Abstract
Maintenance of genomic stability depends on the DNA damage response, a biologic barrier in early stages of cancer development. Failure of this response results in genomic instability and high predisposition toward lymphoma, as seen in patients with ataxia-telangiectasia mutated (ATM) dysfunction. ATM activates multiple cell-cycle checkpoints and DNA repair after DNA damage, but its influence on posttranscriptional gene expression has not been examined on a global level. We show that ionizing radiation modulates the dynamic association of the RNA-binding protein HuR with target mRNAs in an ATM-dependent manner, potentially coordinating the genotoxic response as an RNA operon. Pharmacologic ATM inhibition and use of ATM-null cells revealed a critical role for ATM in this process. Numerous mRNAs encoding cancer-related proteins were differentially associated with HuR depending on the functional state of ATM, in turn affecting expression of encoded proteins. The findings presented here reveal a previously unidentified role of ATM in controlling gene expression posttranscriptionally. Dysregulation of this DNA damage response RNA operon is probably relevant to lymphoma development in ataxia-telangiectasia persons. These novel RNA regulatory modules and genetic networks provide critical insight into the function of ATM in oncogenesis.
Introduction
The DNA damage response (DDR) is essential for maintaining the integrity of the genome, and the failure of this response results in genomic instability and a predisposition to malignancy.1 In response to genotoxic agents, cells remain homeostatic via activation of signaling pathways that turn on specific gene expression programs. A crucial guardian of genomic integrity is the checkpoint kinase ATM (Ataxia Telangiectasia Mutated),2 one of the most often mutated kinases in human cancers.3 A deleted or inactivated ATM gene results in ataxia-telangiectasia (AT), a human genetic disorder characterized by progressive neurodegeneration, immunodeficiency, premature aging, radiation sensitivity, and a high propensity to develop cancer.4,5 A total of 30% to 40% of all AT patients develop neoplasia during their lifetime and 10% to 15%, of those patients present with a lymphoid malignancy in childhood.6 To date, there is no effective therapeutic strategy for this disease, and treatment has focused on slowing the progression of the neurodegeneration and developing approaches for the treatment of tumors while minimizing side effects and treatment for the immunodeficiency.7
ATM is critical for the initial response to double-stranded DNA breaks and is required for subsequent activation of another master checkpoint kinase, the ATM- and Rad3-related protein ATR.8 In response to double-stranded DNA breaks, the ATM kinase phosphorylates and regulates a cascade of downstream effectors, including the checkpoint kinase Chk2 and other components of the DNA repair pathways and the cell-cycle checkpoints, to minimize the risk of genetic damage.7,9 Ionizing radiation (IR)–induced double-stranded DNA breaks sequentially activate ATM and Chk2, which phosphorylates multiple substrate proteins, which in turn delays cell-cycle progression to facilitate DNA repair, or induces cellular apoptosis to eliminate the damaged cells, thereby protecting the organism against cancer.10,11 Recently, it was reported that, in response to H2O2-induced oxidative stress, Chk2 interacts and phosphorylates the RNA-binding protein (RBP) HuR (Hu antigen R, ELAVL1), altering its association with SIRT1 mRNA.12 HuR binds to and regulates posttranscriptionally many mRNAs that encode proteins involved in the stress response, cell proliferation, tumorigenesis, and cell apoptosis.13-26 The effect of HuR on the stability and translation of target mRNAs has been linked to its subcellular localization.15
RBPs and microRNAs are major posttranscriptional regulators of gene expression. They generally regulate subsets of target mRNAs by associating with their 3′- or 5′-untranslated regions (UTRs) in a manner that be modulated by genotoxic stresses and growth conditions.20,27 The coordinated regulation of mRNA subsets is the basis of the posttranscriptional “RNA-operon” model whereby RBPs coregulate multiple mRNAs and thereby regulate the expression of collections of proteins involved in the same function.28 Recently, posttranscriptional regulatory mechanisms were also shown to control the kinetics of proinflammatory cytokine-induced gene expression.29,30
The crucial role of ATM in regulating Chk2 activation and findings that Chk2 regulates HuR binding activity suggests a model in which ATM and Chk2 cooperate to regulate the association of HuR with subsets of target mRNAs and thereby regulates the expression of subsets of genes. Here, we identified a network of interacting proteins encoded by mRNAs differentially bound to HuR in ATM wild-type compared with ATM null lymphocytes after exposure to IR. Our findings establish a hierarchy of molecular events in which IR-triggered activation of the ATM/Chk2 cascade leads to the subsequent phosphorylation of HuR and modulates its posttranscriptional regulation of a network of target mRNAs encoding functionally related proteins. Our results highlight a critical biologic role of ATM and Chk2 in global mRNA-HuR interactions in response to DNA damage and contribute to a better understanding of the attenuated response to irradiation of ATM-deficient lymphocytes.
Methods
Cell culture, treatment, and transfections
B-lymphocyte cell lines GM02184, GM00536 (wild-type, ATM+/+) and GM03332, GM03189, and GM01526 (AT, ATM−/−) were purchased from Coriell Cell Repositories and cultured in RPMI medium 1640 (Quality Biologicals) with 15% fetal bovine serum and penicillin/streptomycin. Human embryonic kidney (HEK-293) cell lines were cultured in Dulbecco modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum and antibiotics. GM5849 AT fibroblast cells stably transfected with the pFLAG empty vector (Vector) or pFLAG-ATM (ATM; generous gifts from Dr F. Carrier) were cultured in minimum essential medium (Invitrogen) supplemented with 15% fetal bovine serum and antibiotics. Cells were exposed to 1 Gy of IR and lysed 6 hours later unless indicated otherwise. ATM kinase inhibitor (KU-55933) was purchased from Calbiochem, and Chk2 inhibitor (Inhibitor II) was from Sigma-Aldrich. HEK-293 cells were transfected either with Lipofectamine 2000 (Invitrogen), when plasmids for HuR overexpression (pcDNA3-TAP) and pHuR-TAP point mutants S88A, S100A, T118A, S202A12,31 were used, or Oligofectamine (Invitrogen), when with small interfering RNAs (siRNAs, control, targeting HuR, QIAGEN; or Chk2, Santa Cruz Biotechnology) were used. Seventy-two hours after transfection, cells were collected for analysis.
