Abstract
In early-stage cutaneous T-cell lymphoma (CTCL), malignant T cells are confined to skin and are difficult to isolate and discriminate from benign reactive cells. We found that T cells from CTCL skin lesions contained a population of large, high-scatter, activated skin homing T cells not observed in other inflammatory skin diseases. High-scatter T (THS) cells were CD4+ in CD4+ mycosis fungoides (MF), CD8+ in CD8+ MF, and contained only clonal T cells in patients with identifiable malignant Vβ clones. THS cells were present in the blood of patients with leukemic CTCL, absent in patients without blood involvement, and contained only clonal malignant T cells. The presence of clonal THS cells correlated with skin disease in patients followed longitudinally. Clonal THS cells underwent apoptosis in patients clearing on extracorporeal photopheresis but persisted in nonresponsive patients. Benign clonal T-cell proliferations mapped to the normal low-scatter T-cell population. Thus, the malignant T cells in both MF and leukemic CTCL can be conclusively identified by a unique scatter profile. This observation will allow selective study of malignant T cells, can be used to discriminate patients with MF from patients with other inflammatory skin diseases, to detect peripheral blood involvement, and to monitor responses to therapy.
Introduction
Cutaneous T-cell lymphoma (CTCL) is a heterogeneous collection of non-Hodgkin lymphomas arising from T cells that home to and inhabit the skin. Patients with nonprogressive early-stage mycosis fungoides (MF) CTCL have stable inflammatory skin lesions and a normal life expectancy.1 In patients with more advanced disease, clonal malignant T cells can spread to involve the blood, lymph nodes, and other peripheral organs. Stage II or greater CTCL has a high mortality rate, and death most commonly occurs as a result of infection.1
The study of skin lesions of MF CTCL has been hindered by the fact that malignant cells are confined to the skin and are difficult to isolate. Moreover, MF CTCL skin lesions contain both malignant and reactive benign T cells, and discriminating between the 2 populations can be difficult.2 Most prior studies have focused on the malignant T cells found in the blood of patients with leukemic disease (L-CTCL), including Sézary syndrome.3-10 Although MF and L-CTCL were previously considered as differing stages of the same disease, recent genetic and phenotypic studies have suggested that they may arise from 2 distinct T-cell subsets, and this may in part explain their differing clinical behaviors.1,11-13 A need therefore exists for more comprehensive studies of the T cells from early-stage MF CTCL skin lesions. Specifically, a biomarker that reliably identifies malignant cells in all stages of CTCL is needed.
In this study, we have isolated the T cells from skin lesions and blood of patients with all stages of MF and L-CTCL. We present here our evidence that the malignant T cells from both blood and skin can be conclusively identified by their unique T-cell scatter profile on flow cytometry and that this biomarker can be used to selectively study the malignant T cells in both early- and late-stage CTCL.
Methods
Skin and blood samples
These studies were performed in accordance with the Declaration of Helsinki and were approved by the institutional review board of the Partners Human Research Committee (Partners Research Management) or the University Medical Center Utrecht Institutional Review Board. Normal human skin was obtained from cosmetic surgery procedures. Blood and lesional skin samples were obtained from patients with CTCL and contact dermatitis seen at the Dana-Farber/Brigham and Women's Cancer Center Cutaneous Lymphoma Program. Since 2002, 443 patients with CTCL have been enrolled in studies that permit the collection of blood or skin or both; of these, 427 patients (96%) have consented to provide samples. There have been 11 publications to date on this patient population. Patients are assigned a unique study number (eg, Pt 188) that is used to identify their experimental data in this and other articles arising from studies of this patient population. Patients with L-CTCL and with MF described in this study met the revised International Society fro Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer criteria for L-CTCL/Sézary syndrome or MF.14 Patient demographics are as shown in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Skin samples from patients with atopic dermatitis or psoriasis were obtained from patients seen at the University Medical Center Utrecht. Peripheral blood mononuclear cells were isolated by Ficoll centrifugation, and T cells were isolated from skin with the use of short-term explant cultures (1-3 weeks) in the absence of exogenous cytokines as described.15 Briefly, Cellfoam matrices 9 mm × 9 mm × 1.5 mm (Cytomatrix Pty Ltd) were incubated in a solution of 100 μg/mL rat tail collagen I (BD Biosciences) in phosphate-buffered saline for 30 minutes, followed by 2 rinses in phosphate-buffered saline. Subcutaneous fat was removed from skin samples, and the tissue was minced into explants ∼ 2 mm × 2 mm in size. Skin explants were placed on the surface of the matrices, and each matrix was placed into 1 well of a 24-well plate. The culture was maintained in 2 mL/well of Iscove modified medium (Mediatech) with 20% heat inactivated fetal bovine serum (Gemini Bio-Products), penicillin, streptomycin, and 3.5 μL/L β-mercaptoethanol. Cultures were fed 3 times a week by aspiration of 1 mL of culture medium and replacement with fresh medium. Best discrimination of the THS population and low-scatter T-cell populations occurred when no exogenous cytokines were added. T cells could be expanded from CTCL skin biopsies by the addition of 100 IU/mL interleukin-2 and 20 ng/mL interleukin-15 to the culture medium, but this favored longer term expansion of benign CD8 T cells and obscured the border between the high- and low-scatter T-cell populations (data not shown).
Flow cytometry
Analysis of T cells was performed with directly conjugated monoclonal antibodies obtained from BD Biosciences, eBioscience, or R&D Systems. FOXP3 staining used the PCH101 antibody (eBioscience). Isotype-matched negative control antibodies were used to set the gates for positive staining. For analysis of cytokine production, T cells were stimulated with either control medium or 50 ng/mL phorbol ester myristate acetate and 750 ng/mL ionomycin for 6 hours plus 10 μg/mL brefeldin A (Calbiochem) for the last 5 hours of incubation. Cells were surface stained, fixed, permeabilized, stained with anti–cytokine antibodies, and examined by flow cytometry. For analyses of Ki-67 expression, cells were stained with directly conjugated anti-Ki67 (Santa Cruz Biotechnology) antibody with the use of the buffers, and the protocol was optimized for intranuclear staining (FOXP3 staining kit; eBioscience). For additional technical parameters for optimal detection of the THS cell population, please reference “Technical advice for the detection of THS cells in the blood of patients with L-CTCL,” included online as supplemental information.
In vitro stimulation of normal skin T cells
Nonexpanded T cells from normal human skin were incubated in 24-well tissue culture plates (Costar; Corning) coated with anti–human CD3 monoclonal antibody (Beckman Coulter) in the presence of 0.1 μg/mL soluble anti-CD28 for 24 hours in RPMI 1640 with 5% human AB serum (Fisher Scientific). In brefeldin A–treated samples, 10 μg/mL brefeldin A (Calbiochem) was added for the last 10 hours of culture. Cells were harvested and stained for surface CD3 and CD4, fixed, permeabilized, stained with directly conjugated anti–γ-interferon antibody (Fastimmune; BD Biosciences) and examined by flow cytometry.
