Abstract
Dendritic cells (DCs) are known to regulate immune responses by inducing both central and peripheral tolerance. DCs play a vital role in negative selection of developing thymocytes by deleting T cells with high-affinity for self-peptide–major histocompatibility complexes. In the periphery, DCs mediate peripheral tolerance by promoting regulatory T-cell development, induction of T-cell unresponsiveness, and deletion of activated T cells. We studied whether allogeneic DCs, obtained from bone marrow cultured with either Flt3L (FLDCs) or granulocyte-macrophage colony-stimulating factor (GMDCs), could induce allospecific central and peripheral tolerance after IV injection; B cells were used as a control. The results showed that only FLDCs reached the thymus after injection and that these cells induced both central and peripheral tolerance to donor major histocompatibility complexes. For central tolerance, injection of FLDCs induced antigen-specific clonal deletion of both CD8 and CD4 single-positive thymocytes. For peripheral tolerance, injection of FLDCs induced donor-specific T-cell unresponsiveness and prolonged survival of donor-derived skin grafts. Tolerance induction by adoptive transfer of FLDCs could be a useful approach for promoting graft acceptance after organ transplantation.
Introduction
Dendritic cells (DCs) are known to be more effective as antigen-presenting cells (APCs) than either B cells or macrophages.1 The principal function of DCs is to promote T-cell activation and differentiation into effector T cells by presenting foreign antigens and providing costimulatory signals.2 DCs also play a critical role in maintaining not only peripheral but also central tolerance.3 Central tolerance is established by negative selection of developing T cells that have high-affinity T-cell receptors (TCRs) for self-peptide major histocompatibility complexes (MHCs) expressed on bone marrow (BM)–derived DCs or thymic epithelial cells in the thymic medulla, which is the main site for negative selection.4-6
Most thymic DCs are localized in the medulla and, like DCs in peripheral lymphoid organs such as lymph nodes and spleen, can be classified into 2 distinct subsets.7 Plasmacytoid DCs (pDCs) express B220 and low levels of CD11c, whereas conventional DCs (cDCs) are CD11c+B220−.8 cDCs are further subdivided by expression of CD8α and signal-regulatory proteinα (SIRPα) as CD8α+SIRPα− DCs and CD8α−SIRPα+ DCs.9 Some DCs are capable of homing to the thymus from the periphery, and these cells are thought to consist of pDCs and CD8α−SIRPα+ DCs, whereas cDCs of intrathymic origin are mostly CD8α+SIRPα− DCs.10 Although conventional DCs are presumed to be crucial for negative selection of developing thymocytes, the involvement of pDCs in selection is unclear.
The relative number of DCs in vivo is low, and isolating sufficient numbers for clinical trials is challenging, so most studies have used ex vivo–generated DCs.11 There are several well-established procedures for generating DCs in culture from BM precursors using granulocyte-macrophage colony-stimulating factor (GM-CSF) or Flt3L.12,13 Culture of BM cells with GM-CSF produces only limited heterogeneity of DCs.14,15 Conversely, culture of BM cells with Flt3L allows the generation of both cDCs and pDCs, which are the close equivalent of steady-state splenic cDCs and pDCs.16
In organ transplantation recipients, repression of antidonor immunity is being investigated as a means to achieve long-lasting, specific tolerance to transplanted organs. Among the strategies being evaluated to attain this goal, much interest has focused on the potential of DCs to regulate immune responses.17 Immature DCs are known to induce antigen-specific peripheral T-cell unresponsiveness or anergy, and they can also generate regulatory T cells.18,19 However, to prevent the release of newly generated, nontolerant T cells from the thymus, DC-based therapies for transplantation must induce central tolerance. To date, DC-based strategies for transplantation have generated only peripheral tolerance, and continuous production of donor-reactive T cells from the thymus could be one of the main reasons for chronic organ rejection.
In the present study, we compared the thymus-homing ability and tolerogenic function of 3 types of APCs: GM-CSF–induced BM-derived DCs (GMDCs), Flt3L-induced BM-derived DCs (FLDCs), and splenic B cells. Only FLDCs were found to be able to traffic to the recipient thymus after IV injection and induce negative selection. Central tolerance induction after IV injection of FLDCs reflected clonal deletion of both CD8 and CD4 single-positive (SP) thymocytes and led to prolonged acceptance of donor skin grafts. These findings suggest a novel clinical strategy for preventing chronic graft rejection.
Methods
Mice
Female C57BL/6(H2b), B6.SJL (Ly5.1, H2b), and BALB/c (H2d) mice were obtained from Sankyo Labo Service. DO11.10 TCR Tg Rag−/− mice were obtained by intercrossing BALB/c background Rag−/− mice and BALB/c DO11.10 TCR Tg mice, which are maintained in our facility. For BM chimera mice, 2C TCR (H2k) Tg mice were kindly provided by Dr Takahama (University of Tokushima, Tokushima, Japan). Mice were maintained under specific pathogen–free conditions and used at 8 to 10 weeks of age. All experiments were performed in accordance with protocols approved by the Animal Care and Use Committee of the Tokyo University of Science.
Antibodies and reagents
FITC-labeled anti–B7-1 (RM80), B7-2 (GL-1), CD11b (M1/70), and anti-rat IgG mAbs, PE-labeled anti-B220 (RA3-6B2) mAb, biotin-labeled anti-CD11c (N418) mAb, and anti-CD172a (P84) mAb were purchased from BD Pharmingen. Biotin-labeled UEA-1 was purchased from Vector Laboratories. The B-cell hybridomas anti-CD40L (MR-1) and anti–class II MHC (M5/114) were obtained from ATCC. Anti-CD40L, anti-CD4 (GK1.5), anti-CD8 (53-6-7), and anti–MHC class II mAbs were purified in our laboratory from the culture supernatant. Human CTLA-4Ig fusion protein was prepared as described previously.20 CellTracker Green carboxyfluorescein diacetate succinimidyl ester (CFSE) and CellTracker Orange 5-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine (CMRA) were purchased from Invitrogen. Recombinant mouse (rm) GM-CSF and rmFlt3L were obtained from the culture supernatant of P3U1 BCMGS-rmFlt3L or P3U1 BCMGS-rmGM-CSF.