IPs of RNP complexes and real-time quantitative RT-PCR
Immunoprecipitations (IPs) of ribonucleoprotein (RNP) complexes followed by microarray analysis (RIP-Chip) was performed as previously described.24,25 In short, lymphocytes were harvested in lysis buffer (150mM KOAc, 2.5mM Mg(OAc)2, 20mM K-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.5, dithiothreitol, phenylmethylsulfonyl fluoride, RNasin, and protease inhibitors), and 3 mg of protein was used for IP for 1 hour at 4°C in the presence of excess antibody (30 μg of either anti-HuR antibody, Santa Cruz Biotechnology; or control immunoglobulin IgG, BD Biosciences PharMingen). After thorough washes with NT2 buffer (50mM Tris-HCl [pH 7.4], 150mM NaCl, 1mM MgCl2, and 0.05% Nonidet P-40) beads were treated with 20 units of RNase-free DNase I (15 minutes at 37°C) and incubated (20 minutes, 55°C) in 100 μL NT2 buffer containing 0.1% sodium dodecyl sulfate and 0.5 mg/mL Proteinase K. RNA from IP material was extracted using phenol and chloroform method in the presence of GlycoBlue (Ambion). For TAP-IP of RNP complexes, cells were transfected with pTAP, pHuR(WT)TAP, pHuR(S88A)TAP, pHuR(S100A)TAP, or pHuR(T118A)TAP, pHuR(S202A)TAP, or pHuR(S100D)TAP lysates were incubated with protein rabbit IgG-agarose (Sigma-Aldrich) and assay was performed as described for HuR IP of RNP. RNA from RNP IP reactions was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). The levels of HuR target mRNAs were quantified by real-time quantitative PCR analysis using iQSYBR Green Supermix (Bio-Rad) and Bio-Rad iCycler instrument. Background binding of the abundant glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as a loading control. Each reaction was carried out in triplicate, and 3 independent experiments were performed.
Microarray data analysis
RNA from IP material was labeled using Illumina TotalPrep RNA Amplification Kit (Ambion). Human HT-12, Version 1.0 gene expression BeadChips containing 48 000 RefSeq transcripts (Illumina), were used for microarray analysis. Raw microarray data were filtered by the detection (P ≤ .02), normalized by Z-score transformation32 and tested for significant differences in signal intensity. The sample quality was analyzed by scatter plot, principal component analysis, and gene sample Z-scores based hierarchy clustering to exclude possible outliers. Analysis of variance test was used to eliminate the genes with larger variances within each comparison group. Genes were considered to be significantly changed after calculating Z ratio, indicating fold difference (Z > 1.5 or < −1.5), false discovery rate, which controls for the expected proportion of false rejected hypothesis (false discovery rate ≤ 0.3) and P < .05. Genes differentially associated with HuR in studied groups were analyzed by DAVID bioinformatics resource (www.david.abcc.ncifcrf.gov) according to predefined pathways annotated by the Kyoto Encyclopedia of Genes and Genomes (KEGG) database33 and were visualized on the KEGG pathway map. Ingenuity Pathways Analysis (IPA; www.analysis.ingenuity.com; Ingenuity Systems) were used to identify the top network functions among IR-dependent differentially associated genes with HuR in ATM wild-type in contrast to ATM null cells. Network analysis uses a curated knowledge base on known functional interactions and protein functions to algorithmically infer biochemical interactions. Significance of functions and pathways was calculated using the right-tailed Fisher test (complete array results: www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE25848).
Western blotting
For Western blot analysis, 20 to 50 μg of protein lysates was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Invitrogen) and transferred onto polyvinylidene difluoride membranes. Blots were probed with polyclonal pChk2 (T68), pChk1 (Ser345), Chk1, FOXO3, MEK2, MEK1, and ZFP36L1 from Cell Signaling, Chk2 from Santa Cruz Biotechnology, and DUSP10 from Sigma-Aldrich, or monoclonal pATM (Ser1981) (Cell Signaling), HuR, p21 (Santa Cruz Biotechnology), γ-H2AX (Upstate), GAPDH, and β-actin (Abcam) antibodies. After incubation with the appropriate secondary antibodies, signals were detected by enhanced chemiluminescence (Pierce Chemical).
Polyribosome fractionation
Cells were incubated for 15 minutes with 100 mg/mL cycloheximide, shortly (1 minute) lysed in cytoplasmic lysis buffer containing 150mM KOAc, 2.5mM Mg(OAc)2, 20mM K-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH. 7.5, RNaseOUT, and protease inhibitor cocktail, and centrifuged for 1 minute (13 000g, 4°C). Lysates were fractionated through linear sucrose gradients, as previously described.24
Oligonucleotides used for real-time quantitative RT-PCR analysis of HuR target mRNAs
Real-time quantitative PCR quantification of selected HuR targets was performed with using following gene-specific primer pairs: ATGAAATTCACCCCCTTTCC and AGGTGAGGGGACTCCAAAGT for p21 mRNA, GATGCATTGCAGAGGCACTA and CCCTAATGCTGGAAGCACTC for FOXO3 mRNA, CCCTTCCAGCTGAGGACAG and ACGGGCAGGAGAGGAGAC for MEK2 mRNA, GCTTGGGGCTATTTGTGTGT and TCCCGAAAATACAGGCAGAC for MEK1 mRNA, ATTACCTCTTCAGCGCCAGA and AATGAGGGAAGGTGCAGTTG for ZFP36L1 mRNA, TCACCTCCCACAAACTGACA and GGAAAAGGGGGAGAAACAAG for DUSP10 mRNA and, CGGAGTCAACGGATTTGGTCGTAT and AGCCTTCTCCATGGTGGTGAAGAC for GAPDH mRNA.
Results
Differential association of HuR with target mRNAs in ATM wild-type versus ATM null cells after IR
In this study, we set out to examine the influence of the ATM/Chk2 signaling pathway on global HuR posttranscriptional gene regulation in response to DNA damage. To ensure that ATM-dependent downstream signaling was not confounded by activation of ATR, we examined the kinetics and dose-dependent phosphorylation of Chk1 (at Ser345) and Chk2 (at Thr68) after IR in both ATM wild-type and null lymphocytes (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Six hours after treatment with 1 Gy of IR induced ATM/Chk2 but not the ATR/Chk1 pathway; therefore, we chose 1 Gy as the optimal dose for further study. Increased levels of γ-H2AX, a DNA double-strand break marker, confirmed DNA damage after 1 Gy of IR (supplemental Figure 1C).