T-cell receptor spectratype analyses
T-cell receptor (TCR)–CDR3 length analysis was performed as previously described.16 Briefly, total RNA was isolated from T cells (SV total RNA isolation system; Promega) and reverse transcribed into cDNA (PowerScript Reverse Transcriptase; BD Biosciences). Polymerase chain reactions (PCRs) were performed with Cβ primers recognizing both the Cβ1 and Cβ2 regions and individual primers for the 26 TCRβ chains as described previously.16 Additional run-off reactions were performed with fluorophore-labeled primers, and labeled products were analyzed with a DNA sequencer and Genescan software.16
Results
CTCL skin lesions contain a unique population of THS cells
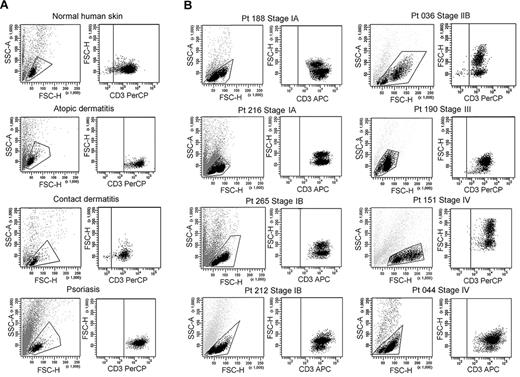
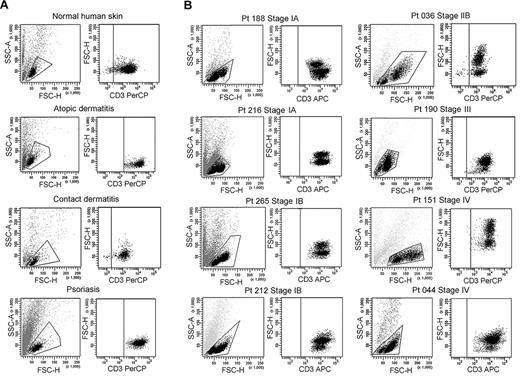
We isolated T cells from normal human skin, CTCL skin lesions, and the skin lesions of atopic dermatitis, contact dermatitis, and psoriasis. Normal human skin, atopic dermatitis, contact dermatitis, and psoriasis all contained a single fairly uniform population of CD3+ T cells by forward scatter (reflecting size) and side scatter (reflecting complexity; Figure 1A). In contrast, T cells isolated from CTCL skin lesions were invariably composed of 2 discrete cell populations, one with a normal scatter profile and a second population with increased side and forward scatter (Figure 1B). These 2 discrete T-cell populations were present in skin lesions from the earliest stages of CTCL and were especially prominent in patients with more advanced disease. Among the patients shown in Figure 1, 6 had skin-localized disease (MF) and 2 had advanced-stage disease with peripheral blood involvement (patients 151 and 044). Two discrete T-cell populations were observed in the 8 patients shown and also confirmed in 30 additional patients. Thirty-eight of 38 patients with CTCL studied had 2 discernible T-cell populations. Staining with 7-amino-actinomycin D confirmed that both T-cell populations were viable (data not shown).
CTCL skin lesions contain a high-scatter population of T cells not seen in normal skin or lesional skin from 3 other T cell–mediated inflammatory skin disorders. (A) T cells were isolated from normal human skin (12 patients) or lesional skin from patients with atopic dermatitis (6 patients), contact dermatitis (1 patient), or psoriasis (6 patients) with the use of short-term explant cultures. All samples tested contained a single population of CD3+ T cells. (B) T cells were then isolated from CTCL skin lesions with the use of short-term explant cultures. Patients with all stages of CTCL exhibited 2 clear populations of T cells. The lower-scatter population had a forward scatter (reflecting size) and a side scatter (reflecting complexity) similar to that of T cells from normal skin, whereas the high-scatter population consisted of larger and more complex cells. Eight representative patients are shown, similar results were obtained in 30 additional patients. SSC-A indicates side-scatter area; FSC-H, forward-scatter height; PerCP, peridin chlorophyll protein; APC, allophycocyanin.
CTCL skin lesions contain a high-scatter population of T cells not seen in normal skin or lesional skin from 3 other T cell–mediated inflammatory skin disorders. (A) T cells were isolated from normal human skin (12 patients) or lesional skin from patients with atopic dermatitis (6 patients), contact dermatitis (1 patient), or psoriasis (6 patients) with the use of short-term explant cultures. All samples tested contained a single population of CD3+ T cells. (B) T cells were then isolated from CTCL skin lesions with the use of short-term explant cultures. Patients with all stages of CTCL exhibited 2 clear populations of T cells. The lower-scatter population had a forward scatter (reflecting size) and a side scatter (reflecting complexity) similar to that of T cells from normal skin, whereas the high-scatter population consisted of larger and more complex cells. Eight representative patients are shown, similar results were obtained in 30 additional patients. SSC-A indicates side-scatter area; FSC-H, forward-scatter height; PerCP, peridin chlorophyll protein; APC, allophycocyanin.
THS cells are activated CD4+ skin homing T cells with elevated expression of FOXP3
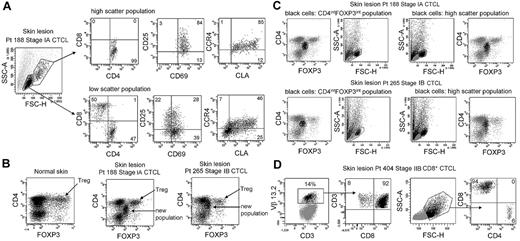
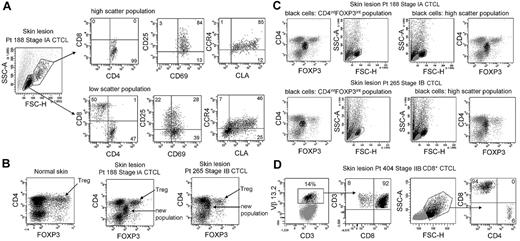
CTCL skin lesions contain a unique THS population of T cells not observed in other inflammatory skin diseases. To determine whether this represented the malignant T-cell population, we compared the phenotype of THS cells and low-scatter T cells in patients with MF (Figure 2A). THS CD3+ cells isolated from skin lesions of MF were uniquely CD4+, whereas low-scatter cells contained a mix of CD4+ and CD8+ T cells. THS cells expressed high levels of the activation markers CD25 and CD69 and the skin homing addressins cutaneous leukocyte antigen (CLA) and CC chemokine receptor 4 (CCR4), compared with lower and more variable expression in low-scatter T cells. Similar findings were observed in 6 additional patients with MF (stages I-III; data not shown).