Preparation of B cells, GMDCs, and FLDCs
For preparation of B cells, splenocytes were harvested and T-cell depletion was carried out by treatment with anti-mThy1 (HO13.4) mAb plus rabbit complement. The B cells were positively panned on plates coated with rabbit anti–mIgM. More than 95% of the cells expressed B220.
BM cells from BALB/c or B6 mice were harvested from bilateral femurs and tibias, and red cells were lysed with ammonium chloride. For GMDC culture, BM cells were cultured with 100 ng/mL rmGM-CSF.12 On day 8, loosely adherent cells were recovered and used as immature GMDCs in our experiments; 65% or more of the nonadherent cells expressed CD11c. For FLDC culture, BM cells were cultured with 100 ng/mL rmFlt3L.13 At day 8, loosely adherent cells were recovered and used as immature FLDCs; 85% or more of the nonadherent cells expressed CD11c+. In some experiments, cDCs from FLDCs were negatively isolated using PE-labeled anti-B220 mAb and anti-PE MACS Microbeads. The pDCs were positively isolated from FLDCs using PE-labeled anti-B220 mAb and anti-PE Microbeads. The purity of cDCs and pDCs was more than 80%.
Preconditioning regimens
To block costimulatory signals through CD28/B7 and CD40/CD40L, hCTLA4-Ig and anti-CD40L mAb (MR-1) (500 μg of each) was injected intraperitoneally on days 0, 2, and 7 after APC transfer. Natural killer (NK) depletion was carried out by injecting 200 μg of mouse anti–NK1.1 mAb (PK136) on days −3 and −1 before APCs were transferred.
In vivo homing assay
APCs were labeled with CFSE at a final concentration of 0.25μM by incubation for 3 minutes at 37°C. After labeling, cells were washed and 1 × 107 APCs were injected IV into B6 recipient mice after the preconditioning regimen. After 24 hours, mice were killed and spleens, lymph nodes, thymi, BM, livers, and lungs were collected. APC migration to various organs and tissues was measured by detection of CFSE+B220+ cells or CFSE+CD11c+ cells by flow cytometric analysis.
Immunohistochemistry
BALB/c FLDCs were labeled with CMRA or CFSE (Invitrogen) according to the manufacturer's instructions, and 1 × 107 cells were infused IV into preconditioned B6 mice. After 24 hours, mice were killed and immunohistochemistry was performed on frozen sections of thymus. Sections of 6-μm thickness were prepared, air-dried, and fixed for 10 minutes with ice-cold acetone. After being blocked with 2% bovine serum albumin, sections were incubated with Cy5-conjugated anti-CD8 (53-12-6) mAb, PE-labeled anti-B220 (RA3-6B2) mAb, lectin UEA-1, and anti-CD11c (N418) mAb. For secondary detection of UEA-1 biotin and CD11c biotin, sections were incubated with Dylight 488–conjugated streptavidin (Pierce). The sections were examined by fluorescence microscopy (BZ9000; Keyence).
Mixed-lymphocyte reaction (MLR) assays
Recipient B6 mice injected with BALB/c APCs (1 × 107) on days 0 and 7 were killed on day 10, and splenocytes and thymocytes were collected. Splenic T cells were prepared by removing B cells and APCs by negative selection on plates coated with rat anti–mMHC class II (M5/114) mAbs. For preparation of mature thymocytes, immature thymocytes were depleted by treatment with anti-CD24 (J11d) mAb plus rabbit complement. Splenic APCs were depleted of T cells with anti-mThy1 mAb plus rabbit complement. Enriched splenic T cells or mature thymocytes (4 × 105) were cocultured with 7 × 105 irradiated (30 Gy) splenic APCs in flat-bottomed, 96-well culture plates for 3 days in 5% CO2 at 37°C. Culture plates were pulsed with 0.5 μCi of 3H-thymidine and cells were harvested after 18 hours.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
FL-pDCs and FL-cDCs were sorted using FACSAria. The purity of sorted cells was more than 95%. RNA was extracted from GMDCs, FLDCs, FL-pDCs, and FL-cDCs, reverse transcribed to cDNA, and then analyzed for the presence of IL-10, TGF-β, TNF-α, and IFN-γ sequences. (IL-10 sense: GGTTGCCAAGCCTTATCGGA, IL-10 antisense: ACCTGCTCCACTGCCTTGCT, TGF-β sense: TACAGGGCTTTCGATTCAGC, TGF-β antisense: GGTTCATGTCATGGATGGTG, TNF-α sense: AATGGCCTCCCTCTCATCAGT, TNF-α antisense: CCTCCACTTGGTGGTTTGCTA, IFN-γ sense: GGCTTTGCAGCTCTTCCTCAT, IFN-γ antisense: TGGCTCTGCAGGATTTTCATG). HPRT was amplified as a control.
Clonal deletion assay
For generation of 2C-B6 chimeras, lethally irradiated recipient B6 mice were injected with a mixture of donor 2C TCR Tg and B6 Ly5.1 BM cells. More than 50 days later, BALB/c or B6 FLDCs was injected IV into the 2C-B6 BM chimera on days 0 and 7, together with costimulation blockade. On day 10, mice were killed and single-cell suspensions were collected from thymi and analyzed by flow cytometry.
For generation of DO-BALB/c chimera mice, BM cells were isolated from donor DO11.10 TCR Tg Rag−/− mice and BALB/c mice and injected IV into lethally irradiated recipient BALB/c mice. More than 50 days later, ovalbumin (OVA) peptide (323-339)–loaded BALB/c FLDCs (incubated with 5mM OVA peptides for 24 hours) or unloaded FLDCs were injected IV into the DO-BALB/c BM chimeras on days 0 and 7 with costimulation blockade. On day 10, mice were killed and single-cell suspensions were collected from thymi and analyzed by flow cytometry.