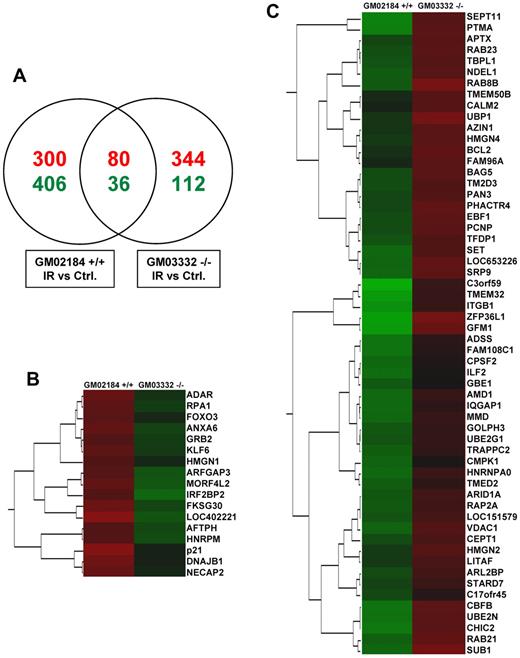
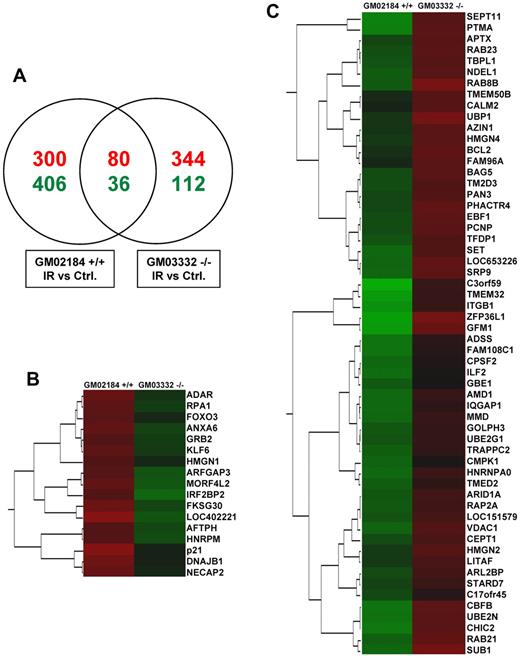
To gain insight into the alteration of HuR binding activity in the presence or absence of ATM/Chk2 signaling, we performed RNP IP analysis using an anti-HuR antibody (supplemental Figure 2A-B) and compared the collection of mRNAs associated with HuR in ATM wild-type and ATM null lymphocytes after exposure to IR. HuR-associated mRNAs were detected by RNP IP of endogenous HuR followed by microarray analysis of the obtained mRNAs. The ratios of mRNA expression were determined for each group at 6 hours after IR, compared with untreated cells. Venn diagram analysis of the common and specific genes whose association with HuR after IR was altered showed a small number of genes overlapping in response to IR between ATM wild-type and ATM null cells (Figure 1A). IR treatment of ATM wild-type cells modulated HuR-mRNA association of 822 genes (380 mRNAs increased and 442 decreased association), and the majority of these messages (706 mRNAs) did not appear to have an altered association with HuR in ATM null cells. This subset of genes is regulated through an IR-triggered activation of ATM/Chk2 pathway, which consequently modulates HuR phosphorylation and its binding activity. Among the top genes with significant changes in the association with HuR after IR in ATM wild-type, we found well-known HuR target genes (eg, p21, PTMA, BCL2; Figure 1B-C), which strongly validated the influence of the ATM signaling cascade on the modulation of HuR-mRNA interactions of genes involved in cellular functions, such as proliferation and apoptosis. IR altered the association of HuR with other target mRNAs in ATM null cells, suggesting that additional, ATM-independent pathways regulate HuR-mRNA interaction.
Global comparison of HuR association with mRNAs triggered by IR (1 Gy, 6 hours) in ATM wild-type (GM02184+/+) and ATM null (GM03332−/−) lymphocytes. (A) Venn diagram comparison of mRNAs whose association with HuR was significantly (Z-ratio difference values > or < ± 1.5; P < .05) increased (red) or decreased (green) in IR-treated compared with untreated (Ctrl) cells. (B-C) Examples of genes demonstrating different association with HuR in response to IR in ATM wild-type and ATM null cells. Clusters of genes showing the biggest differences in association with HuR, measured by Z-ratio values. Values increase from green to red.
Global comparison of HuR association with mRNAs triggered by IR (1 Gy, 6 hours) in ATM wild-type (GM02184+/+) and ATM null (GM03332−/−) lymphocytes. (A) Venn diagram comparison of mRNAs whose association with HuR was significantly (Z-ratio difference values > or < ± 1.5; P < .05) increased (red) or decreased (green) in IR-treated compared with untreated (Ctrl) cells. (B-C) Examples of genes demonstrating different association with HuR in response to IR in ATM wild-type and ATM null cells. Clusters of genes showing the biggest differences in association with HuR, measured by Z-ratio values. Values increase from green to red.
Functional analysis of the mRNAs differentially associated with HuR in response to IR in ATM wild-type and ATM null lymphocytes
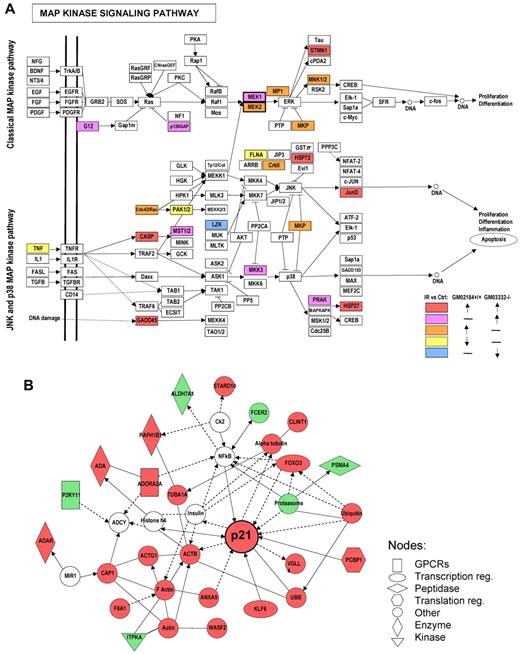
KEGG pathway databases were used to examine whether the genes differentially associated with HuR in IR-treated ATM wild-type and ATM null lymphocytes are components of functionally regulated gene groups. Importantly, KEGG pathway analysis revealed that the mitogen-activated protein kinase signaling pathway was characterized by the most abundant number of transcripts differentially associated with HuR in response to IR in both ATM wild-type and ATM null cells (Figure 2A). Notably, in ATM wild-type cells, IR triggered an increase in MEK2 mRNA association with HuR, whereas ATM null cells showed elevated association of HuR with MEK1 mRNA. To gain insight into the potential functional implications of ATM/Chk2 on HuR-mRNA association, genes markedly affected by IR in ATM wild-type but not ATM null cells were placed in the context of the known molecular interactions using IPA. The top 5 networks revealed molecular functions that were coupled to DNA damage response, cellular growth and proliferation, and cancer (supplemental Table 1). The top ranked network, derived from genes unique for ATM wild-type cells in response to IR treatment, was centered on the increase in HuR binding to p21 mRNA, surrounded by other interacting genes whose association with HuR was affected by IR treatment (Figure 2B). This suggests that the network containing p21 as a hub is indicative of the differences in response to IR between ATM wild-type and null cells in term of ATM/Chk2 influence on HuR binding and posttranscriptional gene regulation.
Networks most highly represented by transcripts differentially associated with HuR after IR treatment. (A) Genes related to the mitogen-activated protein kinase signaling pathway, showing altered association with HuR after IR were visualized on KEGG pathway maps. (B) The top ranked network of genes differentially associated with HuR after IR in ATM wild-type but not in ATM null lymphocytes as defined by IPA “DNA Replication, Recombination, and Repair, Nucleic Acid Metabolism, Small Molecule Biochemistry.” Red represents up-regulated genes; and green, down-regulated genes. Solid lines indicate direct interaction between genes; and dashed lines, indirect interaction between genes.
Networks most highly represented by transcripts differentially associated with HuR after IR treatment. (A) Genes related to the mitogen-activated protein kinase signaling pathway, showing altered association with HuR after IR were visualized on KEGG pathway maps. (B) The top ranked network of genes differentially associated with HuR after IR in ATM wild-type but not in ATM null lymphocytes as defined by IPA “DNA Replication, Recombination, and Repair, Nucleic Acid Metabolism, Small Molecule Biochemistry.” Red represents up-regulated genes; and green, down-regulated genes. Solid lines indicate direct interaction between genes; and dashed lines, indirect interaction between genes.
