Src shRNA knock-down (24 hours, Figure 3E), which also exhibited a marked increase in UCN-01 lethality (48 hours, supplemental Figure 4E). These findings provide genetic evidence that Src kinase function is required for Chk1-inhibitor–induced ERK1/2 activation in MM cells.
Loss-of-function Src mutants or shRNA knock-down prevent ERK1/2 activation and sensitize MM cells to UCN-01, whereas constitutively active Ras or MEK1 diminishes potentiation of UCN-01 lethality by SKI-606. (A) U266 cells were stably transfected with wt or loss-of-function mutants (K297R, kinase-inactive; K296R/Y528F, dominant-negative) of human c-Src. Western blot analysis indicated ectopic expression of these proteins and ERK1/2 phosphorylation. EV, Empty-vector controls. (B-D) U266 cells bearing either wt or mutant c-Src were incubated with the indicated concentrations of UCN-01 for 24 or 48 hours (for blots of PARP [C]), Western blot analysis was performed to monitor phosphorylation of Src (Y418 and Y215) and its downstream protein paxillin (Y118), ERK1/2 phosphorylation, PARP cleavage, BimEL phosphorylation/expression (Calbiochem), and γH2A.X expression. CF, Cleavage fragment; p, phosphorylated; up, unphosphorylated. (E) U266 cells were stably transfected with 2 plasmids (#1 and #2) encoding shRNA targeting different sequences of the human c-Src gene. Western blot analysis demonstrates down-regulation of Src protein expression as well as diminished basal levels of ERK1/2 phosphorylation compared with cells transfected with a plasmid encoding a negative control sequence (left panels). Cells were then exposed to the indicated concentrations of UCN-01 for 24 hours, after which cells were lysed and subjected to Western blot analysis to monitor phosphorylation of ERK1/2, expression of Bim (ProSci) and γH2A.X, and cleavage of PARP (right panels). (F) U266 cells were stably transfected with constitutively active human H-Ras (Q61L) or MEK1 (CA-MEK1). Western blot analysis demonstrated ectopic expression of the mutant proteins and resulting phosphorylation/activation of downstream targets (eg, MEK1/2 and ERK1/2, respectively). These cells were then exposed to 150nM UCN-01 ± 2μM SKI-606 for 48 hours, after which the percentage of apoptotic (annexin V+) cells was determined by flow cytometry (means ± SD, **P < .001 vs EV controls). For blots in panels A, C, E and F, vertical lines have been inserted to indicate repositioned gel lanes.
Loss-of-function Src mutants or shRNA knock-down prevent ERK1/2 activation and sensitize MM cells to UCN-01, whereas constitutively active Ras or MEK1 diminishes potentiation of UCN-01 lethality by SKI-606. (A) U266 cells were stably transfected with wt or loss-of-function mutants (K297R, kinase-inactive; K296R/Y528F, dominant-negative) of human c-Src. Western blot analysis indicated ectopic expression of these proteins and ERK1/2 phosphorylation. EV, Empty-vector controls. (B-D) U266 cells bearing either wt or mutant c-Src were incubated with the indicated concentrations of UCN-01 for 24 or 48 hours (for blots of PARP [C]), Western blot analysis was performed to monitor phosphorylation of Src (Y418 and Y215) and its downstream protein paxillin (Y118), ERK1/2 phosphorylation, PARP cleavage, BimEL phosphorylation/expression (Calbiochem), and γH2A.X expression. CF, Cleavage fragment; p, phosphorylated; up, unphosphorylated. (E) U266 cells were stably transfected with 2 plasmids (#1 and #2) encoding shRNA targeting different sequences of the human c-Src gene. Western blot analysis demonstrates down-regulation of Src protein expression as well as diminished basal levels of ERK1/2 phosphorylation compared with cells transfected with a plasmid encoding a negative control sequence (left panels). Cells were then exposed to the indicated concentrations of UCN-01 for 24 hours, after which cells were lysed and subjected to Western blot analysis to monitor phosphorylation of ERK1/2, expression of Bim (ProSci) and γH2A.X, and cleavage of PARP (right panels). (F) U266 cells were stably transfected with constitutively active human H-Ras (Q61L) or MEK1 (CA-MEK1). Western blot analysis demonstrated ectopic expression of the mutant proteins and resulting phosphorylation/activation of downstream targets (eg, MEK1/2 and ERK1/2, respectively). These cells were then exposed to 150nM UCN-01 ± 2μM SKI-606 for 48 hours, after which the percentage of apoptotic (annexin V+) cells was determined by flow cytometry (means ± SD, **P < .001 vs EV controls). For blots in panels A, C, E and F, vertical lines have been inserted to indicate repositioned gel lanes.
Constitutively active Ras or MEK1 prevents Src/Chk1-inhibitor interactions
To define the functional role of the canonical Ras → ERK1/2 pathway in Src inhibitor–mediated potentiation of Chk1-inhibitor lethality, U266 cells were stably transfected with constitutively active mutants of either Ras (Q61L) or MEK1 (CA-MEK1). Q61L Ras or CA-MEK1 induced a marked increase in MEK1/2 or ERK1/2 phosphorylation (Figure 3F insets). Both mutants displayed significant impairment in the ability of SKI-606 to potentiate UCN-01 lethality (Figure 3F, P < .01 for each case). Similar results were obtained with other Src inhibitors (eg, PP2 [supplemental Figure 4F] or BMS354825 [data not shown]).