High-scatter T cells from CTCL lesional skin are highly activated CD4+ skin homing T cells with elevated FOXP3 expression. (A) Comparison of the phenotype of high- and low-scatter T cells isolated from MF lesional skin. THS cells were uniformly CD4+ and expressed high levels of the activation markers CD69 and CD25 and the skin-homing addressins CCR4 and CLA. In contrast, low-scatter T cells contained a mixed CD4 and CD8 T cells with variable expression of activation markers and skin-homing addressins. Similar results were observed in 6 additional patients with stage I-III CTCL. (B) MF lesional skin contains a unique population of T cells with intermediate CD4 and FOXP3 expression. MF skin lesions contained both FOXP3high regulatory T cells (Tregs), also found in normal skin, and a novel population of T cells expressing intermediate levels of both CD4 and FOXP3. (C) Selective gating on this novel population of T cells (CD4intFOXP3int) showed that it corresponded closely to the THS cell population. Reversing the gating confirmed that the high-scatter T-cell population was comprised entirely of these CD4intFOXP3int T cells. A second patient with stage IB disease is also shown. Similar findings were observed in 4 additional patients. (D) In a patient with CD8+ MF diagnosed by histopathologic studies, a clonal population of CD8+ T cells expressing TCR Vβ 13.2 existed in lesional skin. THS cells in this patient were CD8+. SSC-A indicates side-scatter area; FSC-H, forward-scatter height. In gated histograms, the % total cells in each quadrant are shown.
High-scatter T cells from CTCL lesional skin are highly activated CD4+ skin homing T cells with elevated FOXP3 expression. (A) Comparison of the phenotype of high- and low-scatter T cells isolated from MF lesional skin. THS cells were uniformly CD4+ and expressed high levels of the activation markers CD69 and CD25 and the skin-homing addressins CCR4 and CLA. In contrast, low-scatter T cells contained a mixed CD4 and CD8 T cells with variable expression of activation markers and skin-homing addressins. Similar results were observed in 6 additional patients with stage I-III CTCL. (B) MF lesional skin contains a unique population of T cells with intermediate CD4 and FOXP3 expression. MF skin lesions contained both FOXP3high regulatory T cells (Tregs), also found in normal skin, and a novel population of T cells expressing intermediate levels of both CD4 and FOXP3. (C) Selective gating on this novel population of T cells (CD4intFOXP3int) showed that it corresponded closely to the THS cell population. Reversing the gating confirmed that the high-scatter T-cell population was comprised entirely of these CD4intFOXP3int T cells. A second patient with stage IB disease is also shown. Similar findings were observed in 4 additional patients. (D) In a patient with CD8+ MF diagnosed by histopathologic studies, a clonal population of CD8+ T cells expressing TCR Vβ 13.2 existed in lesional skin. THS cells in this patient were CD8+. SSC-A indicates side-scatter area; FSC-H, forward-scatter height. In gated histograms, the % total cells in each quadrant are shown.
CTCL has been suggested to be a malignancy of FOXP3+ regulatory T cells (Tregs).17 FOXP3 is expressed at high and constant levels by Treg, but non–regulatory human T cells express FOXP3 at lower levels, and expression is transiently up-regulated with T-cell activation.14,18,19 We found previously that skin resident Tregs can be discriminated from activated non-Tregs by their uniquely high levels of FOXP3 expression.19 Normal human skin contains clear populations of CD4+ T cells, CD8+ T cells, and FOXP3hi CD4+ Tregs (Figure 2B). CTCL skin lesions contained these cells and an additional T-cell population that expressed CD4 and FOXP3 at levels intermediate between that of Tregs and non-Tregs (Figure 2B). Selective gating on intermediate FOXP3+ T cells showed that they corresponded to the THS population (Figure 2C). Gating first on THS cells showed that this population uniquely corresponded to the CD4lo+FOXP3intermed T-cell subset (Figure 2C). Decreased CD4 expression is a feature of chronically activated T cells and is consistent with high expression of activation antigens by these cells. Larger cells can appear more positive for surface or intracellular markers because of their increased size; we controlled for this possibility with the use of isotype control antibodies for all stains. Selective gating of high- or low-scatter cells with the use of isotype controls was used to set the threshold for positive staining for all histograms. Increased FOXP3 staining in THS cells was therefore not an artifact of increased cellular size. CTCL skin did contain a clear population of FOXP3high Tregs; however, these cells mapped to the low-scatter population of T cells (Figure 2B-C). THS cells expressed FOXP3 at higher levels than non-Tregs but at lower levels than true Tregs. These findings show that THS cells from MF skin lesions are CD4+ skin homing T cells with a phenotype characteristic of chronic activation.
THS cells in CD8+ CTCL are CD8+ skin homing cells
The malignant T cells in most cases of MF CTCL are CD4+, but occasional cases of CD8+ MF do occur. We studied the T cells isolated from the skin lesions of a patient whose histopathology results were most consistent with CD8+ MF (Figure 2D). T cells from this patient's skin lesions contained a clonal population of T cells that expressed TCR Vβ13.2. Selective gating on the clonal population showed these cells were CD8+ T cells, consistent with the diagnosis of CD8+ MF. In this patient, the THS population was composed almost entirely of CD8+ cells.
In patients with identifiable malignant Vβ clones, THS cells from lesional skin are clonal and express the malignant TCR Vβ clonotype
Clonal T-cell populations can be shown in the skin of most patients with CTCL with the use of highly sensitive PCR–denaturing gradient gel electrophoresis (DGGE) assays for TCRγ chain rearrangements.20 However, most TCRγ gene rearrangements are not productive and thus do not encode proteins that can be used to identify malignant T cells.
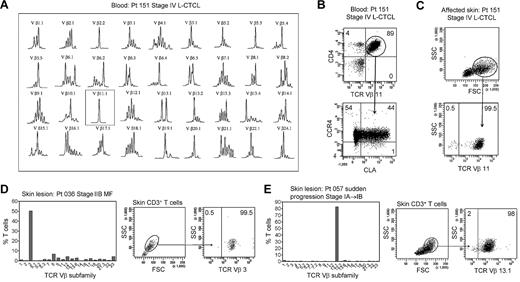
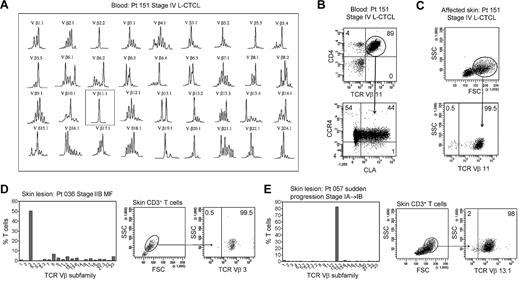
In patients with L-CTCL, malignant T cells can often be identified by their expression of a particular TCR Vβ subfamily.5,21,22 The number of circulating T cells bearing the malignant TCR Vβ in blood can be used to monitor disease activity.5,21 To determine whether THS cells are the malignant T cells in CTCL skin lesions, we studied patients with L-CTCL and known malignant clones. TCR spectratyping was used to identify patients with clonal populations in the peripheral blood (Figure 3A). Flow cytometric studies that used antibodies specific for TCR Vβ subfamilies confirmed that the blood of these patients contained expanded populations of CD4+ T cells carrying the malignant Vβ clonotype (Figure 3B). These cells also expressed high levels of CCR4 and variable CLA as previously reported.4 T cells isolated from skin lesions of these patients showed a clear THS population that was composed solely of T cells carrying the malignant Vβ clonotype (Figure 3C). A representative donor is shown in Figure 3; THS cells isolated from the affected skin of 3 additional patients with L-CTCL were also uniformly positive for that patient's malignant Vβ clonotype (data not shown).