Considering the role of both ATM and HuR in the DNA damage response and cancer, we wished to gain further insight into the cancer-related genes and their interactions that mediate the biologic effect of ATM/Chk2/HuR in response to DNA damage. To this end, we selected cancer-related genes showing altered association with HuR in response to IR treatment in ATM wild-type (functional ATM) in contrast to ATM null cells for analysis of potential molecular networks (supplemental Table 2).
IR-triggered changes in HuR-associated mRNAs influence protein expression patterns
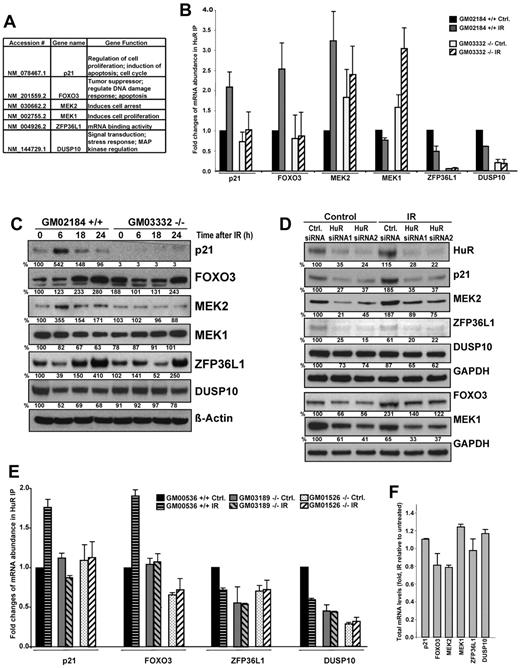
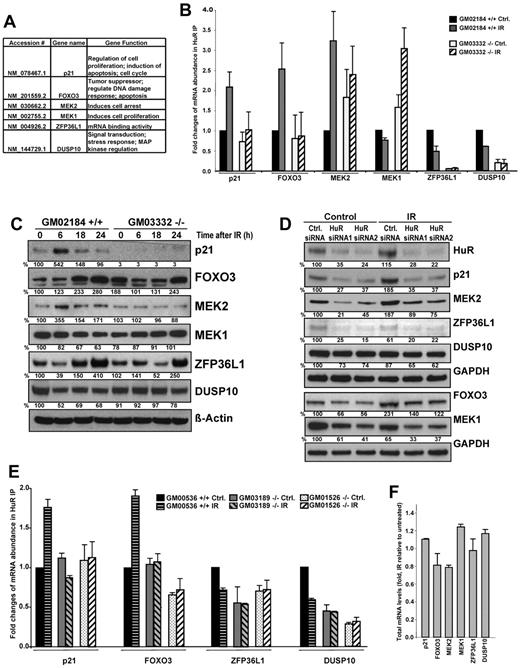
Of the microarray-identified transcripts showing differential association with HuR in ATM wild-type compared with null lymphocytes after IR, we selected 6 cancer-related targets for detailed verification. The genes selected play important roles in the regulation of tumor development pathways, such as the DNA damage response, cell arrest, cell proliferation, and signal transduction (Figure 3A). These HuR target transcripts were validated by quantitative reverse-transcribed polymerase chain reaction (RT-PCR) of mRNA from HuR IP samples in ATM wild-type and null lymphocytes (Figure 3B). To evaluate the functional consequence of the HuR-mRNA associations shown in Figure 3B, we measured the levels of the encoded proteins after IR treatment and after transfecting cells with siRNA targeting HuR. The levels of the proteins examined were elevated (in 6 hours after IR or at the later time points examined) when HuR binding to the corresponding mRNAs increased in IR-treated ATM wild-type lymphocytes (Figure 3C) and were reduced when HuR binding declined or after HuR silencing (Figure 3C-D). The finding that the basal levels of studied mRNAs were lowered in the HuR-silenced cultures before exposure to IR supported the notion that HuR regulates these proteins in unstimulated cultures, and further modifies their alteration after IR irradiation.
Analysis of selected HuR targets after IR exposure. (A-B) ATM wild-type (GM02184+/+) and ATM null (GM03332−/−) lymphocytes were either untreated or exposed to 1 Gy of IR and collected 6 hours later (unless otherwise indicated). The selected mRNAs immunoprecipitated using an anti-HuR antibody (demonstrating changes in association with HuR after IR by microarray analysis) were validated by quantitative RT-PCR; the individual enrichment of each mRNA in HuR IPs was normalized to IgG IPs; afterward, the differences in HuR binding were calculated. Background binding of an abundant transcript encoding a housekeeping protein (GAPDH mRNA) served as a loading control. (C) Western blot analysis of the proteins encoded by the HuR target mRNAs shown in panel B, assayed in whole-cell lysates prepared from lymphocytes that were treated with 1 Gy of IR. β-Actin and GAPDH were assessed as loading markers; relative protein abundance was calculated by densitometry and is expressed as percentage change relative to the levels in nonirradiated ATM wild-type cells. (D) HEK-293 cells were transfected with the indicated siRNAs; cells were either untreated or, 24 hours after transfection, IR treated (1 Gy) and collected 6 hours later (p21, MEK2, ZFP36L1, DUSP10) or 18 hours later (FOXO3, MEK1) for Western blot analysis. (E) HuR binding of target messages in multiple wild-type and ATM null lymphocytes treated with IR (1 Gy, 6 hours); wild-type and ATM null cell lines were purchased from Coriell Cell Repositories. Each cell line was acquired from a different donor. (F) The levels of total mRNA genes validated in panel B were measured by quantitative RT-PCR in ATM wild-type IR-treated compared with untreated lymphocytes. All graphs represent the mean plus or minus SEM from 3 independent experiments.
Analysis of selected HuR targets after IR exposure. (A-B) ATM wild-type (GM02184+/+) and ATM null (GM03332−/−) lymphocytes were either untreated or exposed to 1 Gy of IR and collected 6 hours later (unless otherwise indicated). The selected mRNAs immunoprecipitated using an anti-HuR antibody (demonstrating changes in association with HuR after IR by microarray analysis) were validated by quantitative RT-PCR; the individual enrichment of each mRNA in HuR IPs was normalized to IgG IPs; afterward, the differences in HuR binding were calculated. Background binding of an abundant transcript encoding a housekeeping protein (GAPDH mRNA) served as a loading control. (C) Western blot analysis of the proteins encoded by the HuR target mRNAs shown in panel B, assayed in whole-cell lysates prepared from lymphocytes that were treated with 1 Gy of IR. β-Actin and GAPDH were assessed as loading markers; relative protein abundance was calculated by densitometry and is expressed as percentage change relative to the levels in nonirradiated ATM wild-type cells. (D) HEK-293 cells were transfected with the indicated siRNAs; cells were either untreated or, 24 hours after transfection, IR treated (1 Gy) and collected 6 hours later (p21, MEK2, ZFP36L1, DUSP10) or 18 hours later (FOXO3, MEK1) for Western blot analysis. (E) HuR binding of target messages in multiple wild-type and ATM null lymphocytes treated with IR (1 Gy, 6 hours); wild-type and ATM null cell lines were purchased from Coriell Cell Repositories. Each cell line was acquired from a different donor. (F) The levels of total mRNA genes validated in panel B were measured by quantitative RT-PCR in ATM wild-type IR-treated compared with untreated lymphocytes. All graphs represent the mean plus or minus SEM from 3 independent experiments.