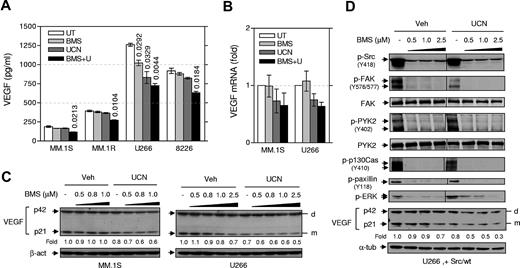
The Src/Chk1-inhibitor regimen reduces VEGF expression and disrupts VEGF-mediated signaling in MM cells
MM cells produce VEGF,20 a growth factor important for both MM-derived angiogenesis33 and MM cell survival/growth.34 qPCR and ELISA indicated that all tested MM cell lines expressed VEGF mRNA (supplemental Figure 5A) and secreted VEGF (Figure 4A and supplemental Figure 5B), respectively. Western blot analysis demonstrated that human MM cells (eg, MM.1S and U266) expressed both VEGF monomer (p21) and dimer (p42) (Figure 4C), a symmetric homodimeric glycoprotein.35,36 Interestingly, UCN-01 treatment clearly reduced VEGF monomer in MM cells (eg, U266 and MM.1S, Figure 4C), consistent with VEGF mRNA down-regulation (Figure 4B) and reduced VEGF secretion (Figure 4A). BMS354825/UCN-01 cotreatment induced more pronounced reductions in VEGF levels in the culture medium (Figure 4A), as well as reductions in VEGF mRNA (Figure 4B) and protein (monomer rather than dimer; Figure 4C) compared with effects of individual treatment. Similar results were observed with SKI-606 (supplemental Figure 4G upper panels). Moreover, genetic disruption of Src function in loss-of-function mutants (particularly K296R/Y528F) modestly but discernibly reduced basal levels of VEGF monomer compared with Src/wt, while UCN-01 further down-regulated VEGF monomers in these mutant cells (supplemental Figure 4G lower panels). Moreover, BMS354825/UCN-01 coadministration also suppressed VEGF expression (particularly the monomer) in Src/wt-expressing U266 cells (Figure 4D). This was associated with disruption of Src-mediated signaling pathways by BMS354825, reflected by substantially diminished autophosphorylation of Src (Y418) and phosphorylation of its substrates (eg, FAK [Y576/577], p130Cas [Y410], and paxillin [Y118]), as well as activation of ERK1/2. Interestingly, in Src/wt-overexpressing cells, BMS354825 also inhibited phosphorylation of another nonreceptor protein tyrosine kinase, PYK2 (Y402, Figure 4D), an event that leads to binding of the SH2 domain of Src to Y402 of Pyk2 and Src activation.37 Finally, Src inhibitors (BMS354825 or SKI-606) also blocked VEGF-induced activation of Src-mediated signaling (eg, p130Cas phosphorylation [Y410] and ERK1/2 activation) in serum-starved MM.1S cells (supplemental Figure 4H). Interestingly, both Src inhibitors diminished UCN-01–induced ERK1/2 activation in serum-starved cells cultured with VEGF165, which was associated with a clear increase in PARP cleavage (supplemental Figure 4H).
Cotreatment with BMS354825 and UCN-01 inhibits VEGF expression in MM cells in association with disruption of Src function. (A) MM cells were exposed (16 hours) to UCN-01 (U266, 150nM; RPMI 8226, MM.1S, and MM.1R, 100nM) ± BMS354825 (0.5μM), after which ELISA assays were performed to monitor VEGF levels, using a standard curve (supplemental Figure 5B), in cell-culture supernatants. Numbers indicate P values versus untreated controls. (B) MM.1S and U266 cells were treated with UCN-01 (MM.1S, 100nM; U266, 150nM) ± 0.5μM BMS354825 for 6 hours (MM.1S) or 16 hours (U266), after which total RNA was isolated and subjected to quantitative PCR analyses to assess human VEGF-A mRNA levels, as described in the supplemental Methods. VEGF expression was expressed as the fold increase relative to values for untreated controls (arbitrarily set at 1). (In panels A and B, results represent the means ± SD for triplicate determinations performed on 3 separate occasions. (C) MM.1S and U266 cells were incubated with UCN-01 (MM.1S, 100nM; U266, 150nM) ± the indicated concentrations of BMS354825 for 16 hours, after which cells were lysed and subjected to Western blot analysis to detect expression of VEGF. d, dimer; m, monomer. (D) U266 cells transfected with wt c-Src were treated with 150nM UCN-01 ± the indicated concentrations of BMS354825 for 16 hours, after which Western blot analysis was performed to monitor phosphorylation of Src (Y418) and its substrates, including FAK (Y576/577), p130Cas (Y410), and paxillin (Y118), as well as phosphorylation of ERK1/2 and PYK2. Vertical lines have been inserted to indicate repositioned gel lanes. In parallel, expression of intracellular VEGF was detected. In panels C and D, the blots for VEGF monomer were quantified by determining integrated density using a densitometer. Values indicate fold changes for drug-treated cells compared with untreated cells.