THS cells from the lesional skin of L-CTCL and advanced MF are clonal and malignant. (A) Spectratype analysis was used to identify clonal populations of T cells in the blood of patients with L-CTCL. The number of individual peaks within each Vβ subfamily is reflective of the T-cell receptor diversity within that subfamily. Patient 151 had a clonal T-cell population expressing TCR Vβ 11.1. (B) Flow cytometric studies confirmed the presence of an expanded clonal population of CD4+ T cells in the blood that expressed TCR Vβ 11.1. This population had high expression of CCR4 and variable CLA expression. (C) T cells isolated from the involved skin of this patient contained 2 clear T-cell populations. The THS cell population was composed entirely of clonal and malignant T cells. Similar findings were observed in 3 additional patients with L-CTCL. (D-E) High-scatter lesional skin T cells from patients with MF with identifiable T-cell clones were uniformly clonal and malignant. T cells were isolated from lesional skin and TCR diversity was analyzed with the use of flow cytometry. In a subset of patients, an identifiable clone was found. Selectively gating on the high-scatter population of lesional skin T cells showed that the THS cell population was uniformly composed of clonal malignant T cells. SSC indicates side scatter. In gated histograms, the % total cells in each quadrant are shown.
THS cells from the lesional skin of L-CTCL and advanced MF are clonal and malignant. (A) Spectratype analysis was used to identify clonal populations of T cells in the blood of patients with L-CTCL. The number of individual peaks within each Vβ subfamily is reflective of the T-cell receptor diversity within that subfamily. Patient 151 had a clonal T-cell population expressing TCR Vβ 11.1. (B) Flow cytometric studies confirmed the presence of an expanded clonal population of CD4+ T cells in the blood that expressed TCR Vβ 11.1. This population had high expression of CCR4 and variable CLA expression. (C) T cells isolated from the involved skin of this patient contained 2 clear T-cell populations. The THS cell population was composed entirely of clonal and malignant T cells. Similar findings were observed in 3 additional patients with L-CTCL. (D-E) High-scatter lesional skin T cells from patients with MF with identifiable T-cell clones were uniformly clonal and malignant. T cells were isolated from lesional skin and TCR diversity was analyzed with the use of flow cytometry. In a subset of patients, an identifiable clone was found. Selectively gating on the high-scatter population of lesional skin T cells showed that the THS cell population was uniformly composed of clonal malignant T cells. SSC indicates side scatter. In gated histograms, the % total cells in each quadrant are shown.
As mentioned previously, the malignant T cells in L-CTCL and MF have significant phenotypic and functional differences. To determine whether the THS cells in MF skin lesions were also clonal and malignant, we next studied patients with stable established MF without evidence of peripheral blood involvement. Although PCR-DGGE can often show clonal populations in patients with early-stage MF, identification of the malignant clone with the use of TCR Vβ–specific antibodies requires that the clone is present at levels significantly above what would be expected in the normal T-cell repertoire. We therefore studied a subset of patients with more-advanced MF in whom the malignant clone was clearly detectable with the use of flow cytometry (Figure 3D-E). The clonal T-cell population in skin was first identified by staining with a panel of antibodies specific for TCR Vβ subunits. We then selectively gated on the THS population and found that they contained a uniform population of clonal T cells bearing the malignant clonotype (Figure 3D-E).
THS cells are oligoclonal in patients with early-stage MF
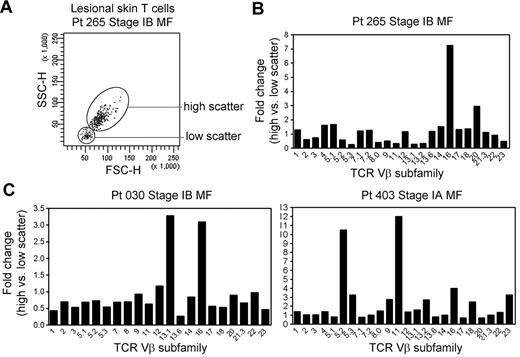
THS cells are clonal in patients with MF in whom the malignant clone can be conclusively identified. In patients with early-stage MF, THS cells were not clonal (Figure 4). However, when we analyzed for comparative usage of particular TCR Vβ subunits in the high-scatter versus low-scatter population, we found a preferential oligoclonal enrichment for particular Vβ subunits in the THS population, suggesting the presence of expanded T-cell clones within this population (Figure 4).
THS cells in early-stage MF are enriched for clonal or oligoclonal T-cell populations. (A) THS and low-scatter T cells were isolated from a panel of patients with stage IA-stage IB MF. (B) The preferential presence of T cells expressing particular TCR Vβ subfamilies was analyzed by comparing the fold change in the frequency of each TCR Vβ subfamily in the high- versus low-scatter population (percentage of TCR Vβ high-scatter/percentage of low-scatter). There was a trend toward oligoclonality or clonality in the high-scatter population. The 3 patients shown had identifiable T-cell clones on TCRγ PCR. SSC-H indicates side-scatter height; FSC-H, forward scatter-height.
THS cells in early-stage MF are enriched for clonal or oligoclonal T-cell populations. (A) THS and low-scatter T cells were isolated from a panel of patients with stage IA-stage IB MF. (B) The preferential presence of T cells expressing particular TCR Vβ subfamilies was analyzed by comparing the fold change in the frequency of each TCR Vβ subfamily in the high- versus low-scatter population (percentage of TCR Vβ high-scatter/percentage of low-scatter). There was a trend toward oligoclonality or clonality in the high-scatter population. The 3 patients shown had identifiable T-cell clones on TCRγ PCR. SSC-H indicates side-scatter height; FSC-H, forward scatter-height.
Lesional skin samples from these patients were additionally studied by PCR-DGGE for TCRγ-chain rearrangements as a part of their clinical evaluation. Patient 030 had a clonal population with the use of the Vγ1 primer, patient 265 had a detectable clone with the use of the Vγ(1-8) primer set, and patient 403 had a clonal population with the use of the Vγ10 primer. Three additional patients with stage IA MF with no detectable clonal populations by TCRγ PCR-DGGE had a diverse pattern of TCR Vβ use in the THS cell population (data not shown).
THS cells are present in the blood of patients with CTCL with peripheral blood involvement
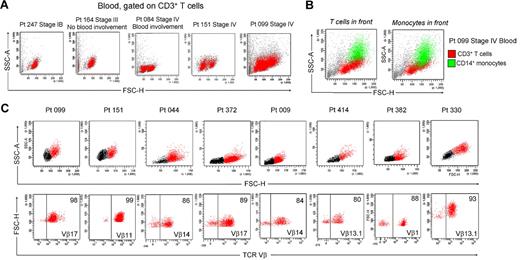
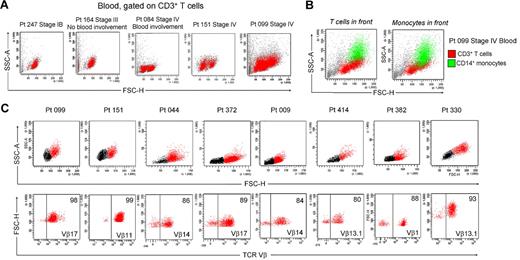
Patients with L-CTCL by definition have detectable circulating malignant cells in the blood. Peripheral blood involvement can be determined by the presence of large atypical cells in blood smears, by an increased CD4/CD8 ratio, increased absolute CD4 T-cell counts, or loss of CD7 and CD26 expression.23 Blood involvement is rarely observed in earlier stages of MF CTCL.24 We examined the blood of patients with different stages of CTCL and found that a THS population was also present in the blood of patients with documented blood involvement (Figure 5A). For example, patients 164 and 084 had comparable levels of skin disease, but patient 084 also had peripheral blood involvement as determined by an elevated CD4/CD8 ratio of 13.8 and a modestly increased absolute CD4 count of 1969/mm3. Blood from patient 164 had a single low-scatter population of CD3+ T cells, but patient 084 had an additional population of THS cells. Patients 151 and 099 both had stage IV disease with known blood involvement and both had 2 clear populations of T cells.