To gain biologic insight into these findings, we verified HuR binding to several selected target mRNAs in multiple ATM wild-type and ATM null lymphocytes under basal and IR-treatment conditions. Although we observed variations in basal binding of HuR to some transcripts (ie, ZFP36L1 mRNAs), the response to IR HuR-mRNA association changed only in ATM wild-type cells (Figure 3E). To understand the molecular process whereby IR regulates HuR target mRNAs expression in the presence of intact ATM/Chk2 signaling, we first investigated whether IRs influence abundance of their total mRNA levels. We compared IR-treated relative to untreated ATM wild-type lymphocytes by quantitative RT-PCR analysis of total RNA; by contrast to HuR-mRNA association data (Figure 3B), IR had minimal influence on total mRNAs levels of all studied HuR's target transcripts (Figure 3F). We observed that IR-induced changes in studied proteins expression were HuR modulated (Figure 3C-D) but were not accompanied by a concomitant differences in total mRNAs abundance (Figure 3F). Because HuR has other roles in posttranscriptional regulation of gene expression in addition to message stability, we investigated other potential mechanisms. Therefore, we asked whether IR affected the translation of examined HuR target mRNAs by monitoring the fractions of mRNAs associated with the polysomes in each untreated and IR-treated ATM wild-type and compared with null lymphocytes. As shown in supplemental Figure 3, consistent with the IR-triggered increase in HuR binding to p21 and FOXO3 mRNAs (Figure 3B), we observed increased levels of those transcripts in the actively translating fractions of the gradient (fractions 9-11), and lower levels in nontranslating and low-translating fractions of the gradient after treatment with IR. Correspondingly, mRNAs with decreased binding to HuR after IR treatment (as ZFP36L1 and DUSP10 mRNAs), were found to be decreased in the translationally engaged, polysomal fractions (fractions 6-12) of IR-treated cells (supplemental Figure 3). These differences were not seen when testing the distribution of those mRNAs in ATM null lymphocytes or the housekeeping GAPDH mRNA in both ATM wild-type or null cells. Through its ability to regulate several antiapoptotic effector proteins, HuR was shown to promote an antiapoptotic function in unstimulated and short-wavelength ultraviolet light-irradiated cells.14,22 Our study of the influence of HuR function, vis-à-vis cell survival in response to IR, demonstrated increased cell death in populations expressing reduced HuR levels (supplemental Figure 4A-B), which strongly supports HuR's prosurvival role.
Pharmacologic inhibition of ATM and Chk2, silencing of Chk2, and reintroduction of ATM gene into ATM null cells affect HuR-mRNA association
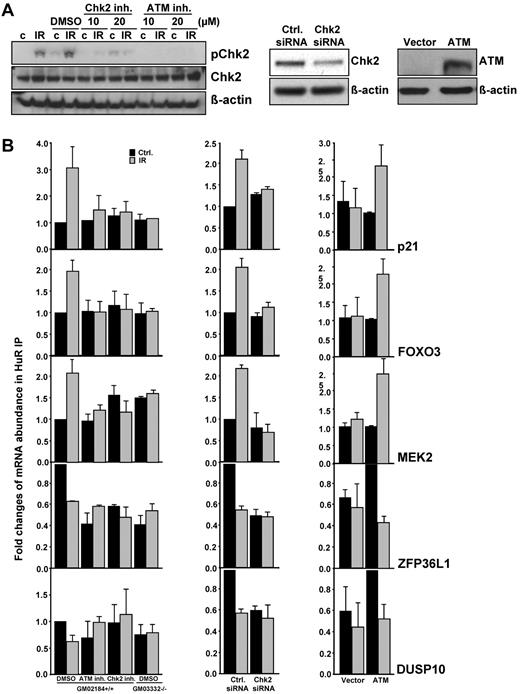
To confirm the specificity of the influence ATM/Chk2 signaling exerts on IR-induced modulation of HuR/mRNA association, small-molecule inhibitors of both ATM and Chk2 were used (Figure 4A). As measured by mRNA IP, IR altered the association of HuR with mRNAs in ATM wild-type lymphocytes but not in the cells pretreated with either ATM or Chk2 inhibitors, or in ATM null cells (Figure 4B left). A similar outcome to that obtained using small-molecule inhibitors was found when analyzing cells in which Chk2 was knocked down by siRNA (Figure 4A-B middle). In addition, IR-altered association of HuR with its target mRNAs was observed when using ATM null cells stably reintroduced with ATM gene (Figure 4A-B right). These results lend strong support to the view that inhibiting the function of either ATM or Chk2 directly influences HuR-mRNAs complexes, and underscore the influence of the ATM/Chk2 signaling pathway in regulating the expression of HuR targets after IR.
Specificity of ATM/Chk2 signaling on HuR-mRNA association. (A) Left: ATM wild-type (GM02184+/+) lymphocytes were pretreated for 2 hours with Chk2 or ATM inhibitors and then exposed to 1 Gy of IR and harvested after 6 hours of further incubation. pChk2 and Chk2 levels were assessed by Western blotting. Middle: Chk2 levels by 72 hours after transfection of HEK-293 cells with either control (Ctrl) or Chk2-directed siRNAs. Right: ATM levels in GM5849 AT fibroblasts stably reintroduced with ATM gene. (B) The association of HuR-mRNA complexes after IR (1 Gy, 6 hours) of cells pretreated with Chk2 or ATM inhibitor (10μM) (left), Chk2 knockdown (middle), and ATM reintroduction (right) was tested by mRNP-IP followed by quantitative RT-PCR. Each reaction was carried out in triplicate and was repeated 3 times. The individual enrichment of each mRNA in HuR IPs was normalized to IgG IPs; afterward, the differences in HuR binding were calculated. GAPDH mRNA background amplification in the IP material served as the internal control. Data are the mean ± SEM from 3 independent experiments.
Specificity of ATM/Chk2 signaling on HuR-mRNA association. (A) Left: ATM wild-type (GM02184+/+) lymphocytes were pretreated for 2 hours with Chk2 or ATM inhibitors and then exposed to 1 Gy of IR and harvested after 6 hours of further incubation. pChk2 and Chk2 levels were assessed by Western blotting. Middle: Chk2 levels by 72 hours after transfection of HEK-293 cells with either control (Ctrl) or Chk2-directed siRNAs. Right: ATM levels in GM5849 AT fibroblasts stably reintroduced with ATM gene. (B) The association of HuR-mRNA complexes after IR (1 Gy, 6 hours) of cells pretreated with Chk2 or ATM inhibitor (10μM) (left), Chk2 knockdown (middle), and ATM reintroduction (right) was tested by mRNP-IP followed by quantitative RT-PCR. Each reaction was carried out in triplicate and was repeated 3 times. The individual enrichment of each mRNA in HuR IPs was normalized to IgG IPs; afterward, the differences in HuR binding were calculated. GAPDH mRNA background amplification in the IP material served as the internal control. Data are the mean ± SEM from 3 independent experiments.