Cotreatment with BMS354825 and UCN-01 inhibits VEGF expression in MM cells in association with disruption of Src function. (A) MM cells were exposed (16 hours) to UCN-01 (U266, 150nM; RPMI 8226, MM.1S, and MM.1R, 100nM) ± BMS354825 (0.5μM), after which ELISA assays were performed to monitor VEGF levels, using a standard curve (supplemental Figure 5B), in cell-culture supernatants. Numbers indicate P values versus untreated controls. (B) MM.1S and U266 cells were treated with UCN-01 (MM.1S, 100nM; U266, 150nM) ± 0.5μM BMS354825 for 6 hours (MM.1S) or 16 hours (U266), after which total RNA was isolated and subjected to quantitative PCR analyses to assess human VEGF-A mRNA levels, as described in the supplemental Methods. VEGF expression was expressed as the fold increase relative to values for untreated controls (arbitrarily set at 1). (In panels A and B, results represent the means ± SD for triplicate determinations performed on 3 separate occasions. (C) MM.1S and U266 cells were incubated with UCN-01 (MM.1S, 100nM; U266, 150nM) ± the indicated concentrations of BMS354825 for 16 hours, after which cells were lysed and subjected to Western blot analysis to detect expression of VEGF. d, dimer; m, monomer. (D) U266 cells transfected with wt c-Src were treated with 150nM UCN-01 ± the indicated concentrations of BMS354825 for 16 hours, after which Western blot analysis was performed to monitor phosphorylation of Src (Y418) and its substrates, including FAK (Y576/577), p130Cas (Y410), and paxillin (Y118), as well as phosphorylation of ERK1/2 and PYK2. Vertical lines have been inserted to indicate repositioned gel lanes. In parallel, expression of intracellular VEGF was detected. In panels C and D, the blots for VEGF monomer were quantified by determining integrated density using a densitometer. Values indicate fold changes for drug-treated cells compared with untreated cells.
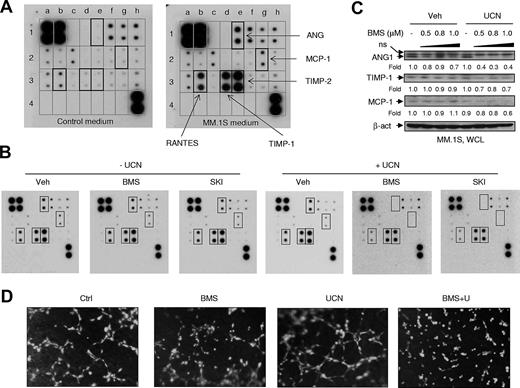
The Src/Chk1-inhibitor regimen inhibits production of angiogenic factors in MM cells and in vitro angiogenesis
In addition to VEGF, human MM cells also produce multiple other angiogenic factors.20,38 Accordingly, angiogenesis antibody arrays (supplemental Figure 5C) were performed using fetal bovine serum (FBS)–containing medium obtained from a 24-hour culture of MM.1S cells (MM.1S medium). Compared with control medium (RPMI 1640 + 10% FBS), MM.1S medium robustly expressed ANG, RANTES (regulated on activation normal T-cell expressed and secreted), TIMP-1, and TIMP-2, and modestly expressed monocyte chemotactic protein 1 (MCP-1) (Figure 5A). In contrast to the detection of VEGF in MM.1S cell-culture medium by ELISA (Figure 4A), of VEGF mRNA expression by qPCR (Figure 4B), or of protein by Western blot analysis (Figure 4C), the antibody array failed to detect VEGF in MM.1S medium (Figure 5A), possibly because of low sensitivity of the latter assay. Whereas treatment with agents alone had minimal effects, cotreatment of MM.1S cells with either BMS354825 or SKI-606 with UCN-01 substantially diminished the production of ANG, RANTES, and MCP-1 and modestly reduced levels of TIMP-1 and TIMP-2 (Figure 5B). Moreover, whereas BMS354825 or UCN-01 alone had little effect, cotreatment discernibly inhibited intracellular expression of ANG1, TIMP-1, and MCP-1 (Figure 5C). Finally, BMS354825/UCN-01 cotreatment dramatically suppressed angiogenesis in vitro, manifested by marked disruption of 3-dimensional tube formation of primary HUVECs cultured on Geltrex Reduced Growth Factor Basement Membrane Matrix (2 hours, Figure 5D; 4 hours, supplemental Figure 5D), in association with diminished pro-liferation and increased apoptosis of HUVECs (supplemental Figure 5E).
Cotreatment with Src inhibitors and UCN-01 inhibits both production of multiple angiogenic factors by MM cells and in vitro tube formation of HUVECs. (A) MM.1S cells were washed twice with serum-free medium and then cultured for 24 hours in fresh RPMI 1640 medium containing 10% FBS, after which the supernatants (MM.1S medium) were prepared by centrifuging twice at 500g for 10 minutes each. Control (fresh RPMI 1640 medium with 10% FBS) and MM.1S media were then subjected to the angiogenesis antibody array. According to the table of corresponding factors (supplemental Figure 5C), the angiogenic factors expressed in MM.1S medium, compared with control medium, are highlighted by black squares. (B) MM.1S cells were incubated (16 hours) with 1μM BMS354825 or SKI-606 ± 100nM UCN-01, after which the same array was performed to assess the effects of the drug treatments on the production of angiogenic factors. (C) In parallel, whole cells were lysed and subjected to Western blot analysis to monitor the expression of corresponding factors. The blots were quantified by determining integrated density. Values indicate fold changes for drug-treated cells compared with untreated cells. WCL, Whole-cell lysate; ns, nonspecific bands. (D) Primary HUVECs were preincubated with the cell-permeable dye Calcein AM, and then seeded and incubated (2 hours) with 0.1μM BMS354825 ± 100nM UCN-01 using reduced growth factor basement membrane matrix. Images were captured using an inverted fluorescent microscope (10× magnification). Results are representative of triplicate experiments.