Clonal and malignant THS cells are present in the blood of patients with L-CTCL. (A) THS cells were present in the blood of patients with CTCL with known blood involvement but absent in patients without evidence of peripheral blood disease. Representative patients are shown; a THS cell population has been confirmed in 16 patients with L-CTCL. (B) Blood THS cells were obscured by monocytes in some patients. Staining for CD3 in addition to CD4 was required to show these cells. (C) THS cells were uniformly clonal and malignant in patients with L-CTCL and identifiable malignant T-cell clones. Shown are 8 patients with L-CTCL with identifiable T-cell clones. THS cell populations (red) were present in all patients. Selective gating on THS cells showed that most were clonal malignant T cells. SSC-A indicates side-scatter area; FSC-H, forward-scatter height.
Clonal and malignant THS cells are present in the blood of patients with L-CTCL. (A) THS cells were present in the blood of patients with CTCL with known blood involvement but absent in patients without evidence of peripheral blood disease. Representative patients are shown; a THS cell population has been confirmed in 16 patients with L-CTCL. (B) Blood THS cells were obscured by monocytes in some patients. Staining for CD3 in addition to CD4 was required to show these cells. (C) THS cells were uniformly clonal and malignant in patients with L-CTCL and identifiable malignant T-cell clones. Shown are 8 patients with L-CTCL with identifiable T-cell clones. THS cell populations (red) were present in all patients. Selective gating on THS cells showed that most were clonal malignant T cells. SSC-A indicates side-scatter area; FSC-H, forward-scatter height.
The presence of 2 T-cell populations in the blood of patients with CTCL has been reported but is not an acknowledged feature of blood involvement in CTCL.3 One possible reason is that many analyses are carried out by automatically gating on the low-scatter lymphocyte population and staining for CD4 only. We found that THS CD3+ T cells were often obscured by monocytes that have a similar forward- and side-scatter properties and also express CD4 (Figure 5B). A CD4 stain would therefore fail to discriminate between THS cells and normal monocytes. A CD3 stain and a wider scatter gate is therefore necessary to detect THS cells in CTCL blood.
THS cells from the blood of patients with L-CTCL are clonal and malignant
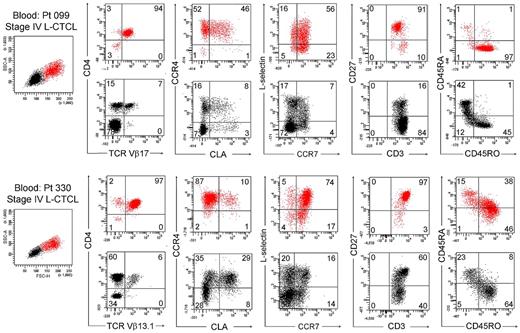
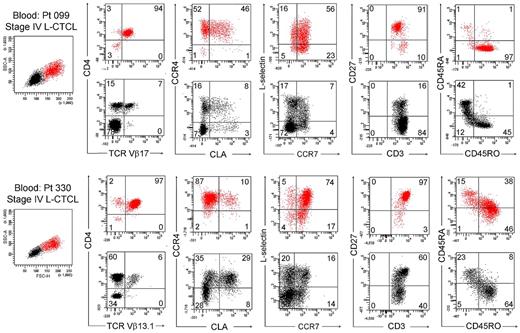
We next studied T cells from the blood of patients with L-CTCL in whom the malignant T-cell clones were identifiable by monoclonal TCR Vβ antibodies. Without exception, most THS cells from the blood of these patients were clonal and expressed the malignant TCR Vβ subunit (Figure 5C). THS cells were uniformly CD4+ and CCR4+ and expressed the malignant clonal TCR Vβ subunit, and most coexpressed the central memory T-cell markers L-selectin and CCR7 and retained CD27 expression, as previously described (Figure 6).4,13 CLA was expressed at high levels by THS cells from donor 099 (Figure 6) but was variably expressed by the 5 other donors studied (data not shown). In marked contrast, low-scatter T cells contained in mixed population of CD4+ and CD8+ cells, most of which lacked expression of CCR4, CLA, L-selectin, and CCR7. A minority of T cells expressing the malignant TCR Vβ subunit were also present in the low-scatter T-cell population. In both groups, there was little expression of the activation antigens CD25 and CD69 (data not shown). There was some heterogeneity of CD45RO and CD45RA expression among clonal THS cells (Figure 6), as previously reported.13
THS cells from the blood of patients with L-CTCL were clonal proliferative CD4+ CCR4+ memory T cells expressing central memory T-cell markers. The phenotype of high-scatter (red) and low-scatter (black) T cells of 2 patients with L-CTCL are shown. In both patients, THS cells virtually all clonal CD4+ cells expressing high levels of CCR4 and variable levels of CLA. Most THS cells coexpressed the TCM cell markers L-selectin and CCR7 and retained CD27 expression. Two representative patients are shown; similar results were found in 6 additional patients with L-CTCL. In gated histograms, the % total cells in each quadrant are shown.
THS cells from the blood of patients with L-CTCL were clonal proliferative CD4+ CCR4+ memory T cells expressing central memory T-cell markers. The phenotype of high-scatter (red) and low-scatter (black) T cells of 2 patients with L-CTCL are shown. In both patients, THS cells virtually all clonal CD4+ cells expressing high levels of CCR4 and variable levels of CLA. Most THS cells coexpressed the TCM cell markers L-selectin and CCR7 and retained CD27 expression. Two representative patients are shown; similar results were found in 6 additional patients with L-CTCL. In gated histograms, the % total cells in each quadrant are shown.
The presence of THS cells in blood correlates with skin disease severity
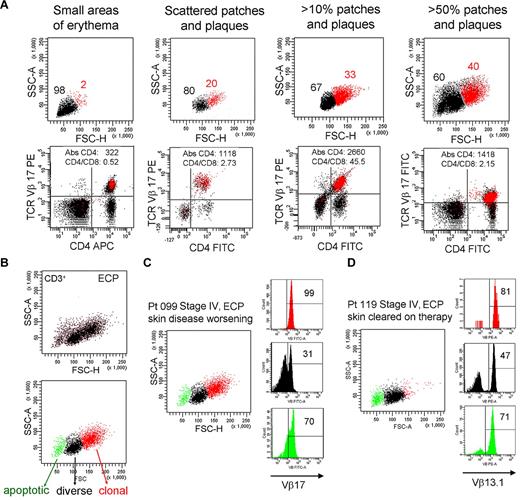
To determine whether THS cells correlated with any facet of disease activity, we studied patient 099 for more than a 4-year period during which time the extent of the patient's skin disease fluctuated (Figure 7A). There was a clear correlation between the number of malignant, THS cells and the severity of skin disease. At all time points, THS cells were a uniform population of clonal T cells expressing the previously identified malignant TCR Vβ subunit.