HuR residues phosphorylated by Chk2 in response to IR
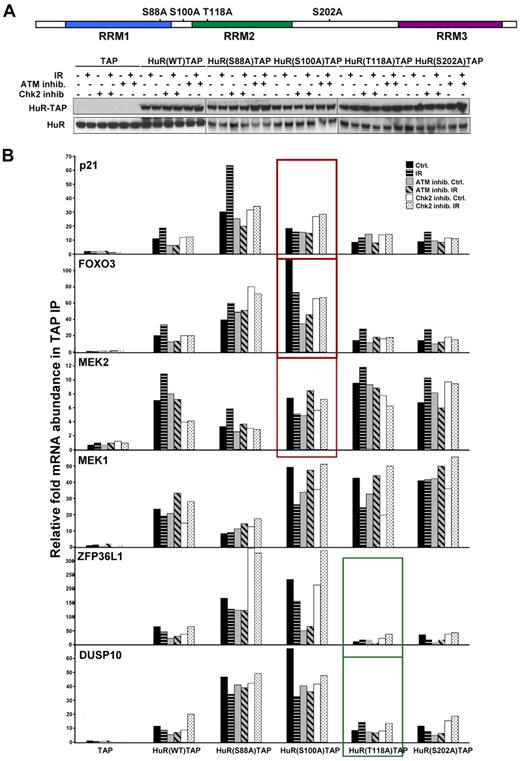
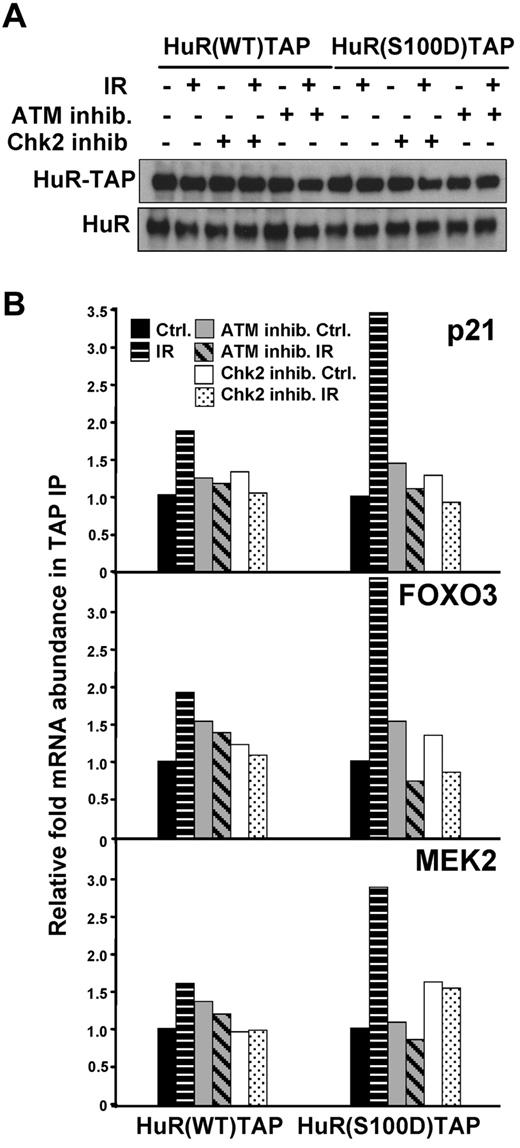
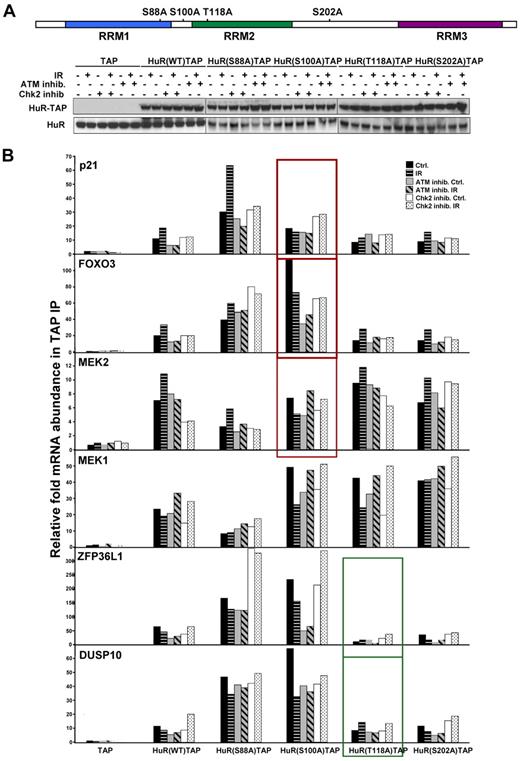
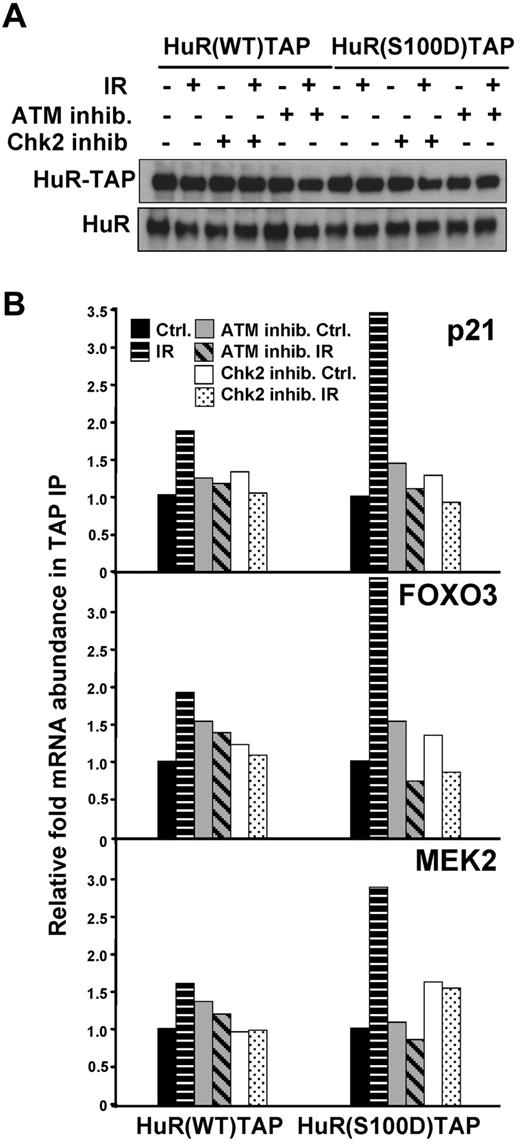
A recent study identified Chk2 as a protein that interacted with HuR after oxidative stress, phosphorylated HuR at residues Ser-88, Ser-100, and Thr-118, and impacted on the ability of HuR's ability to bind SIRT mRNA.12 We examined the Chk2-HuR interaction and HuR phosphorylation at serine and threonine in lymphocytes in response to IR, by co-IP of HuR followed by Western blotting (supplemental Figure 5A-B). A total of 1 Gy of IR did not significantly affect total protein levels or the subcellular localization of HuR (supplemental Figures 1B and 5C), which excludes the possibility that 1 Gy of IR triggers HuR phosphorylation at S158 and S221 by PKC and S202 by Cdk1, which was previously shown to influence its subcellular distribution.32,34 To identify the HuR phosphorylation residue(s) regulated by ATM-induced Chk2 activity and that are critical for HuR-mRNA interaction in response to IR exposure, we examined HuR-TAP fusion proteins mutated at Chk2 phosphorylation sites: HuR(S88A), HuR(S100A), HuR(T118A)12 (Figure 5A). As shown in Figure 4B, treatment with either ATM or Chk2 inhibitors could effectively inhibit the function of these molecules and subsequently modulate HuR binding activity. Therefore, cells pretreated either with ATM or Chk2 inhibitors served us as controls, confirming contribution of ATM/Chk2 pathway in the HuR-mRNA association. Importantly, analysis of the binding capacity of each HuR-TAP mutant protein to the validated mRNAs, which increased its association with HuR after IR (p21, FOXO3, and MEK2), consistently showed a lower association with HuR(S100A) compared with HuR(WT), and other mutants (Figure 5B). By contrast, binding of mutant HuR(T118A) to mRNAs after IR was generally similar to binding of HuR(WT). To corroborate the role of Ser-100 site, the binding of fusion proteins mimicking phosphorylated residues S100, HuR(S100D)TAP, was also tested and demonstrated increased association with validated transcripts after IR compared with HuR(WT) (Figure 6).