Skin grafts
Recipient B6 mice injected with BALB/c APCs (1 × 107) on days 0 and 7 were transplanted with skin allografts as described previously.21 Briefly, full-thickness BALB/c or C3H skin grafts (∼ 1 cm2) were transplanted onto the dorsal thorax of recipient mice and secured with a plastic adhesive bandage for 7 days. Graft survival was followed by daily visual inspection. Rejection was defined as a complete loss of viable epidermal graft tissue.
Results
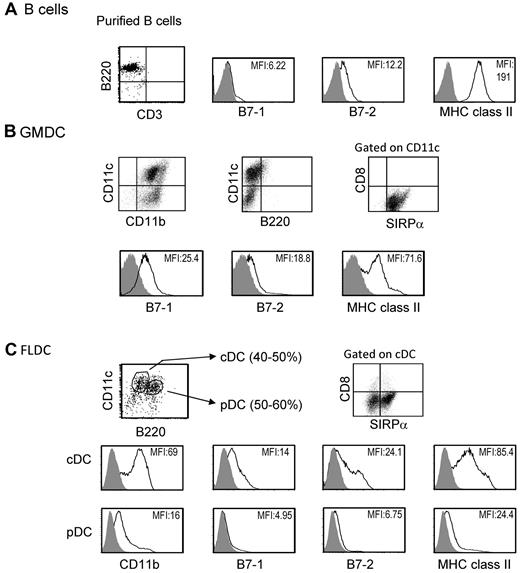
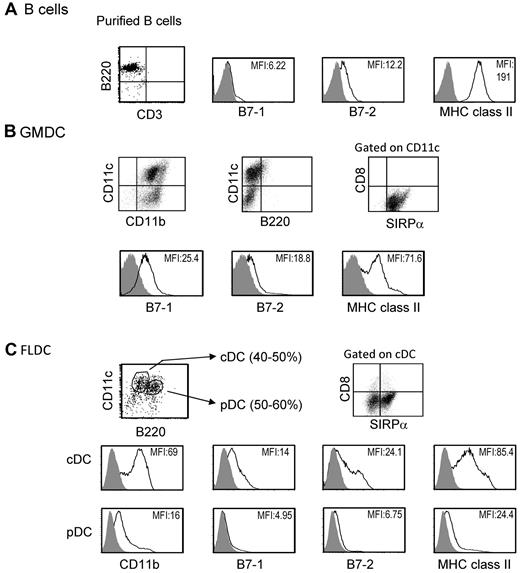
Previous studies have reported that under certain conditions, adoptive transfer of MHC-different donor splenocytes can induce donor-specific tolerance.22 We studied induction of donor microchimerism and tolerance after IV injection of 3 types of APCs, GMDCs, FLDCs, and splenic B cells. As described elsewhere, freshly isolated splenic B cells expressed high levels of MHC class II and low levels of CD80 (B7-1) and CD86 (B7-2) costimulatory molecules (Figure 1A).23 GMDCs were prepared by culture of BM cells with GM-CSF for 8 days, and were a relatively homogeneous population of 65%-75% CD11c+ cells that expressed SIRPa+ but did not express B220 or CD8α. The expression of MHC class II on GMDCs was low to intermediate and expression levels of CD80 and CD86 were low (Figure 1B). The FLDCs generated by BM culture with Flt3L showed 80%-90% CD11c+ cells, and these cells were subdivided into pDCs (CD11cintB220+) and cDCs (CD11chiB220−). Approximately 50% of cDCs in FLDCs were SIRPa+ and expressed low to intermediate levels of MHC class II and low levels of costimulatory molecules, which were comparable with the levels on GMDCs (Figure 1C). In contrast, the levels of MHC class II and costimulatory molecules on pDCs were extremely low (Figure 1C). Based on this phenotypic analysis, the GMDC and FLDC populations used in this study could both be classified as immature DCs.15
Characteristics of APCs. The data show representative expression of B7-1, B7-2, and MHC II on purified BALB/c splenic B cells (A), GMDCs (B), and FLDCs (C) as measured by flow cytometric analysis. The FLDCs were subdivided into pDCs (CD11cintB220+) and cDCs (CD11chiB220−). Data in all panels are representative of at least 5 independent experiments.
Characteristics of APCs. The data show representative expression of B7-1, B7-2, and MHC II on purified BALB/c splenic B cells (A), GMDCs (B), and FLDCs (C) as measured by flow cytometric analysis. The FLDCs were subdivided into pDCs (CD11cintB220+) and cDCs (CD11chiB220−). Data in all panels are representative of at least 5 independent experiments.
Analysis of the ability of various APCs to home to the thymus
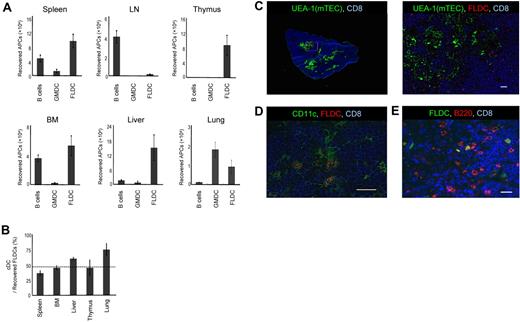
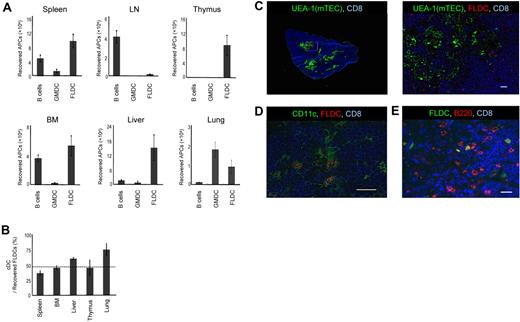
The presence of donor APCs in the thymus is considered to be critical for the induction of central tolerance.24 Therefore, we examined the ability of allogeneic APCs to migrate to the thymus after donor APCs were labeled with CFSE and 1 × 107 cells were injected into B6 mice. To inhibit acute rejection of donor APCs, recipient mice were first treated with anti-NK1.1 mAb to deplete NK cells. In addition, costimulation blockade was carried out by the combined administration of hCTLA4-Ig and anti-CD40L mAb. At 24 hours after injection, the thymus and various organs were collected and single-cell suspensions were analyzed by flow cytometry. When GMDCs and B cells were injected, localization of CFSE+ cells in the thymus was virtually undetectable (Figure 2A); B cells migrated mainly to the spleen, lymph nodes, and BM, whereas GMDCs lodged largely in the lungs. In marked contrast, FLDCs migrated well to the thymus and also to the spleen, BM, liver, and lungs, although not to the lymph nodes (Figure 2A). Thus, homing to the thymus applied only to FLDCs and not to GMDCs or B cells.