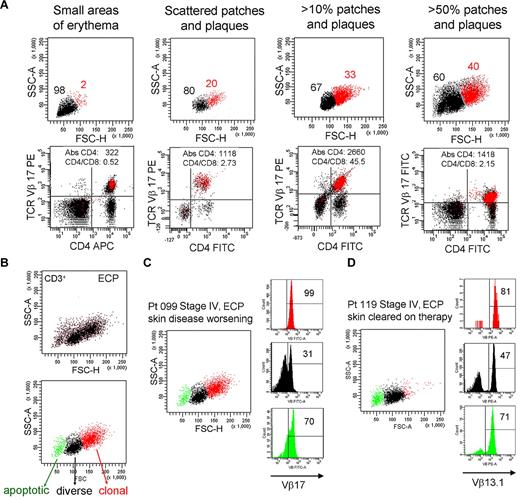
The presence of THS cells in blood correlates with skin disease severity. (A) Peripheral blood T cells were drawn from patient 099 (stage IV L-CTCL) over a 4-year period during which the extent of her skin disease fluctuated. The presence of THS cells (red) correlated with the severity of skin disease. At all time points, THS cells were clonal CD4+ T cells expressing the previously identified malignant TCR Vβ17 clonotype. The absolute CD4 T-cell count and CD4/CD8 ratio are also shown. (B-D) Successful clearing of skin lesions on ECP is associated with loss of the high-scatter malignant T-cell clone in patients with L-CTCL. (B) Three T-cell populations were typically evident in patients with CTCL with blood involvement on ECP. The lowest-scatter T-cell population, shown in green, had high levels of caspase 6 activation, consistent with apoptosis (data not shown). Two patients with stage IV L-CTCL with identifiable malignant clones and extensive skin involvement were studied. (C) Patient 099 had worsening of skin disease on ECP and had large numbers of clonal THS cells demonstrable in the blood. (D) Patient 119 experienced complete clearing of skin lesions on ECP, and study of her blood at the time of skin clearing showed very few high-scatter clonal T cells. Both patients also had some cells expressing the malignant Vβ subunit in the low-scatter T-cell population. SSC-A indicates side-scatter area; FSC-H, forward-scatter height; PE, phycoerythrin; APC, allophycocyanin; and FITC, fluorescein isothiocyanate. In gated histograms, the % total cells in each quadrant are shown.
The presence of THS cells in blood correlates with skin disease severity. (A) Peripheral blood T cells were drawn from patient 099 (stage IV L-CTCL) over a 4-year period during which the extent of her skin disease fluctuated. The presence of THS cells (red) correlated with the severity of skin disease. At all time points, THS cells were clonal CD4+ T cells expressing the previously identified malignant TCR Vβ17 clonotype. The absolute CD4 T-cell count and CD4/CD8 ratio are also shown. (B-D) Successful clearing of skin lesions on ECP is associated with loss of the high-scatter malignant T-cell clone in patients with L-CTCL. (B) Three T-cell populations were typically evident in patients with CTCL with blood involvement on ECP. The lowest-scatter T-cell population, shown in green, had high levels of caspase 6 activation, consistent with apoptosis (data not shown). Two patients with stage IV L-CTCL with identifiable malignant clones and extensive skin involvement were studied. (C) Patient 099 had worsening of skin disease on ECP and had large numbers of clonal THS cells demonstrable in the blood. (D) Patient 119 experienced complete clearing of skin lesions on ECP, and study of her blood at the time of skin clearing showed very few high-scatter clonal T cells. Both patients also had some cells expressing the malignant Vβ subunit in the low-scatter T-cell population. SSC-A indicates side-scatter area; FSC-H, forward-scatter height; PE, phycoerythrin; APC, allophycocyanin; and FITC, fluorescein isothiocyanate. In gated histograms, the % total cells in each quadrant are shown.
To further study the question of whether the presence of THS cells correlated with skin disease, we studied the blood T cells from 2 patients with similar stages of L-CTCL; 1 of these patients responded to extracorporeal photopheresis (ECP) and 1 did not (Figure 7C-D). First, we observed that blood samples taken from patients undergoing ECP often had 3 populations of CD3+ T cells (Figure 7B). A population of shrunken, very low-scatter cells expressed high levels of caspase 6 and annexin V, suggesting that they represented apoptotic T cells (Figure 7B; data not shown). A second population of T cells was present that contained both CD4 and CD8 T cells with a diverse TCR Vβ repertoire as assessed by flow cytometry (Figure 7B; additional data not shown), and the third, high-scatter population was composed uniformly of T cells bearing the malignant T-cell clonotype.
Patients 099 and 119 both had, at the time of the blood draw shown, stage IV disease with peripheral blood involvement (Figure 7C-D). Both had severe skin disease that had required the initiation of ECP. Patient 099 had skin disease that became refractory to ECP; at the time point shown (Figure 7C), she had patches, plaques, and macular erythema covering > 50% of her body surface area as well as intractable pruritus. By contrast, patient 119 had almost complete clearing of her skin disease on ECP. At the time of the blood draw shown (Figure 7D), she had no clinically evident skin disease. A THS population was obvious in patient 099 but scarcely evident in patient 119, in whom skin disease was in remission. In both patients, THS cells were virtually all clonal and malignant as determined by expression of the malignant Vβ TCR (Vβ17 for patient 099, Vβ13.1 for patient 119). Low-scatter T cells were more diverse. Malignant T cells made up most of the apoptotic T cells in both patients, lending credence to the concept that ECP induces selective apoptosis of malignant T cells in CTCL.
Benign clonal T cells are confined to the low-scatter T-cell population
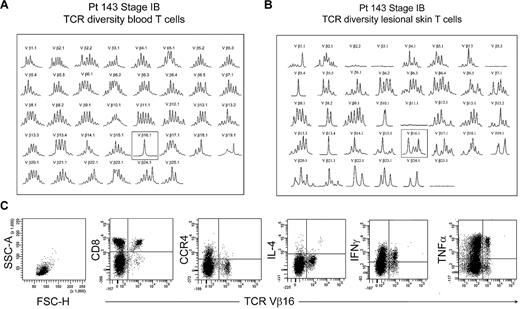
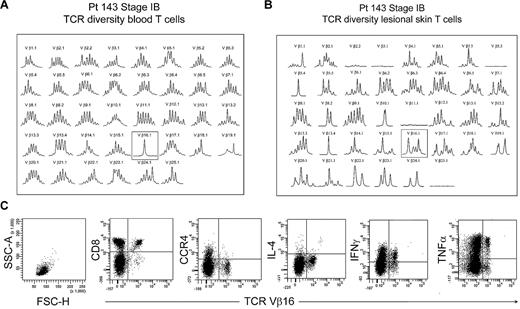
The peripheral blood of most patients with established MF had no identifiable clonal T-cell populations, with rare exceptions. One such exception, patient 143, had an expanded clonal population of T cells in the peripheral blood that expressed TCR Vβ 16.1 (Figure 8A). However, spectratyping of T cells isolated from the patient's lesional skin failed to show a clone and instead showed that a diverse population of T cells expressing TCR Vβ 16.1 existed in the skin (Figure 8B). Analysis of peripheral blood by flow cytometry showed no THS cell population in this patient. Further analysis showed that the expanded population of Vβ 16 T cells in blood was composed of CD8+ T cells, although the patient's skin lesions contained atypical CD4+ T cells by histopathology. Moreover, these clonal Vβ 16–expressing CD8+ T cells lacked expression of CCR4 and produced high levels of tumor necrosis factor-α and interferon γ (IFN-γ). Thus, the clonal population in this patient's peripheral blood was composed of benign expanded CD8 effector cells, was not malignant, and fell into the low-scatter T-cell population.