Cotreatment with Src inhibitors and UCN-01 inhibits both production of multiple angiogenic factors by MM cells and in vitro tube formation of HUVECs. (A) MM.1S cells were washed twice with serum-free medium and then cultured for 24 hours in fresh RPMI 1640 medium containing 10% FBS, after which the supernatants (MM.1S medium) were prepared by centrifuging twice at 500g for 10 minutes each. Control (fresh RPMI 1640 medium with 10% FBS) and MM.1S media were then subjected to the angiogenesis antibody array. According to the table of corresponding factors (supplemental Figure 5C), the angiogenic factors expressed in MM.1S medium, compared with control medium, are highlighted by black squares. (B) MM.1S cells were incubated (16 hours) with 1μM BMS354825 or SKI-606 ± 100nM UCN-01, after which the same array was performed to assess the effects of the drug treatments on the production of angiogenic factors. (C) In parallel, whole cells were lysed and subjected to Western blot analysis to monitor the expression of corresponding factors. The blots were quantified by determining integrated density. Values indicate fold changes for drug-treated cells compared with untreated cells. WCL, Whole-cell lysate; ns, nonspecific bands. (D) Primary HUVECs were preincubated with the cell-permeable dye Calcein AM, and then seeded and incubated (2 hours) with 0.1μM BMS354825 ± 100nM UCN-01 using reduced growth factor basement membrane matrix. Images were captured using an inverted fluorescent microscope (10× magnification). Results are representative of triplicate experiments.
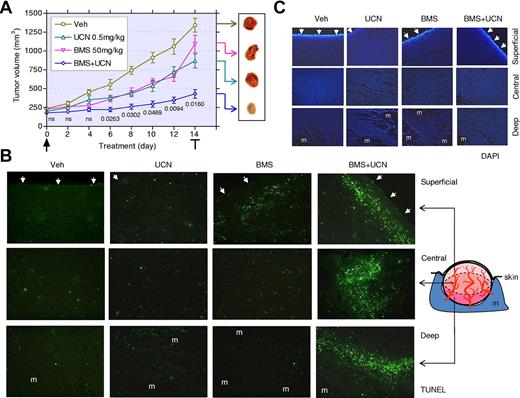
The Src/Chk1-inhibitor regimen induces MM-cell apoptosis and suppresses tumor growth in vivo
To assess the in vivo activity of the Src/Chk1-inhibitor strategy, athymic nude mice were inoculated with MM.1S cells in the flank. After the appearance of measurable tumors (5-7 days), mice were treated daily with BMS354825 (50 mg/kg) ± UCN-01 (0.5 mg/kg). Tumor size was monitored every 2 days. Whereas BMS354825 or UCN-01 administrated alone had modest effects, cotreatment with both agents significantly suppressed tumor growth (Figure 6A, P < .05 after 6-day treatment, compared with UCN-01 alone, n = 8 per group). Weight loss for all conditions was minimal (eg, < 8%; data not shown). After 14 days, tumors were excised and cryosections stained by TUNEL to monitor apoptosis. Microphotographs were taken from superficial, central, and deep regions within tumors (Figure 6B). BMS354825 alone modestly induced apoptosis in the superficial region and had significantly less effect elsewhere, whereas UCN-01 alone had little effect in each region. Cotreatment with BMS354825 and UCN-01 dramatically increased apoptosis in all regions. Figure 6C shows the corresponding 4,6-diamidino-2-phenylindole, dihydrochloride images of the tumor regions.
Coadministration of BMS354825 and UCN-01 markedly suppresses tumor growth in association with a striking induction of MM-cell apoptosis in vivo. (A) NCr-nu/nu mice were subcutaneously inoculated with 107 MM.1S cells in the right rear flank. After tumors were measurable, 50 mg/kg of BMS354825 ± 0.5 mg/kg of UCN-01 were administrated daily for 14 days. Tumor size was monitored every 2 days. Values represent the means ± SD for 8 mice, and numbers indicate P values vs UCN-01 administrated alone. After the final drug dose, tumors were excised (inset). (B-C) Cryosections of the excised tumors were stained by TUNEL with 4,6-diamidino-2-phenylindole, dihydrochloride counter-staining. Fluorescent images were captured at 20×/0.50 magnification in the superficial, central, and deep regions within tumors. Arrows indicate the dermal facet of tumors. m, muscle.
Coadministration of BMS354825 and UCN-01 markedly suppresses tumor growth in association with a striking induction of MM-cell apoptosis in vivo. (A) NCr-nu/nu mice were subcutaneously inoculated with 107 MM.1S cells in the right rear flank. After tumors were measurable, 50 mg/kg of BMS354825 ± 0.5 mg/kg of UCN-01 were administrated daily for 14 days. Tumor size was monitored every 2 days. Values represent the means ± SD for 8 mice, and numbers indicate P values vs UCN-01 administrated alone. After the final drug dose, tumors were excised (inset). (B-C) Cryosections of the excised tumors were stained by TUNEL with 4,6-diamidino-2-phenylindole, dihydrochloride counter-staining. Fluorescent images were captured at 20×/0.50 magnification in the superficial, central, and deep regions within tumors. Arrows indicate the dermal facet of tumors. m, muscle.