Migration of APCs. (A) Donor BALB/c APC subsets were labeled with CFSE and injected IV into B6 mice (n = 4); 24 hours later, migration of APCs to various organs and tissues was determined by detecting CFSE+ cells using flow cytometric analysis. (B) Comparison of homing of cDCs and pDCs (n = 4). The horizontal dashed line indicates the percentage of cDCs in the input sample. (C-E) Immunostaining of frozen sections of the thymus injected with CMRA- or CFSE-labeled FLDCs. Frozen sections of thymus were stained with Cy5–anti-CD8 (53-6.7) mAb, the lectin UEA-1, biotin–anti-CD11c (N418) mAb, and PE–anti-B220 mAb, respectively. Scale bars represent 200μm (C left) or 20μm (C right, D-E). Data in all panels are representative of 3 independent experiments.
Migration of APCs. (A) Donor BALB/c APC subsets were labeled with CFSE and injected IV into B6 mice (n = 4); 24 hours later, migration of APCs to various organs and tissues was determined by detecting CFSE+ cells using flow cytometric analysis. (B) Comparison of homing of cDCs and pDCs (n = 4). The horizontal dashed line indicates the percentage of cDCs in the input sample. (C-E) Immunostaining of frozen sections of the thymus injected with CMRA- or CFSE-labeled FLDCs. Frozen sections of thymus were stained with Cy5–anti-CD8 (53-6.7) mAb, the lectin UEA-1, biotin–anti-CD11c (N418) mAb, and PE–anti-B220 mAb, respectively. Scale bars represent 200μm (C left) or 20μm (C right, D-E). Data in all panels are representative of 3 independent experiments.
Because FLDCs are composed of 2 different types of DCs, cDCs and pDCs, we measured the homing properties of each of these subsets. Our results showed that both cDCs and pDCs migrated to the recipient thymus at close to the same ratio (Figure 2B), and that both SIRPα+ and SIRPα− DCs migrated equally to the recipient thymus (data not shown). Furthermore, the DCs that reached the thymus had a relatively immature phenotype (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To determine the localization of the transferred donor FLDCs in the thymus, we analyzed thymic cryosections by immunofluorescence 24 hours after injection of CMRA- or CFSE-labeled FLDCs (Figure 2C-E). Donor FLDCs localized in the corticomedullary junction, the site of negative selection. Costaining with anti-CD11c mAb confirmed that most of the CMRA+ donor cells in the thymus were CD11c+ DCs. Costaining with anti-B220 revealed that both cDCs and pDCs migrated to the area around the corticomedullary junction. In terms of their homing ability, both subpopulations of FLDCs demonstrated equivalent capacity to localize in the thymus after IV transfer.
Donor-specific T-cell tolerance induced by adoptive transfer of FLDCs
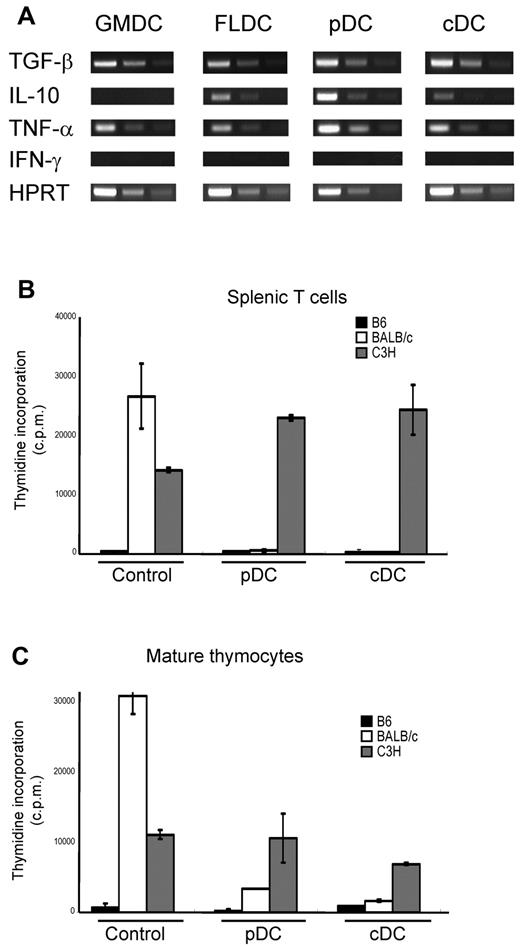
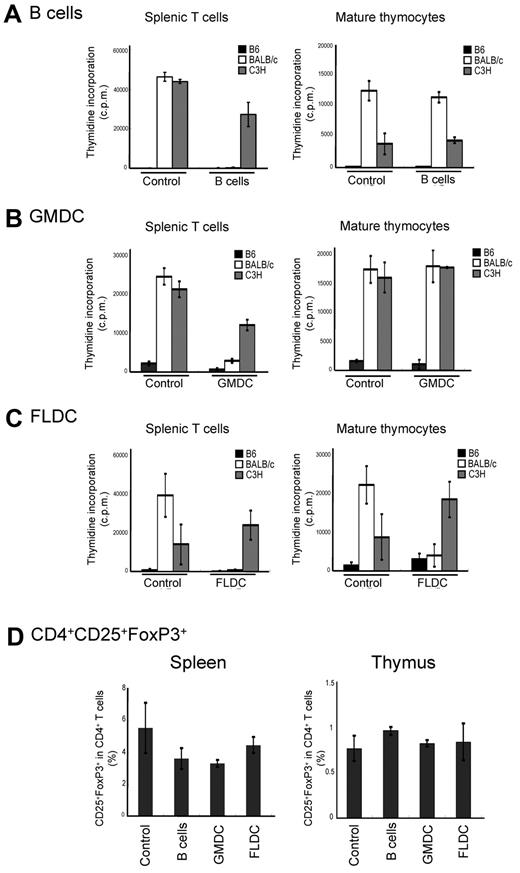
Other investigators have reported that adoptive transfer of immature DCs or B cells induces antigen-specific tolerance in vivo.25,26 Therefore, we assessed whether the infusion of donor APCs could induce donor-specific tolerance as measured by MLR assays. For this experiment, doses of 1 × 107 APC subsets from BALB/c mice were injected twice on days 0 and 7 into B6 mice that were pretreated to deplete NK cells and block costimulatory signals. At 10 days after APC subset infusion, the alloreactivity of T cells from recipient mice was assayed by MLR. Compared with control mice, splenic T cells recovered from mice injected with BALB/c FLDCs, GMDCs, or B cells responded poorly to BALB/c APCs relative to control T cells, but retained reactivity against C3H APCs (Figure 3A-C). For mature thymocytes as responder cells, injection of either B cells or GMDCs failed to reduce the response to BALB/c APCs relative to control C3H APCs (Figure 3C). In marked contrast, thymocytes from recipients of BALB/c FLDCs exhibited almost no response to BALB/c APCs, but retained reactivity to C3H APCs. Furthermore, thymocytes from FLDC-injected mice did not display activation markers (ie, up-regulation of CD44) after coculture with BALB/c APC, indicating the induction of specific tolerance at the level of mature thymocytes (supplemental Figure 2). Thus, injection of FLDCs induced both peripheral tolerance and central tolerance, whereas donor B cells and GMDCs infusion induced only peripheral tolerance. Injection of each type of APC caused no increase of CD4+CD25+FoxP3+-regulatory T cells in spleen and thymus, suggesting that peripheral and central tolerance induction was not mediated by regulatory T cells (Figure 3D).
