Abstract
Hodgkin lymphoma (HL) incidence with HIV infection may have increased with the introduction of combination antiretroviral therapy (cART), suggesting that immune reconstitution may contribute to some cases. We evaluated HL risk with cART during the first months of treatment. With 187 HL cases among 64 368 HIV patients in France, relative rates (RRs) and 95% confidence intervals (CIs) of HL were estimated using Poisson models for duration of cART, CD4 count, and HIV load, with and without adjustment for demographic/clinical covariates. HL risk was unrelated to cART use overall, but it was related to time intervals after cART initiation (P = .006). Risk was especially and significantly elevated in months 1-3 on cART (RR 2.95, CI 1.64-5.31), lower in months 4-6 (RR 1.63), and null with longer use (RR 1.00). CD4 count was strongly associated with HL risk (P < 10−6), with the highest HL incidence at 50-99 CD4 cells/mm3. With adjustment for CD4 count and covariates, HL risk was elevated, but not significantly (RR 1.42), in months 1-3 on cART. HIV load had no added effect. HL risk increased significantly soon after cART initiation, which was largely explained by the CD4 count. Further studies of HIV-associated HL are needed.
Introduction
Hodgkin lymphoma (HL) incidence is significantly elevated in the HIV-infected population.1-3 In addition, since the advent of combination antiretroviral therapy (cART) in 1996, studies in the United States4 and the United Kingdom5 have reported an increase in the incidence of HL compared with the pre-cART era. For example, among people with AIDS in the United States, the incidence of HL was shown to be elevated 8-fold compared with the general population during the pre-cART era, and this increased significantly to 13-fold during the cART era.1 Excess risk of HL in a second US population and among the HIV-infected population in France was shown to be very high during the pre-cART era.2,6 In the French study, HL incidence increased even more (by 1.4-fold) during the cART era compared with the pre-cART era.2 In the Swiss HIV Cohort Study, HL risk was 3-fold higher in the cART era compared with the pre-cART era in an analysis of the first 18 cases,7 but this was not statistically significant and no increment in the cART era was found in an updated analysis of 47 HL cases.8
Increased HL in the cART era has been postulated to be related to the actual use of cART, with a potential role for immunologic mechanisms.2,4,9 Some support for an effect of cART on HL risk was provided by the British and initial Swiss studies, but these findings were marginally significant and were adjusted little or not at all for immune deficiency.5,7 The British study also suggested that the excess risk of HL might specifically relate to the use of non-nucleoside reverse transcriptase inhibitors (NNRTIs).5 Immune perturbation, and its control by cART, is likely to be integral to the development of HIV-associated HL. The relative risk (RR) of HIV-associated HL is 3.5-fold higher after AIDS onset,10 and a lower CD4 lymphocyte count generally predicts a higher incidence of HL.11-13 However, with the most severe immune deficiency (< 50 CD4 cells/mm3), HL risk appears to be paradoxically lower.11,12
Measures of immunity and their relationship to HL risk are highly dynamic. In the 2009 report from the Swiss study, HL risk was inversely related to CD4/CD8 lymphocyte ratio 1-2 years before diagnosis, whereas within 1 year of diagnosis, the overwhelming correlate of risk was T-cell and total lymphopenia.8 Within-subject changes in blood counts and effects of initiating cART were not evaluated.8 Relatively abrupt changes occurring with cART initiation could, in the short term, tip the balance to increase HL risk. In addition to a rapid decrease in HIV load and a more gradual, clinically beneficial increase in CD4 count, the initiation of cART induces marked decreases in circulating levels of activated CD4 and CD8 cells.14 Moreover, within 6 months of starting cART, 10% or more of patients develop immune reconstitution inflammatory syndrome (IRIS).15 Classically, IRIS is described as the appearance or progression of opportunistic infections, especially tuberculous and nontuberculous mycobacteria, cryptococcosis, uveitis, and progressive multifocal leukoencephalopathy, within weeks to months of cART initiation.16 Myriad other conditions, both infectious and autoimmune and including systemic lupus erythematous, polymyositis, rheumatoid arthritis, and Graves disease, have been attributed to IRIS.15
Considering the possibility that HL may be a manifestation of IRIS, the present study evaluated the risk of HL during the first months after cART initiation in an HIV-infected population followed in France using data from the French Hospital Database on HIV infection (FHDH-ANRS CO4), with careful modeling of the relationship between risk of HL and level of immunity as reflected by the CD4 cell count.