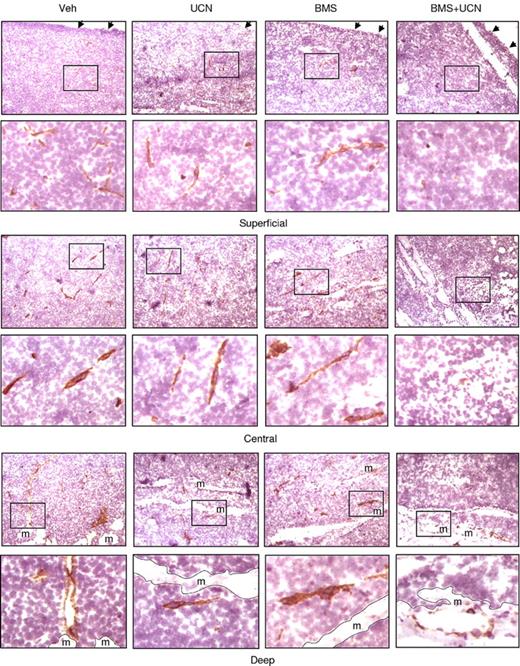
The Src/Chk1-inhibitor regimen diminishes angiogenesis in vivo
The preceding in vitro studies demonstrated that Src/Chk1-inhibitor cotreatment diminished the expression/production of angiogenic factors in MM cells and in vitro angiogenesis of HUVECs, raising the possibility that this regimen might perturb angiogenesis in vivo. To address this, cryosections of excised MM tumors were stained by immunohistochemistry using antibodies specifically recognizing murine CD31, an established vascular endothelial cell marker.17 Microphotographs were obtained from superficial, central, and deep tumor regions (Figure 7). Treatment with BMS354825 alone modestly reduced CD31+ microvessels only in the superficial region, whereas UCN-01 alone had minimal effects. Notably, CD31+ microvessels were essentially absent in all tumor regions after coadministration of BMS354825 and UCN-01. In addition, tumors removed from mice cotreated with BMS354825 and UCN-01 displayed a whitish appearance, suggesting avascularity (Figure 6A inset). Interestingly, regions exhibiting diminished CD31+ microvessels (Figure 7) coincided with those displaying increased apoptosis (TUNEL positivity) (Figure 6B), raising the possibility that that antiangiogenic effects contribute to the marked induction of MM cell apoptosis and thus suppression of tumor growth by the Src/Chk1-inhibitor regimen in vivo.
Coadministration of BMS354825 and UCN-01 inhibits angiogenesis in vivo. Following the experiments described in Figure 6, cryosections of the excised tumors were stained by immunohistochemistry using antibodies specifically recognizing murine CD31, an established vascular endothelial marker. Images were captured at 20×/0.50 magnification in the superficial, central, and deep regions within tumors. For each region, areas marked by black squares (top panels) were amplified and shown in the bottom panels. Dashed lines correspond to the borders between tumor and muscle (m). Arrows indicate the dermal facets of tumors.
Coadministration of BMS354825 and UCN-01 inhibits angiogenesis in vivo. Following the experiments described in Figure 6, cryosections of the excised tumors were stained by immunohistochemistry using antibodies specifically recognizing murine CD31, an established vascular endothelial marker. Images were captured at 20×/0.50 magnification in the superficial, central, and deep regions within tumors. For each region, areas marked by black squares (top panels) were amplified and shown in the bottom panels. Dashed lines correspond to the borders between tumor and muscle (m). Arrows indicate the dermal facets of tumors.
Discussion
Chk1, a key component of the DNA-damage–checkpoint machinery, represents an important therapeutic target within the DNA-damage–response network.2 Chk1 inhibitors, including the prototype UCN-01 and newer, more specific inhibitors, have primarily been developed to potentiate the activity of conventional genotoxic agents or radiation.2,5,6 However, new insights into the DNA-damage checkpoint provide a mechanistic foundation for alternative Chk1-inhibitor strategies. Recent findings indicate that Chk1 plays a critical role not only in checkpoints triggered by exogenous DNA insults (eg, DNA-damaging agents or radiation), but also those activated by endogenous DNA-replication stress.4 Chk1 inhibitors by themselves induce DNA breaks, presumably reflecting aberrant events associated with DNA initiation and/or elongation during DNA replication.39,40 Moreover, Chk1 inhibitors activate compensatory mechanisms (eg, negative feedback responses through activation of the Chk1 upstream kinase ATR39 ), which may limit lethality. We previously reported that Chk1 inhibitors activate the pro-survival MEK/ERK pathway, whereas MEK1/2 inhibitors strikingly potentiate Chk1-inhibitor lethality in human MM8 and leukemia cells.7 This suggests that activation of the Ras/Raf/MEK/ERK pathway represents an important compensatory, cytoprotective response to Chk1 inhibitors, and suggests an alternative strategy for Chk1-inhibitor development.