Benign clonally expanded T cells are found in the low-scatter T-cell population and can be discriminated from malignant T-cell clones. (A) Spectratype analysis of blood T cells showed a clonal T-cell population expressing TCR Vβ16.1 in a patient with long-standing stable stage IB MF and no other evidence of peripheral blood involvement. (B) Spectratype analysis of T cells isolated from skin lesions showed a diverse T-cell population expressing TCR Vβ16.1. (C) Further analysis of peripheral blood showed a lack of THS cells and showed that the expanded TCR Vβ16.1+ T-cell population was CD8+, lacked expression of CCR4, and produced effector cytokines, including interferon γ (IFNγ) and tumor necrosis factor-α (TNFα), suggesting that these cells represented a benign expanded CD8 clonal T-cell population. SSC-A indicates side-scatter area; FSC-H, forward-scatter height; and IL-4, interleukin-4.
Benign clonally expanded T cells are found in the low-scatter T-cell population and can be discriminated from malignant T-cell clones. (A) Spectratype analysis of blood T cells showed a clonal T-cell population expressing TCR Vβ16.1 in a patient with long-standing stable stage IB MF and no other evidence of peripheral blood involvement. (B) Spectratype analysis of T cells isolated from skin lesions showed a diverse T-cell population expressing TCR Vβ16.1. (C) Further analysis of peripheral blood showed a lack of THS cells and showed that the expanded TCR Vβ16.1+ T-cell population was CD8+, lacked expression of CCR4, and produced effector cytokines, including interferon γ (IFNγ) and tumor necrosis factor-α (TNFα), suggesting that these cells represented a benign expanded CD8 clonal T-cell population. SSC-A indicates side-scatter area; FSC-H, forward-scatter height; and IL-4, interleukin-4.
THS phenotype does not correlate with recent cellular activation or proliferation
We conducted additional experiments to determine whether recent cellular activation or proliferation or both gives rise to the separate and discernible THS cell population that we observe in CTCL. T cells undergoing a cellular activation and proliferation are larger and more complex. We isolated nonexpanded T cells from human skin and cultured them on CD3-coated plates in the presence of soluble anti-CD28 antibody for 48 hours. We stained the cells for expression of early- and late-activation markers CD69 and CD25, gated selectively on the activated T cells within this population, and compared their scatter characteristics to nonactivated T cells. We found that the activated T cells did not form a clear second high-scatter population but were instead admixed with the low-scatter population (supplemental Figure 1A).
We next addressed the question of whether THS cells in the blood of patients with L-CTCL may have a high-scatter phenotype as a result of recent proliferation. We stained blood T cells from patients with L-CTCL with identifiable malignant clones for the expression of Ki-67, a marker of recent cellular proliferation. Only a small proportion of the high-scatter, clonal malignant T cells in these patients had recently proliferated, suggesting that ongoing or recent proliferation was not responsible for the increased size and complexity of these cells (supplemental Figure 1B).
Discussion
CTCL is a heterogeneous group of lymphomas with differing clinical manifestations and prognoses. Early-stage MF CTCL is characterized by inflammatory skin lesions that can remain stable in location and extent for many years. In MF, malignant T cells are confined to inflammatory skin lesions and are responsive to skin-directed therapies used to treat nonmalignant inflammatory skin diseases, including topical steroids and UV light. Malignant T cells are present in the blood in L-CTCL and can also form tumors in the skin, lymph nodes, and internal organs in advanced stages of the disease. Malignant T cells in late-stage disease are typically refractory to therapy. Patients with advanced stages of MF CTCL or L-CTCL require systemic therapies, including oral and intravenous chemotherapies, and long-term remissions are rare without stem cell transplantation.25
Gene expression analyses and differing responses to therapy support the concept that MF and L-CTCL may be distinct diseases.1,11,12 We have recently shown that malignant T cells in MF phenotypically resemble skin-resident effector memory T cells, a subset of nonmigratory T cells that remains long term in fixed positions within the skin.13,26,27 In contrast, malignant T cells in L-CTCL have a phenotype suggestive of central memory T (TCM) cells. TCM cells are highly migratory T cells that recirculate between the blood and lymph nodes and a subset can also enter the skin.28,29 TCM cells are long-lived, capable of multiple cell divisions, and are resistant to apoptosis.28 Thus, although studies of clonal malignant T cells in patients with L-CTCL have provided valuable insights, it is not clear how applicable these findings are to the malignant T cells in MF.
Two difficulties have prevented comprehensive studies of the malignant T cells in skin lesions of MF CTCL, regardless of stage. First, malignant T cells are confined to skin lesions and are therefore difficult to isolate and study. Second, MF inflammatory skin lesions contain both clonal malignant T cells and a heterogeneous population of benign-activated infiltrating T cells, and discriminating these 2 populations in the absence of an identifiable clone has been impossible with currently available biomarkers. Sensitive PCR-DGGE assays for TCR γ chain rearrangements can often show clonal populations in early-stage CTCL skin lesions, but, in virtually all cases of MF CTCL, malignant cells express αβ TCRs on their surface. PCR studies have been designed to study the TCR Vβ subunit use of T cells in CTCL.30 This method has the potential to identify malignant cells on a genomic basis that could subsequently be identified with Vβ monoclonal antibodies, but the large number of TCR Vβ subunits makes this PCR too cumbersome for routine use. Monoclonal antibodies to particular TCR Vβ subunits can be used to identify the clonal malignant T cells in CTCL.5,21,22 However, only a subset of patients have malignant T cells identifiable by commercially available TCR Vβ antibodies, and these antibodies can only detect a malignant clone when the percentage of clonal cells is sufficiently above the expected frequency of normal T cells bearing that Vβ subunit.31,32 There is therefore a need for a reliable biomarker to isolate the malignant T cells in early-stage CTCL and to discriminate them from benign T cells.