Tolerance induction by injection of allogeneic APCs. Recipient splenic T cells or mature thymocytes from APC-injected mice were cultured in vitro with splenic APCs derived from B6, BALB/c, or C3H mice for 90 hours. MLR (incorporation of 3H-thymidine) for B6 recipients of B cells (A), GMDCs (B), and BALB/c (C) FLDCs are shown. The data represent the means of triplicate cultures. (D) Percentages of CD4+CD25+Foxp3+ cells in splenocytes of B6 recipients of BALB/c APC subsets (n = 4). Data in all panels are representative of at least 3 independent experiments.
Tolerance induction by injection of allogeneic APCs. Recipient splenic T cells or mature thymocytes from APC-injected mice were cultured in vitro with splenic APCs derived from B6, BALB/c, or C3H mice for 90 hours. MLR (incorporation of 3H-thymidine) for B6 recipients of B cells (A), GMDCs (B), and BALB/c (C) FLDCs are shown. The data represent the means of triplicate cultures. (D) Percentages of CD4+CD25+Foxp3+ cells in splenocytes of B6 recipients of BALB/c APC subsets (n = 4). Data in all panels are representative of at least 3 independent experiments.
Induction of donor-specific tolerance by injection of Flt3L-induced cDCs and pDCs
Other investigators have reported that various DCs subsets derived from BM differ in their cytokine profiles.14 Therefore, we assayed expression of suppressive cytokines (ie, TGF-β and IL-10) by RT-PCR in each DC subset. RT-PCR revealed that TGF-β was detectable in all DC subsets, whereas IL-10 was detected in FLDCs, FL-pDCs, and FL-cDCs, but not in GMDCs. We also assayed expression of other cytokines, including TNF-α and IFN-γ, by RT-PCR. TNF-α was detected in GMDCs, unfractionated FLDCs, and both FL-pDCs and FL-cDCs, but IFN-γ mRNA was not detected in any DC subset (Figure 4A). To assess which subset of FLDCs induced tolerance, mice were injected with either enriched FL-cDCs or FL-pDCs; these subsets were negatively or positively enriched using PE-labeled anti-B220 mAb, followed by incubation with anti-PE Microbeads. The resulting purity of cDCs and pDCs was more than 80% (supplemental Figure 3). Splenic T cells and mature thymocytes from mice injected with either FL-cDCs or FL-pDCs were assayed by MLR as described in “Mixed-lymphocyte reaction (MLR) assays.” Unlike control T cells, T cells from mice injected with FL-cDCs or FL-pDCs proliferated poorly to BALB/c APCs but responded well to C3H (Figure 4B-C); such tolerance to BALB/c applied to both spleen T cells and mature thymocytes. These results indicate that injection of either FL-cDCs or FL-pDCs induced strong donor-specific central and peripheral tolerance.
Contribution of Flt3L-induced cDCs and pDCs to the induction of donor-specific central and peripheral tolerance. Enriched FL-cDCs and FL-pDCs from BALB/c mice were IV transferred 10 days after DC infusion, and alloreactivity of T cells from the recipient mice was assayed by MLR. (A) RNA was extracted from each DC subset to analyze the expression of various cytokines by RT-PCR. (B-C) MLR to B6, BALB/c, and C3H spleen APCs by recipient T cells prepared from (B) spleen and (C) mature thymocytes. Data in all panels are representative of at least 3 independent experiments.
Contribution of Flt3L-induced cDCs and pDCs to the induction of donor-specific central and peripheral tolerance. Enriched FL-cDCs and FL-pDCs from BALB/c mice were IV transferred 10 days after DC infusion, and alloreactivity of T cells from the recipient mice was assayed by MLR. (A) RNA was extracted from each DC subset to analyze the expression of various cytokines by RT-PCR. (B-C) MLR to B6, BALB/c, and C3H spleen APCs by recipient T cells prepared from (B) spleen and (C) mature thymocytes. Data in all panels are representative of at least 3 independent experiments.