Recent evidence indicates that Chk1 inhibitors activate ERK1/2 through a Ras-dependent mechanism.26 In this context, Src plays an important role upstream of this signaling cascade.12,13 For example, disruption of Src kinase function by Src inhibitors (eg, PP2 or PP1) or the dominant-negative Src mutant (K296R/Y528F) blocks ERK1/2 activation induced by various stimuli,32,41-43 raising the possibility that Src might be involved in ERK1/2 activation in the present setting. Indeed, interruption of Src kinase function by either pharmacologic (eg, Src/Abl inhibitors BMS354825 or SKI606) or genetic (eg, loss-of-function Src mutants including kinase-inactive K297R,41 and dominant-negative K296R/Y528F,32 or Src shRNA) means markedly attenuated Chk1-inhibitor–induced ERK1/2 activation in MM cells. To date, at least 3 SFK members (ie, Hck, Lyn, and Src) have been implicated in MM-cell growth; of these, Hck and Lyn, in contrast to Src, are predominantly involved in IL-6–induced signaling pathways. For example, exposure of human MM cells to IL-6 triggers tyrosine phosphorylation/activation of Hck, Lyn and Fyn, but not Src.44 Moreover, kinase-inactive mutants of Hck (K269E or K269R), but not Src (K297R), prevent IL-6–mediated activation of ERK1/2 and inhibit MM-cell proliferation.15,16 Recently, exposure of serum-starved MM cells to VEGF165 has been shown to induce activation of Src (manifested by Y418 phosphorylation) and ERK1/2, events abrogated by BMS354825.17 Such findings suggest that the Src → ERK1/2 signaling cascade operates to promote survival mediated by certain growth factors (eg, VEGF and PDGF) in human MM cells. Interestingly, exposure of MM cells to Chk1 inhibitors did not clearly induce Src phosphorylation at its active sites (eg, Y418 and Y215). However, disruption of Src function by pharmacologic inhibitors or specifically by genetic means (ie, loss-of-function Src mutants and Src shRNA) dramatically blocked Chk1-inhibitor–induced ERK1/2 activation, suggesting that Src kinase is required for the compensatory ERK1/2 activation induced by Chk1 inhibitors. While these results provide evidence that Src is required for ERK1/2 activation by Chk1 inhibitors, the possibility that other SFK members may also be involved cannot be excluded.
BMS354825 has recently been shown to inhibit MM-cell proliferation in standard culture medium containing 10% FBS, while inducing MM-cell apoptosis in serum-deprived medium containing VEGF165 or PDGF-BB.17 The latter phenomenon may reflect inhibition of VEGF- and PDGF-BB–mediated signaling (eg, activation of Src and ERK1/2) by BMS354825.17 We also observed that in 10% FBS medium, BMS354825 alone at concentrations < 500nM only reduced MM-cell proliferation, but induced apoptosis at higher concentrations (eg, > 5μM) and longer exposure intervals (eg, > 48-72 hours). However, serum-starved MM cells cultured in the presence or absence of VEGF165 were more susceptible to BMS354825 or SKI-606 than cells cultured in 10% FBS medium (Dai and Grant, unpublished observations). In contrast, coadministration of marginally toxic concentrations of BMS354825 or other Src inhibitors (eg, SKI-606 and PP2) with Chk1 inhibitors strikingly induced apoptosis in MM cell lines and in primary CD138+ MM cells cultured under standard conditions. Furthermore, 24 hours of pretreatment with lower BMS354825 concentrations (eg, 50-200nM) moderately enhanced the lethality (reflected by increased PARP cleavage) of longer UCN-01 exposures (ie, an additional 48 hours) in association with reduced ERK1/2 activation and prevention of Bim degradation (supplemental Figure 1A). These disparate results suggest that ERK1/2-dependent cytoprotective responses to Chk1 inhibitors may be distinct from SFK-mediated downstream actions responsible for growth factor (eg, VEGF)–mediated proliferation/survival. In support of this notion, in the presence of VEGF165 concentrations that induced ERK1/2 phosphorylation in serum-starved MM cells, UCN-01 further activated ERK1/2, whereas BMS354825 or SKI-606 substantially diminished this event and increased apoptosis. Finally, constitutively active Ras (Q61L) or MEK1 markedly attenuated interactions between Src and Chk1 inhibitors, indicating that Src inhibitors act upstream of the Ras → ERK1/2 cascade. These findings suggest that while Src may signal to other downstream survival cascades (eg, PI3K/Akt16 ), interference with compensatory activation of the Ras/MEK/ERK pathway plays a critical functional role in the potentiation of Chk1-inhibitor lethality by Src inhibitors.
The present findings suggest that, along with inactivation of the Ras → ERK1/2 signaling cascade, Src kinase inhibition potentiates Chk1-inhibitor lethality through multiple downstream mechanisms. First, as observed in the case of MEK1/2 inhibition,29 interruption of Src function by either pharmacologic (eg, Src/Abl inhibitors) or genetic (eg, loss-of-function Src mutants, or Src shRNA) means substantially blocked Chk1-inhibitor–induced BimEL phosphorylation and degradation, resulting in accumulation of the unphosphorylated, more active form of BimEL.30 Second, interruption of Src function markedly potentiated Chk1-inhibitor–induced γH2A.X expression, a DNA double-strand–break marker.26 Finally, interruption of Src function enhanced Chk1-inhibitor–mediated pro-death p34cdc2 activation (reflected by dephosphorylation at inhibitory sites [Y15]).45 It is possible that these events cooperate to kill MM cells in the setting of simultaneous Chk1 and Src kinase inhibition. For example, Bim accumulation may lower the death threshold for MM cells, rendering them more susceptible to the lethal consequences of DNA damage and p34cdc2 activation.