Association of HuR's point mutated at Chk2 phosphorylation sites with target mRNAs. (A) Schematic of point-mutated HuR residues phosphorylated by Chk2 (top) and HuR-TAP proteins expression in transfected HEK-293 cells. Cells were left untreated, treated with ATM or Chk2 inhibitors (10μM, 8 hours), or pretreated with ATM or Chk2 inhibitors for 2 hours, and then irradiated (1 Gy, 6 hours). (B) Binding of HuR-TAP chimeric proteins HuR target mRNAs was measured by TAP IP followed by quantitative RT-PCR. The individual enrichment of each mRNA in HuR-TAP fusion proteins IPs compared with TAP (vector control) IPs is indicated. The average of 2 similar experiments is shown.
Association of HuR's point mutated at Chk2 phosphorylation sites with target mRNAs. (A) Schematic of point-mutated HuR residues phosphorylated by Chk2 (top) and HuR-TAP proteins expression in transfected HEK-293 cells. Cells were left untreated, treated with ATM or Chk2 inhibitors (10μM, 8 hours), or pretreated with ATM or Chk2 inhibitors for 2 hours, and then irradiated (1 Gy, 6 hours). (B) Binding of HuR-TAP chimeric proteins HuR target mRNAs was measured by TAP IP followed by quantitative RT-PCR. The individual enrichment of each mRNA in HuR-TAP fusion proteins IPs compared with TAP (vector control) IPs is indicated. The average of 2 similar experiments is shown.
Association of HuR-TAP fusion protein mimicking phosphorylated residue S100 with HuR target mRNAs. (A) HEK-293 cells were transfected with plasmids to express HuR-TAP fusion proteins (WT or bearing a phosphomimic mutation S100D). (B) HuR binding was assessed as explained in Figure 5. The enrichment of each mRNA in HuR(S100D)TAP IPs compared with HuR(WT)TAP IPs is indicated. The average of 2 similar experiments is shown.
Association of HuR-TAP fusion protein mimicking phosphorylated residue S100 with HuR target mRNAs. (A) HEK-293 cells were transfected with plasmids to express HuR-TAP fusion proteins (WT or bearing a phosphomimic mutation S100D). (B) HuR binding was assessed as explained in Figure 5. The enrichment of each mRNA in HuR(S100D)TAP IPs compared with HuR(WT)TAP IPs is indicated. The average of 2 similar experiments is shown.
In summary, mutation of the HuR residues phosphorylated by Chk2 suggests that phosphorylation at Ser-100 is important for increased association of HuR with target mRNAs and Thr118 can be relevant for the dissociation of HuR-mRNA complexes after IR. These results demonstrate that ATM/Chk2-mediated phosphorylation of HuR is required for optimal HuR binding in response to IR, providing an important functional linkage between ATM/Chk2 signaling and HuR activity after DNA damage.
Discussion
In response to DNA double-strand breaks, ATM phosphorylates multiple proteins involved in cell-cycle checkpoints, including the checkpoint kinase Chk2.35,36 ATM-activated Chk2 prevents replication of damaged DNA and controls cell fate by subsequent phosphorylation of multiple downstream proteins involved in gene transcription, DNA repair, growth arrest, and apoptosis, such as E2F1,37 BRCA1,38 Cdc25C and Cdc25A,35,39 and p53.40 Given that modulation of phosphorylation sites on RBPs has been shown to influence the association of mRNAs with the protein,41 here we propose a model in which ATM and Chk2 cooperate to phosphorylate HuR and thereby modulate HuR association with its target mRNAs. We set out to examine this regulation experimentally, using AT patient-derived B-cell lines as well as normal donors that differ in their ATM status and exposing them to IR. The relatively low dose we used (1 Gy) precludes the confounding influence of parallel pathways, such as the ATR/Chk1 signaling (supplemental Figure 1A), which was not affected at these doses. We identified cadres of mRNAs whose association with HuR was altered in cells with wild-type ATM but not in cells without a functional ATM in response to IR-triggered DNA damage stimuli. These data support the view HuR functions as a downstream effector of ATM/Chk2 phosphorylation, enhancing its association with one subset of mRNAs while reducing its association with others.
After genotoxic stress, the levels of expressed genes are controlled through both transcriptional and posttranscriptional events. HuR has been shown to stabilize target mRNAs, modulate their translation, or affect splicing and export.15,17 Recently, phosphorylation of HuR at a region of RNA recognition motifs 1 and 2 by the checkpoint kinase Chk2 was shown to affect HuR's ability to bind to the SIRT1 mRNA, in turn affecting the stability of the transcript.12 However, there are scant data examining the modulation of HuR binding to target RNAs after IR exposure. Although the impact on transcription of the AT null state has previously been explored,42-44 there are limited data on post-transcriptional events in AT null cells in response to IR. In our study, IR exposure triggered reproducible changes in HuR binding of target mRNAs, and subsequent protein synthesis, accompanied by minor or no significant changes in total mRNA levels of validated genes (Figure 3B-F; supplemental Figure 3). These novel data demonstrated that, in response to IR, the main influence of HuR is exerted on the translation of its targets. However, this control may be specific to subsets of target mRNAs and needs to be confirmed by additional experiments in future studies. Based on our findings, we propose that HuR plays a central role in ATM-mediated Chk2 regulation of genes after DNA damage. This regulation probably occurs by Chk2-mediated HuR phosphorylation resulting in modulation of HuR binding activity to target mRNAs encoding proteins involved in control of cell fate after DNA damage, and subsequent modulation of their expression via posttranscriptional mechanisms.
