In this issue of Blood, Cranmer and colleagues demonstrate that the linkage between the cytoskeletal protein filamin A and the platelet receptor glycoprotein (GP) Ibα provides structural integrity to the plasma membrane during platelet adhesion to von Willebrand factor (VWF) under high shear.1
On damage to vessel walls, exposed extracellular matrix (ECM) proteins trigger a series of events leading to the formation of a vascular plug. ECM-associated VWF plays a critical role in mediating initial platelet recruitment under flow because of the rapid on-rate of binding between VWF and the platelet receptor GPIb/V/IX.2 A conformational change in VWF induced by binding to ECM proteins is required for GPIb-VWF interactions, restricting platelet recruitment to sites of vascular injury. Alternatively, high-affinity binding of GPIb to VWF can be induced by exogenous modulators such as the bacterial glycopeptide ristocetin, the snake C-type lectin botrocetin, or pathologic levels of high shear as is found in stenosed vessels.2,3
Concomitant with mediating platelet recruitment to VWF, the GPIb/V/IX receptor complex regulates the cytoskeletal architecture of platelets. The GPIb/V/IX complex is physically anchored to the membrane skeleton through the cytoskeletal protein filamin A, and is intimately involved in maintaining platelet size and shape.4,5 Disrupted expression or mutations in the GPIb/V/IX complex in Bernard-Soulier syndrome results in a bleeding phenotype characterized by giant platelets and thrombocytopenia.6 Similarly, mice with filamin-deficient platelets have macrothrombocytopenia, increased bleeding times, and decreased expression and altered surface distribution of GPIbα.7
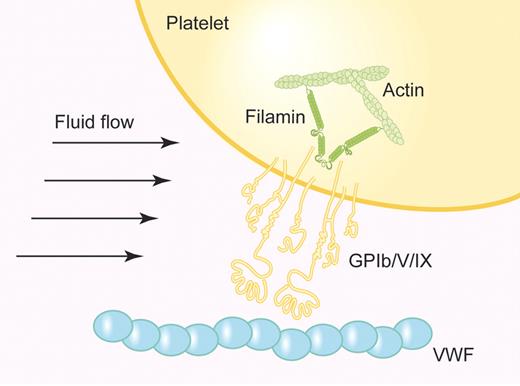
Filamin A is composed of 2 identical subunits, each containing an N-terminal actin-binding domain followed by 24 tandem immunoglobulin-like domains (IgFLN1-24). Dimerization of filamin through IgFLN24 results in a flexible parallel homodimer that can induce high-angle branching of actin filaments, promoting the formation of orthogonal actin networks. With more than 50 binding partners, filamin A plays an integral role in anchoring cell-surface receptors to the actin cytoskeleton and localizing cytoplasmic signaling proteins. In platelets, the protein tyrosine kinase Syk binds at IgFLN5,7 whereas the GPIbα subunit recognition site in filamin A resides in IgFLN17.8 The cytoplasmic residues 557 to 575 on the GPIbα subunit constitute the GPIb/V/IX-filamin interaction site.9 In this issue, Cranmer and colleagues demonstrate that GPIbα-filamin binding is required for maintaining the mechanical integrity of the platelet membrane during platelet adhesion to VWF under shear flow (see figure).1
Representation of GPIb/V/IX-mediated platelet recruitment to VWF under flow. The GPIbα subunit of GPIb/V/IX links to the actin membrane skeleton through filamin A, stabilizing the plasma membrane and supporting the adhesion of platelets to VWF under high shear. (Professional illustration by Paulette Dennis.)
Representation of GPIb/V/IX-mediated platelet recruitment to VWF under flow. The GPIbα subunit of GPIb/V/IX links to the actin membrane skeleton through filamin A, stabilizing the plasma membrane and supporting the adhesion of platelets to VWF under high shear. (Professional illustration by Paulette Dennis.)
Using a transgenic mouse model with human GPIbα (hGPIbα)–expressing platelets, Cranmer et al engineered the substitution of 2 residues in the cytoplasmic domain (Phe568Ala and Trp570Ala) of hGPIbα to disrupt the hGPIbα-filamin linkage (hGPIbαFW). The authors demonstrate that platelet recruitment to human VWF under shear remained unchanged in the absence of the hGPIbα-filamin linkage, suggesting that the GPIbα-filamin A interaction is dispensable for the intrinsic VWF binding function of GPIbα. However, once bound to VWF and subjected to high shear, hGPIbαFW platelets formed extensive membrane tethers that detached from the main body of the platelet, leading to progressive reductions in the size of translocating platelets resulting from the deposition of platelet membrane on VWF. Ultimately, hGPIbαFW platelets disintegrated under shear conditions relevant to arterial thrombus formation.
The results presented by Cranmer et al differ from previous studies that have shown that GPIb-cytoskeletal interactions can directly regulate the binding properties of the receptor. For instance, Feng et al demonstrated that blocking GPIbα-filamin interactions inhibited VWF-induced platelet aggregation, while Englund et al found that filamin-bound GPIbα had reduced VWF-binding capacity.4,9 Further investigation will be required to thoroughly address these discrepancies, because the transgenic mice generated by Cramer et al express both human and endogenous mouse GPIbα, and thus, in the hGPIbαFW mice, the endogenous mouse GPIbα-filamin linkage remains intact. Removal of mouse GPIbα from hGPIbαFW platelets or use of the newly generated filamin A-null mouse platelets may help to resolve the discrepancies. Nonetheless, these new findings from Cranmer et al establish a key function for filamin A in regulating the mechanoreceptor properties of the GPIb/V/IX complex required for the maintenance of platelet integrity under high shear.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■