Angiogenesis represents a critical microenvironmental factor in MM progression.38 In MM patients, bone-marrow angiogenesis, manifested by higher microvessel density, correlates with disease stage and poor prognosis.46,47 MM cells induce angiogenesis directly through the production of angiogenic factors (eg, VEGF)19 and interactions with the microenvironment (eg, endothelial cells, stromal cells, and osteoclasts).38 VEGF and bone-marrow angiogenesis support the survival, proliferation, and migration of MM cells while contributing to drug resistance and MM-associated bone disease.38,48 Consequently, angiogenesis represents a potentially important target in MM. Src plays a critical role in tumor angiogenesis by targeting VEGF expression/production of tumor cells18 and angiogenesis of endothelial cells.17,49 Src inhibitors such as BMS354825 exert antiangiogenic effects in diverse human tumor types, including MM.17 Administration of 150 mg/kg/d of BMS354825 significantly decreased microvessel density in an OPM-1 murine xenograft model.17 In contrast, the relatively lower BMS354825 dose (50 mg/kg/d) used here, when administered alone, exhibited only modest antiangiogenic effect within the superficial region distal to muscle tissue from which the tumor blood supply originates. Significantly, UCN-01 coadministration with this BMS354825 dose effectively abrogated CD31+ microvessels throughout the tumors. Consistent with these findings, combined treatment with Chk1 inhibitors and BMS354825 or SKI-606 clearly reduced production of VEGF in MM cells, in association with diminished VEGF mRNA and protein expression. Interestingly, angiogenesis antibody array and Western blot analysis revealed that cotreatment with BMS354825 or SKI-606 and UCN-01 also substantially decreased production of other angiogenic factors, including ANG, RANTES, TIMP-1/2, and MCP-1, by MM.1S cells. These findings raise the possibility that diminished ability of MM cells to produce multiple angiogenic factors may contribute to the antiangiogenesis of this regimen. On the other hand, BMS354825 administrated as a single agent disrupts VEGF- and/or PDGF-mediated signaling in vascular endothelial cells and/or induction of endothelial cell death.17 In this context, BMS354825/UCN-01 coadministration strikingly suppressed in vitro angiogenesis, manifested by marked disruption of HUVEC tube formation (2-4 hours exposure), while inhibiting the proliferation of and promoting apoptosis in HUVECs (24 hours exposure). One caveat is that the murine subcutaneous model used here may not fully recapitulate disease within the bone marrow. Consequently, the functional significance of the observed angiogenic effects of this regimen in human MM remains to be determined.
In summary, the present findings demonstrate that Src inhibitors interact synergistically with Chk1 inhibitors to induce apoptosis and to diminish angiogenesis, resulting in a dramatic increase in anti-MM activity in vitro and in vivo. They further indicate that intact Src kinase function is required for ERK1/2 activation–mediated compensatory and cytoprotective responses in MM cells exposed to Chk1 inhibitors. Conversely, disruption of Src kinase function blocks Chk1-inhibitor–induced activation of the Ras → ERK1/2 signaling cascade, preventing phosphorylation/degradation of BimEL, accompanied by potentiation of DNA damage and p34cdc2 activation, culminating in apoptosis. Results of in vitro and in vivo studies raise the possibility that, in addition to these events, antiangiogenic actions may also contribute to the pronounced in vivo anti-MM activity of the Src/Chk1-inhibitor regimen. The therapeutic significance of these findings is highlighted by the expanded preclinical and clinical development of new-generation, more specific Chk1 inhibitors,2,5,6 as well as emerging evidence of the anti-MM activity of Src inhibitors (eg, BMS354825).17 Finally, it is noteworthy that the Src/Chk1-inhibitor regimen selectively targeted primary CD138+ MM cells but not their normal CD138− counterparts, as was previously observed in the case of MEK1/2 inhibitors.8 Collectively, the present findings provide a theoretical basis for further investigation of a strategy combining Chk1 and Src inhibitors in MM and potentially other hematopoietic malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by awards CA63753, CA93738, CA100866, and P50CA130805 from the National Institutes of Health; award 61181-10 from the Leukemia & Lymphoma Society of America; Lymphoma SPORE award P50CA130805; Multiple Myeloma SPORE award 1P50CA142509-01; and awards from the V Foundation.
National Institutes of Health
Authorship
Contribution: Y.D. designed and performed research, analyzed data, and wrote the manuscript; S.C., R.S., X.-Y.P., L.W., J.A.A., and L.B.K. performed research and analyzed data; P.D. helped to design research; and S.G. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Steven Grant, Division of Hematology/Oncology, Virginia Commonwealth University/Medical College of Virginia, MCV Station Box 230, Richmond, VA 23298; e-mail: stgrant@vcu.edu.