Using biologic pathway analysis software, we classified mRNAs that associate with HuR in response to IR into subsets of functionally related genes to define the relationships among proteins involved in the DNA damage response. Many proteins implicated in the MEK/extracellular signal-regulated kinase (ERK) signaling pathway, which is often dysregulated in cancer, were encoded by transcripts differentially associated with HuR in an ATM/Chk2-dependent manner in response to IR. Mitogen-activated protein kinase signaling works in concert to balance cell death with growth and survival, and the deregulation of this pathway leads to genomic instability and cancer.45 Furthermore, the MEK/ERK signaling pathway is constitutively activated in a large number of cancers, including lymphomas.46-49 An exciting finding in this study was that 2 major components of this pathway, MEK1 and MEK2, were found to be regulated by HuR in response to IR in a reciprocal manner when using ATM wild-type and ATM null cells (Figures 2A, 3B-C). MEK1 and MEK2 contribute to the divergent effects of ERK signaling, whereas MEK1-activated ERK2 induces cell proliferation, MEK2-activated ERK2 initiates growth arrest.50-52 In our study, the HuR-MEK2 mRNA interaction, and MEK2 protein levels increased in ATM wild-type but not in ATM null cells after treatment with IR. These results support the importance of ATM/Chk2 signaling on the role of HuR in regulation of MEK2 levels and its contribution to IR-induced cell arrest, an attenuated cellular response in the absence of an ATM protein (in ATM null cells). The IR-induced increase in the binding of HuR to MEK1 mRNA (and modulation of HuR binding to subset mRNAs) that was observed in ATM null cells indicates the existence of other ATM-independent mechanisms influencing HuR binding capacity, leading to an IR-induced increase in cell proliferation in ATM null cells. Our previous finding that increased MEK1 mRNA-HuR association is implicated in cellular transformation24 in conjunction with presented results support the notion that the dysregulation of HuR activity in the ATM null phenotype may contribute to the high propensity of AT patients to develop malignancies.
Although it is well known that HuR regulates numerous genes posttranscriptionally, of significant interest to us was to identify specific genes/pathways that are dependent on and selectively participate in the radioresponse through the ATM/Chk2/HuR regulation cascade. The cyclin-dependent kinase inhibitor p21/CDKN1A (a well-characterized HuR target) appeared as the primary hub, in the top functional network derived from the genes, which changed association with HuR in response to IR exclusively in the ATM wild-type cells. p21 plays a major role in regulating cell-cycle progression, and it has been shown to be involved in mediating growth arrest in response to a variety of stressors, including DNA damage.53,54 Expression of p21 protein is tightly regulated at multiple levels. Although the best-understood induction of p21 response to DNA damage occurs through a transcriptional mechanism by the tumor suppressor p53,53-56 HuR has been reported as a major player in regulating p21 expression post-transcriptionally in response to short-wavelength ultraviolet light.14 Our study discovered the impact of ATM/Chk2 cascade on IR-mediated HuR regulation of the p21 network by post-transcriptional regulation of p21 mRNA and the mRNAs encoding multiple p21-interacting proteins in the network. Notably, a recent paper demonstrated the clinical relevance of p21 protein levels in predicting clinical outcome of DLBCL patients older than 60 years after treatment with R-CHOP.57
In addition, other data show that IR modifies gene expression at the level of translation and suggests that IR-induced translational control of a subset of mRNAs is a fundamental component of cellular radioresponse.58 Linking presented findings to the well-established role of HuR in regulating translation of target mRNAs in the damage response in concert with examining the upstream participation of ATM in this regulation provides us with a unique opportunity to precisely define the role of HuR in the DNA damage response (DDR).
Chk2 was found to be activated by H2O2-induced free radical damage, physically interacted with HuR, and was shown to phosphorylate HuR at residues Ser-88, Ser-100, and Thr-118.12 Our data build on these earlier findings and suggest that, in response to IR exposure, HuR associates with target mRNAs when Ser-100 is phosphorylated and dissociates when site Thr-118 is phosphorylated. In addition, HuR phosphorylation at Ser-202 by the G2-phase kinase Cdk1 was shown to influence its subcellular distribution.31 Analyzed transcripts demonstrated the same binding affinity to HuR(S202) mutant compared with wild-type HuR (Figure 5B) and HuR subcellular localization did not change after 1 Gy of IR (supplemental Figure 5C) establishing an important role of ATM/Chk2 signaling on HuR gene regulation in the double-stranded DDR.
Together, our studies are the first to demonstrate biologically relevant differences in genome-wide ATM/Chk2/HuR-modulated transcripts occurring in response to IR treatment. Using a combination of genetic and pharmacologic approaches, we established that the ATM/Chk2-induced HuR phosphorylation is able to induce a complex and dynamic posttranscriptional RNA operon that mediates in part the DNA damage response. This response most probably involves the coordinated action of additional RBPs and microRNAs and probably additional factors in coordinating an intricate functional program. Nevertheless, identifying HuR as a main regulator in the posttranscriptional DNA damage response in lymphocytes reveals an important area for future studies. The newly identified role of ATM in Chk2 phosphorylation of HuR during the DNA damage response provides a functional link between ATM and HuRs oncogenic, survival, and antiapoptotic activities. The findings presented here reveal a previously unidentified defect in posttranscriptional regulation secondary to a lack of ATM activity, thereby contributing to the inappropriate DNA damage response reported in ATM null cells. Because AT patients show a hypersensitivity to radiotherapy and an extraordinary high risk of developing lymphoid malignancies, gaining a better understanding of this hitherto unreported perturbation in the translation of cancer-related mRNAs will provide additional insight into the high predisposition to lymphoma of patients with AT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr F. Carrier for AT and ATM stably transfected GM5849 fibroblasts and S. Corl for technical assistance.
This work was supported in part by the American Cancer Society (Institutional Research Grant; K.M.-M), the Department of Veterans Affairs (Merit Review Award; R.B.G.), and the National Institutes of Health (R01AA017972; R.B.G.). K.G.B. and M.G. were supported by the National Institute on Aging-Intramural Research Program, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: K.M.-M. performed research, analyzed data, and wrote the paper; P.R.H. performed research and wrote the paper; Y.Z., B.D., E.L., and K.G.B. performed research and analyzed data; J.D.K. wrote the paper; M.G. provided reagents and wrote the paper; Z.L. analyzed data and wrote the paper; and R.B.G. conceived of and designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: J.D.K. has financial relationships with Ribonomics Inc and MBL Inc, which hold licenses to technologies relevant to aspects of this study. The remaining authors declare no competing financial interests.
Correspondence: Ronald B. Gartenhaus, Marlene and Stewart Greenebaum Cancer Center, University of Maryland, Baltimore, MD, 21201; e-mail: rgartenhaus@som.umaryland.edu.