Clonal deletion of antigen-specific thymocytes induced by injection of FLDCs
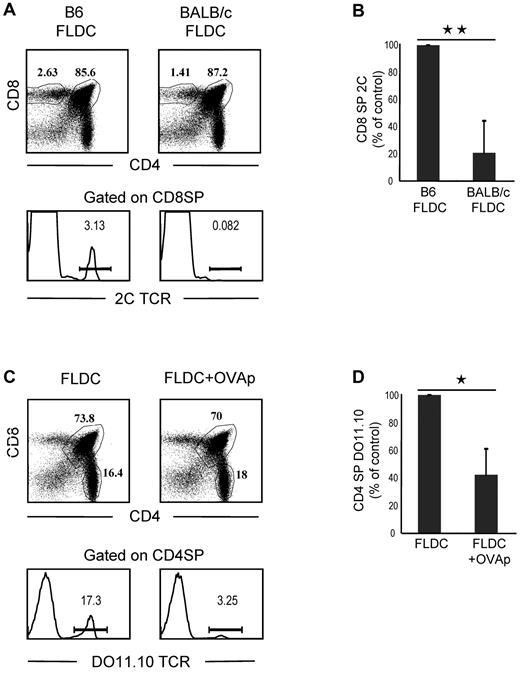
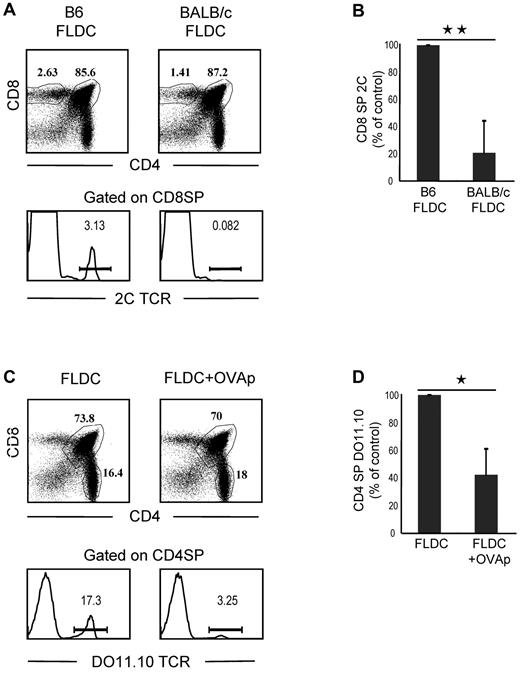
To seek further information on negative selection induced by transfer of FLDCs, we prepared mixed-BM chimeras by reconstituting lethally irradiated B6 mice with a mixture of 2C TCR TgBM cells and wild-type B6 Ly5.1 BM cells. After more than 50 days, BM chimeras were injected with B6 or BALB/c FLDCs. Because 2C CD8+ T cells have alloreactivity for MHC I Ld ligands on BALB/c APCs and BALB/c FLDCs home to the thymus, injecting the mixed chimeras with BALB/c APCs would be expected to cause selective deletion of the subset of 2C CD8+ thymocytes in the chimeras. On day 10 after APC injection, the chimeras were killed to quantitate the frequency and phenotype of wild-type and 2C thymocytes by flow cytometry. For mice injected with syngeneic B6 FLDCs, 2C CD8+ SP thymocytes accounted for approximately 3% of total CD8+ SP cells, whereas < 1% of CD8+ SP thymocytes expressed the 2C TCR in mice that received BALB/c FLDCs (Figure 5A-B). The proportion of 2C CD8+ SP thymocytes in mice injected with BALB/c FLDCs was reduced by a mean of approximately 80% (range 53%-97%) relative to B6 FLDC–injected mice. Therefore, marked negative selection of Ld-reactive 2C thymocytes occurred after transfer of BALB/c FLDCs.
Migration of FLDCs to the thymus induces clonal deletion of antigen-specific SP thymocytes. (A) 2C-B6 BM chimeras were prepared by reconstituting heavily irradiated B6 mice with a mixture of 2C TCR Tg BM cells and B6 Ly5.1 BM cells. BM chimeras were then injected with B6 FLDCs or BALB/c FLDCs. The mice were analyzed for the frequency of wild-type and 2C thymocytes by flow cytometry (n = 5); 3 of 5 FLDC-injected mice exhibited an extensive deletion of 2C cells, as shown in the lower portion of the figure. (B) Mean percentage of 2C thymocytes of 5 individual mice. **P < .01. (C) DO11.10-BALB/c BM chimera mice were prepared by reconstituting irradiated BALB/c mice with a mixture of DO11.10 TCR Tg BM cells and BALB/c BM cells. BM chimeras were then injected with unloaded or OVAp-loaded FLDCs. The mice were analyzed for the frequency of wild-type and DO11.10 thymocytes by flow cytometry (n = 4); 2 of 4 mice showed the marked deletion of DO11.10 SP CD4 cells illustrated in the lower portion of the figure. (D) Data are means from 4 mice. *P < .05; **P < .01.
Migration of FLDCs to the thymus induces clonal deletion of antigen-specific SP thymocytes. (A) 2C-B6 BM chimeras were prepared by reconstituting heavily irradiated B6 mice with a mixture of 2C TCR Tg BM cells and B6 Ly5.1 BM cells. BM chimeras were then injected with B6 FLDCs or BALB/c FLDCs. The mice were analyzed for the frequency of wild-type and 2C thymocytes by flow cytometry (n = 5); 3 of 5 FLDC-injected mice exhibited an extensive deletion of 2C cells, as shown in the lower portion of the figure. (B) Mean percentage of 2C thymocytes of 5 individual mice. **P < .01. (C) DO11.10-BALB/c BM chimera mice were prepared by reconstituting irradiated BALB/c mice with a mixture of DO11.10 TCR Tg BM cells and BALB/c BM cells. BM chimeras were then injected with unloaded or OVAp-loaded FLDCs. The mice were analyzed for the frequency of wild-type and DO11.10 thymocytes by flow cytometry (n = 4); 2 of 4 mice showed the marked deletion of DO11.10 SP CD4 cells illustrated in the lower portion of the figure. (D) Data are means from 4 mice. *P < .05; **P < .01.