![Figure 1. Src inhibitors block ERK1/2 activation in human MM cells exposed to UCN-01, in association with synergistic induction of apoptosis. (A-B) Human MM cells were exposed (24 hours) to UCN-01 (U266, 150nM; RPMI 8226, MM.1S, and MM.1R, 100nM) ± BMS354825 (0.5-2.5μM [A]) or SKI606 (U266 and RPMI 8226, 2μM; MM.1S and MM.1R, 1μM [B]), after which Western blot analysis was performed to detect expression of total and/or phosphorylated c-Src (Y418) and ERK1/2. (C) RPMI 8226 and U266 cells were treated as described in panel A for 24 hours and 48 hours, respectively. (D) MM.1S and MM.1R cells were exposed to 100nM UCN-01 ± 1μM BMS354825 for 24 hours. (E) MM cell lines were treated as described in (B) for 24 hours (RPMI 8226, MM.1S, and MM.1R) or 48 hours (U266). For C-E, after drug treatment, the percentage of apoptotic (annexin V+) cells was determined by flow cytometry (means ± SD, *P < .01 and **P < .001 vs UCN-01 alone). (C) Inset: RPMI 8226 cells were exposed to a range of BMS354825 (0.5-1.25μM) and UCN-01 (50-125nM) concentrations alone and in combination at fixed ratio (10:1) for 24 hours. At the end of this period, the percentage of annexin V+ cells was determined for each condition. Fractional effect values were obtained by comparing results with those of untreated controls, and median dose-effect analysis was used to characterize the nature of the interaction between these agents. Combination index values less than 1.0 denote a synergistic interaction. The results of a representative experiment are shown; 2 additional studies yielded equivalent results. (F) CD138+ cells isolated from the bone marrow of a patient (patient #2) with MM were exposed (24 hours) to 100nM UCN-01 ± 0.5 or 1.0μM BMS354825, after which the extent of apoptosis was determined by annexin V/propidium iodide (PI) staining and flow cytometry. Values shown represent the percentage of annexin V+ cells including early (annexin V+/PI−, bottom right quadrants) and late apoptosis (annexin V+/PI+, top right quadrants). Two additional experiments yielded equivalent results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/6/10.1182_blood-2010-06-291146/4/m_zh89991066390001.jpeg?Expires=1767870783&Signature=bzL4S2UDX5TfEgnT4Y6R427io53Vnt8tfDDQSJ4X4kSm4dB~s0wsT5bO3xDvfJzb8G45y9K-q8Nm-33eT9SgLZBUzi5gO9~HXt177zcgmzY~aUvTSR1401g6pBqHHWh0fOltaXlwvO6UC3wV3HALtCkiymCFwk0SjtRGd-FRuS6SGQBy4fiEJJBraHlE2vwzCGhVberTR1FyaQyvxNlobvQipefA3~U8pFLOK3pCbvLi8ITq6jWxBT5n9W5sd7z-oygpvV6-e0bnbnI7Le65y0by1Q4sChnMEqZ3nEmBHQ9w766k17AZscy3ePOvcbs9oLfBiaF3GjJzmiijcX5AKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Loss-of-function Src mutants or shRNA knock-down prevent ERK1/2 activation and sensitize MM cells to UCN-01, whereas constitutively active Ras or MEK1 diminishes potentiation of UCN-01 lethality by SKI-606. (A) U266 cells were stably transfected with wt or loss-of-function mutants (K297R, kinase-inactive; K296R/Y528F, dominant-negative) of human c-Src. Western blot analysis indicated ectopic expression of these proteins and ERK1/2 phosphorylation. EV, Empty-vector controls. (B-D) U266 cells bearing either wt or mutant c-Src were incubated with the indicated concentrations of UCN-01 for 24 or 48 hours (for blots of PARP [C]), Western blot analysis was performed to monitor phosphorylation of Src (Y418 and Y215) and its downstream protein paxillin (Y118), ERK1/2 phosphorylation, PARP cleavage, BimEL phosphorylation/expression (Calbiochem), and γH2A.X expression. CF, Cleavage fragment; p, phosphorylated; up, unphosphorylated. (E) U266 cells were stably transfected with 2 plasmids (#1 and #2) encoding shRNA targeting different sequences of the human c-Src gene. Western blot analysis demonstrates down-regulation of Src protein expression as well as diminished basal levels of ERK1/2 phosphorylation compared with cells transfected with a plasmid encoding a negative control sequence (left panels). Cells were then exposed to the indicated concentrations of UCN-01 for 24 hours, after which cells were lysed and subjected to Western blot analysis to monitor phosphorylation of ERK1/2, expression of Bim (ProSci) and γH2A.X, and cleavage of PARP (right panels). (F) U266 cells were stably transfected with constitutively active human H-Ras (Q61L) or MEK1 (CA-MEK1). Western blot analysis demonstrated ectopic expression of the mutant proteins and resulting phosphorylation/activation of downstream targets (eg, MEK1/2 and ERK1/2, respectively). These cells were then exposed to 150nM UCN-01 ± 2μM SKI-606 for 48 hours, after which the percentage of apoptotic (annexin V+) cells was determined by flow cytometry (means ± SD, **P < .001 vs EV controls). For blots in panels A, C, E and F, vertical lines have been inserted to indicate repositioned gel lanes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/6/10.1182_blood-2010-06-291146/4/m_zh89991066390003.jpeg?Expires=1767870783&Signature=iTGdLjXwilc7y0HfDudyMxjnULQjcobWZKzzULeGfE-6z5QFrPhIBnWEWHJZ6bTT9a8iiyyl7bBQbFv3IQ7M0hJ5U1GaefGsxOK8E9N~j30aTv3tCMFHfJ0rSOe8Pt9Q8CYhkfgV4sHz7dFiot1SHoc3Y98hIuNrHyj7wxKatwDb1o1I9uZrDYlhUw4VrGvJKX0kZwe4hLNwwZMty5z~WJ3cafGKgWA~qLFwIhpzrIe4bc-zy52rM-JPUDEqx09327tQy1hRiPSqU0-9vo-r~FizxEkD44-lbWWC~deM5morkLq9PyzzYiZdko6CC~vEFjhAeCUI5ikWyGL-b3uwEA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)