We isolated T cells from skin lesions of early-stage CTCL with the use of short-term explant cultures, a method used previously to study the T cells from normal skin, psoriatic skin lesions, and skin cancers, including melanoma and squamous cell carcinoma.15,29,33-35 T cells isolated in this manner maintain expression of skin-homing addressins, TCR diversity, and the ability to divide and produce inflammatory cytokines after antigen exposure.15,29,33,34 T cells isolated from the skin lesions of patients with CTCL contained 2 separate populations of CD3+ T cells discernible by their differing forward- and side-scatter profiles on flow cytometry. First, there was a population of T cells with lower forward and side scatter (low-scatter) that contained a mixed population of CD4 and CD8 T cells. This population of T cells contained a distinct FOXP3+ population of Tregs, similar to those observed in normal skin (Figure 2B). In patients with CTCL, there was a second T-cell population with increased forward scatter, indicating increased cellular size, and increased side scatter, indicating increased cellular complexity. The high-scatter population was composed of only CD4+ T cells in CD4 MF and CD8+ T cells in CD8 MF (Figure 2). THS cells from MF skin lesions showed higher expression of 2 activation markers, CD69 and CD25, than T cells in the low-scatter population. CD4 expression was also decreased in THS cells, a phenotype that has been previously reported on circulating malignant T cells in L-CTCL.3 However, decreased CD4 expression can also result from cellular activation or viral infection and so is not specific for CTCL.36,37
The increased cellular size and complexity of the THS population could be a result of intense cellular activation, viral infection, or other insults. Highly activated T cells are larger and more complex than resting cells. However, atopic dermatitis, contact dermatitis, and psoriasis skin lesions all contain activated T cells, yet these skin conditions do not contain a separate discernible population of THS cells as observed in CTCL.38-40
To determine whether THS cells represented the malignant T cells in CTCL skin lesions, we studied patients in whom the malignant clone could be identified by staining with antibodies specific for the TCR Vβ subunit of the malignant clone. T cells isolated from the skin lesions of patients with L-CTCL fell into 2 discernible T-cell populations (Figure 3C). The THS populations in these patients were comprised solely of clonal malignant T cells. However, as discussed previously, the malignant T cells in L-CTCL are demonstrably different from those in MF. We also studied patients with MF that had identifiable clonal populations within the skin and no peripheral blood involvement. In these patients also, we observed that the THS population was composed solely of clonal malignant T cells (Figure 3D-E). In patients with earlier stage MF in whom a malignant T-cell clone could not be conclusively identified with the use of flow cytometry, we nonetheless observed a tendency toward clonality or oligoclonality in the THS population (Figure 4). These results suggest that even in early-stage MF, malignant T cells are contained within the THS population.
We observed a similar THS population in the blood of patients with CTCL with known peripheral blood involvement but not in patients without blood involvement (Figure 5A). THS cells often overlapped with monocytes on flow cytometric profiles and could not be detected unless cells were costained for CD3 and CD4; the forward- and side-scatter gates were opened to include the monocyte population. Studies that used only CD4 staining and gating on the known lymphocyte scatter would fail to detect these T cells. The THS cells in patients with L-CTCL was composed entirely of clonal malignant T cells (Figure 5). We have now replicated these findings in 14 patients with L-CTCL and identifiable malignant T-cell clones.
The THS cells in patients with L-CTCL shared many of the characteristics previously identified in clonal malignant CTCL T cells (Figure 6). These cells were CD4+ and CCR4+ but expressed variable levels of the skin-homing addressin CLA. THS cells had a phenotype consistent with TCM cells, namely coexpression of L-selectin and CCR7 as well as maintenance of CD27 expression.13,28 As previously described, there was some variability in expression of CD45RO and CD45RA by malignant T cells.13
To establish the functional significance of THS cells, we studied the presence of this population in patients during differing phases of disease activity. The presence and number of THS cells correlated with the extent of skin involvement (Figure 7A). Moreover, the number of THS cells decreased in patients with good clinical response to ECP but remained unchanged in patients who did not respond to this treatment modality (Figure 7B-D). In responding patients, malignant T cells were found preferentially within the apoptotic T-cell population, providing additional evidence that, regardless of the mechanism, ECP does induce selective apoptosis of malignant T cells in CTCL.
Finally, we reported a patient with presumably skin-limited stage IB MF in whom a clonal T-cell population was nonetheless identified in the peripheral blood (Figure 8). This patient did not have a THS population in the blood, and spectratype analysis of T cells from skin lesions failed to show a similar clonal population in the skin. This clone was CD8+, did not express CCR4, and produced significant amounts of interferon γ and tumor necrosis factor-α on stimulation, suggesting that it represented an expanded CD8 T-cell clone similar to those frequently observed in healthy persons.41 Thus, benign clonally expanded T cells fall into the normal low-scatter T-cell population and can be discriminated from the malignant clonal T cells in CTCL.
Our findings show that the malignant T cells in both MF and L-CTCL can be conclusively identified by their unique high-scatter profile (THS). The use of this novel biomarker to discriminate malignant from benign T cells in CTCL will facilitate comprehensive and comparative studies of malignant versus benign T cells, including identification of commonly mutated genes. When T cells are isolated from lesional skin of patients with psoriasis, atopic dermatitis, and contact dermatitis, a THS population is observed only in patients with CTCL. Moreover, it is invariably present in patients with CTCL, even in the earliest stages of disease. However, additional studies are needed to determine whether a THS population is present in other disorders such as lymphomatoid papulosis and lymphomatoid drug reactions. Thus, although a THS population is sensitive for the diagnosis of CTCL, it may not be specific and needs to be used in combination with other confirmatory studies (eg, clonality, loss of CD26 expression). We find that the presence of a THS population in the blood of a patient with a confirmed diagnosis of CTCL is a highly sensitive way to detect peripheral blood involvement in patients in whom other hematologic parameters are still within normal values. Finally, the number of blood T cells in the THS population can be used to follow the extent of peripheral blood involvement in patients in whom the malignant clone cannot be identified with the use of commercially available TCR Vβ antibodies. In summary, this novel biomarker will allow more comprehensive studies of malignant T cells in both early- and late-stage CTCL, can be used in combination with other tests to help discriminate patients with early-stage CTCL from patients with other inflammatory skin diseases, and can be used to detect and follow the extent of peripheral blood involvement in patients with L-CTCL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients who donated the samples that made this work possible. Dr Thomas Cochran of the Boston Center for Plastic Surgery and Dr Elof Eriksson of Brigham and Women's Hospital generously provided normal skin.
This work was supported by the SPORE in Skin Cancer P50 CA9368305 National Institutes of Health/NCI (to T.S.K.), R01 A1025082 National Institutes of Health/NIAID (to T.S.K.), and R01AR056720 (to R.A.C.) and by a Damon Runyon Clinical Investigator Award (to R.A.C.).
National Institutes of Health
Authorship
Contribution: R.A.C. and T.S.K. designed the experiments; R.A.C. conducted experiments, analyzed data, prepared figures, and drafted the manuscript; J.B.S., A.C., and R.W. performed experiments and assisted in figure preparation; K.Y. performed spectratyping analyses; J.E.T. conducted experiments and edited the manuscript; D.H., J.J.C., and H.P.K. provided blood, psoriatic skin, atopic dermatitis skin and clinical information. In addition to guiding experimental approaches, T.S.K. recruited patients with CTCL, supported sample collection, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rachael A. Clark, Brigham and Women's Hospital Department of Dermatology and the Harvard Skin Disease Research Center, EBRC Rm 505A, 221 Longwood Ave, Boston, MA 02115; e-mail: rclark1@partners.org.