To determine whether IV injection of FLDCs induces antigen-specific clonal deletion of CD4 SP cells, we reconstituted lethally irradiated BALB/c mice with a mixture of DO11.10 TCR Tg (on a BALB/c background) BM cells and BALB/c BM cells; in these chimeras, we measured deletion of OVA peptide (OVAp)–reactive DO11.10 CD4+ T cells. After more than 50 days, the mixed-BM chimeras were injected with 2 × 107 OVAp (OVA323-339)–loaded FLDCs or unloaded FLDCs, together with costimulation blockade. On day 10, mice were killed to quantitate the frequency and phenotype of the wild-type and DO11.10 thymocytes by flow cytometry. With injection of unloaded FLDCs, TCR-clonotype–positive DO11.10 CD4 SP thymocytes accounted for approximately 17% of total CD4 SP cells (Figure 5C-D). In contrast, the proportion of DO11.10 CD4 SP cells was reduced by approximately 60% (range 36%-82%) in chimeras injected with OVAp-loaded FLDCs, indicating marked clonal deletion. Note that in both of the above experiments, injection of antigen-loaded FLDCs caused little or no depletion of double-positive thymocytes (Figure 5A-C), which ruled out nonspecific deletion of cells mediated by production of inflammatory cytokines and/or steroid hormones.27
Injections of FLDCs induce long-term survival of allogeneic skin grafts
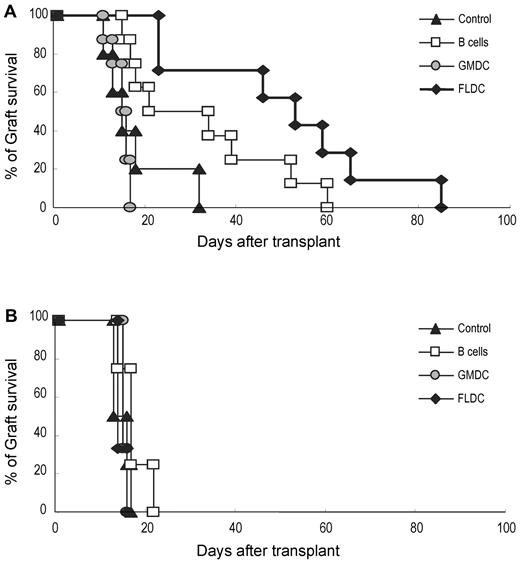
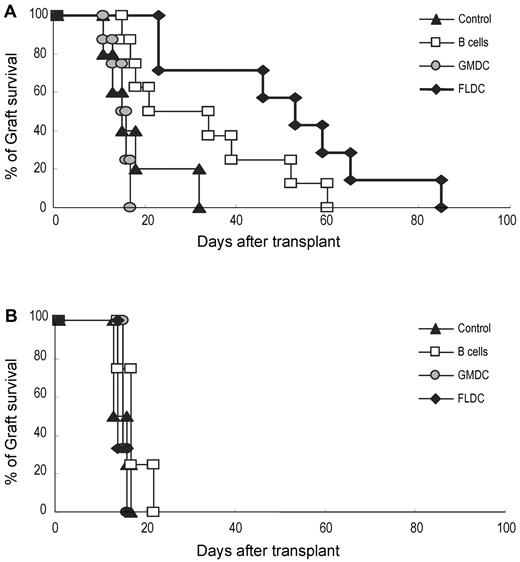
To assess whether adoptive transfer of donor APCs could prolong the survival of donor skin grafts, B6 mice were injected with BALB/c FLDCs, GMDCs, or B cells, as for the experiments summarized in Figure 3. After 10 days, full-thickness BALB/c or C3H skin grafts were transplanted onto the dorsal thorax of recipient mice. In control mice, BALB/c and C3H grafts were all rejected rapidly, with a mean survival time of 17.8 (n = 5) or 14.8 days (n = 4), respectively (Figure 6A-B). With injection of BALB/c APCs, GMDCs had no effect on the tempo of rejection, whereas B-cell injection extended the mean survival time of BALB/c grafts to 32 days (n = 8) compared with 17.5 days (n = 4) for C3H grafts. Better tolerance occurred after injection of BALB/c FLDCs, in which the mean survival time for BALB/c grafts was extended to 50.6 days (n = 7) compared with 14.7 days (n = 3) for C3H grafts (Figure 6A-B).
Prolonged acceptance of allogeneic skin grafts was induced by adoptive transfer of donor FLDCs. Recipient B6 mice were transplanted with BALB/c (5-8 mice/group; A) or C3H skin (3-4 mice/group; B) on the dorsal thorax on day 10. Graft survival was followed daily by visual inspection. Rejection was defined as a complete loss of viable epidermal graft tissue.
Prolonged acceptance of allogeneic skin grafts was induced by adoptive transfer of donor FLDCs. Recipient B6 mice were transplanted with BALB/c (5-8 mice/group; A) or C3H skin (3-4 mice/group; B) on the dorsal thorax on day 10. Graft survival was followed daily by visual inspection. Rejection was defined as a complete loss of viable epidermal graft tissue.
Discussion
The establishment of mixed-hematopoietic chimerism is regarded as an ideal method for inducing long-term transplantation tolerance,28,29 but this generally requires treating the recipient with either whole-body irradiation or high doses of myeloablative drugs, both of which have serious side effects.30 An alternative approach is to condition the host by inducing tolerance to the donor by adoptive transfer of host-derived regulatory T cells or donor-derived DCs.31,32 However, previous attempts to induce tolerance by injecting DCs have succeeded in inducing only peripheral tolerance and not central tolerance, so tolerance was not observed at the level of newly generated thymocytes. This might be one of the main reasons that DC treatment often leads to chronic rejection.
In the present study, we found that ex vivo–expanded, donor BM-derived FLDCs could home to the thymus of fully allogeneic recipient mice and induce donor-specific central tolerance and peripheral tolerance. We compared the thymic homing ability of B cells, GMDCs, and FLDCs, and found that only FLDCs could localize in the recipient thymus. Several possibilities exist to explain this observation. First, thymic trafficking might depend on cell size. According to forward scatter in flow cytometric analysis, the cell size of GMDCs was approximately 50% larger than that of FLDCs, and most GMDCs remained trapped in the lungs 24 hours after IV injection (Figure 2A). However, this explanation is not applicable to B cells, which are small and fail to localize in the lungs. A second possibility is that splenic B cells and GMDCs differ from FLDCs in lacking the homing molecules required for reaching the thymus. It has been reported that the P-selectin and VLA-4–VCAM-1 interaction is critical for splenic DCs to migrate to the thymus.33 However, although B cells and GMDCs express the P-selectin ligand PGSL-1 and VLA-4,34 they failed to migrate to the thymus in our studies, so the specific molecules regulating entry of FLDCs into the thymus remain to be elucidated.
It is well known that, although T-cell reactivity to MHC alloantigens is intense, blockade of the CD40 and CD28 costimulatory pathways can significantly prolong allograft survival in both mice and primates in the absence of further immunosuppression.35-37 Nevertheless, CD40/CD28 costimulation blockade alone has not been shown to induce indefinite graft survival, perhaps because other costimulatory molecules can play a role in graft rejection.21,38 Another possibility is that costimulation blockade fails because of the activity of newly generated, nontolerant T cells released from the host thymus, which agrees with the view that central tolerance is a prerequisite for long-term graft acceptance in the hematopoietic chimera model.39 In the present study, we have shown that IV allogeneic FLDC infusion together with costimulatory blockade induced effective donor-specific central and peripheral tolerance, as evaluated by MLR (Figure 3C). However, donor B-cell and GMDC infusion did not induce central tolerance, reflecting the failure of these cells to reach the thymus, which is in contrast to prominent thymic homing by FLDCs. Therefore, the induction of central tolerance by DC infusion is critically dependent on the injected cells reaching the host thymus (Figure 3A-B).
Culturing BM cells with Flt3L generated 2 major subsets of DCs, cDCs and pDCs. We found that after IV injection, both subsets could induce donor-specific central and peripheral tolerance (Figure 4C). The finding that central tolerance induction was almost as efficient with pDCs as with cDCs was surprising, because the role of pDCs in the thymus is unclear and these cells have relatively poor APC function.4,40 However, efficient APC function may be less important for deleting immature T cells in the thymus than for stimulating mature T cells in the periphery; as mentioned earlier, the FLDC subsets of cDCs and pDCs used here both had an immature phenotype. Our finding that both subsets of DCs could induce negative selection is in agreement with the previous report that selective depletion of cDCs in mice did not induce a defect in central tolerance,41 whereas combined depletion of both cDCs and pDCs did impair negative selection and caused onset of spontaneous severe autoimmunity.42
After IV injection, FLDCs were found to localize in the thymus at the corticomedullary junction (Figure 2C). This result shows that the donor FLDCs reached the known main site of negative selection. We confirmed that IV injection of antigen-loaded FLDCs resulted in antigen-specific clonal deletion of thymocytes at the level of both CD8 and CD4 SP thymocytes, but with little effect on double-positive thymocytes (Figure 4A-B). The key conclusion from our combined findings is that IV injection of donor FLDCs led to efficient deletion (negative selection) of immature thymocytes with reactivity for alloantigens on the donor APCs. These data are in agreement with reports that splenic or thymic DCs can traffic to the recipient thymus and induce thymocyte deletion.33,43 However, the use of splenic or thymic DCs to induce tolerance in the clinical setting is not realistic. Our data show that strong intrathymic tolerance can be induced by the injection of in vitro–expanded FLDCs derived from BM precursor cells, and this approach could be clinically relevant.
It is notable that pretreatment of B6 mice with BALB/c FLDCs led to prolonged specific tolerance to donor skin allografts. FLDCs were clearly more effective than B cells in inducing graft tolerance, whereas GMDCs were totally ineffective. Previous studies have suggested that regulatory T cells can play an effective role in inducing transplantation tolerance.32,44 It has also been reported that allogeneic DCs are able to promote the generation of regulatory T cells.45 In our studies, culturing splenic T cells with FLDCs in vitro without costimulatory blockade led to division of Foxp3+ regulatory T cells (supplementary Figure 4); however, this division was abolished by costimulatory blockade, which was an essential part of our in vivo protocol. We did not see an increase in the numbers of regulatory T cells in vivo, suggesting that prolongation of graft survival in FLDC-injected mice was not mediated by regulatory T cells. In the case of mice injected with B cells or GMDCs, newly formed thymocytes were not tolerized to the donor antigens and there was only partial tolerance of T cells in the periphery, thereby leading to only a mild delay in donor skin graft rejection. Thus, peripheral tolerance without central tolerance was ineffective for inducing long-term graft acceptance.
Recently, much research interest has been focused on DC therapy, and there are ongoing clinical trials using DC vaccines for tumor immunotherapy.31 It has been reported that ex vivo–expanded human DCs can be obtained by culturing peripheral blood monocytes with Flt3L and IL-4.46 In the present study, we established that IV injection of mice with ex vivo–expanded donor FLDCs combined with costimulation blockade led to efficient trafficking of these DCs to the recipient thymus, and that such treatment induced donor-specific central and peripheral tolerance, leading to prolonged survival of donor skin grafts. Therefore, it is plausible that in human patients, tolerance induction by adoptive transfer of Flt3L-induced DCs could be a useful approach for promoting graft acceptance after organ transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Yousuke Takahama for providing us with 2C TCR Tg mice, Drs Shuhei Ogawa and Kazunobu Ohnuki for helpful discussions, and Mr Yuki Akieda and Ms Sakiko Kobayashi for skillful technical assistance.
Authorship
Contribution: T.Y. performed research, analyzed data, and wrote the paper; S.W., H.H., T.S., T.N., and N.I. performed research; H.T. provided the Flt3L construct used in the study; R.A. provided essential advice and analyzed data; J.S. provided essential advice, analyzed data from the clonal deletion assay, and wrote the paper; and H.K. supervised the project and wrote the paper. All authors read and commented on the draft versions of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hidehiro Kishimoto, MD, PhD, Division of Immunobiology, Research Institute for Biological Sciences, Tokyo University of Science, 2669 Yamazaki, Noda City, Chiba, 278-0022, Japan; e-mail: hidek@rs.noda.tus.ac.jp.