Methods
Patients
The FHDH-ANRS CO4 cohort database is described in detail elsewhere.17 Briefly, FHDH-ANRS CO4 is an open cohort study conducted by a clinical epidemiologic network that was initiated in 1992 and that includes 70 French teaching hospitals belonging to 26 HIV treatment and information centers (COREVIH) located in both mainland France and French overseas territories. The cohort includes patients who have documented HIV-1 or HIV-2 infection and who have given their written informed consent to participate in the database in accordance with the Declaration of Helsinki. The FHDH-ANRS CO4 database and its use have been approved by the French computer watchdog group Commission nationale de l'informatique et des libertés (CNIL) in accordance with French law. Data at each visit are recorded prospectively by trained research assistants. Visits are typically scheduled and data collected at months 1 and 3 after starting ART and then every 3-4 months. Intervals up to 6 months between visits are accepted for patients whose viral load has been well controlled for several years. In the database used for the analysis, the follow-up was through June 2009. The standardized FHDH-ANRS CO4 data collection form includes baseline characteristics, standard biologic markers such as CD4 cell count and plasma HIV RNA level, clinical manifestations, treatments, clinical trials in which the patients are enrolled, deaths, and causes of death, as reported in the medical records. By the end of 2009, more than 117 000 HIV-infected subjects who had attended at least one follow-up visit between 1992 and December 2009 were included in the database, with a mean follow-up of 81 months.
There were 2 exclusions from the current analysis: patients with a history of HL before their first visit in FHDH-ANRS CO4 (n = 74) and those who had received single or dual antiretroviral medications before cART (n = 26 444).
Variables
The numbers of patients evaluated each month at each COREVIH center were aggregated (person-time) and analyzed in strata. We considered the following explanatory variables, some of which were fixed and some of which were time-varying. The use and duration of cART for each patient in each stratum was divided into 5 time-interval categories: (1) no cART and year < 1996, (2) no cART and year ≥ 1996 (the reference category), (3) ≤ 3 months since starting cART, (4) 4-6 months since starting cART, and (5) > 6 months since starting cART. A patient was presumed to have stayed on cART once it was started. In a sensitivity analysis, HL risk in each of the first 3 months after cART initiation was examined. CD4 cell counts (per mm3) were categorized as follows: 0-49, 50-99, 100-199, 200-349, 350-499, and ≥ 500. For each patient, time was accumulated between each CD4 count. As expected, with follow-up every 3-4 months, the median time between 2 CD4 values was 3.2 months (interquartile range, 2.0-5.2 months). Plasma HIV RNA in copies per milliliter was classified as follows: < 500, 500-4999, 5000-49 999, and ≥ 50 000; time between HIV RNA values was accumulated as for CD4 counts. Past history of an AIDS-defining clinical condition (European definition for AIDS stage18 ) for each patient in each stratum was a binary variable. Sex and exposure group were described in a combined variable as follows: men who have sex with men (MSM), injectable drug users (IDU), other men, and other women. The patients also were classified based on geographic origin: migration from sub-Saharan Africa, migration from other countries, and no migration. Age in each stratum was divided into 3 groups: 15-34 years, 35-49 years, and ≥ 50 years. Calendar years were grouped into 3 periods: < 1996 (availability of single and dual antiretroviral therapy), 1996-1999 (the early cART period), and ≥ 2000 (the current cART period).
Age, calendar period, CD4 cell count, AIDS, HIV RNA, and duration of cART treatment were included as time-dependent variables. The first cART regimen received by each patient was classified as follows: no cART or calendar period before cART, regimen exclusively containing nucleosida reverse transcriptase inhibitors (NRTIs), protease inhibitor (PI)–containing regimen, or nonNRTI-containing regimen without PI. Patients who developed HL during follow-up were censored at that visit, and therefore the CD4 count and other values were the most recent before HL diagnosis.
Statistical analyses
The incidence of HL (number of HL cases divided by person-time) was computed for each stratum of the different variables. The effect of the variables—including duration of cART exposure with no cART and year ≥1996 at the reference category—on difference in incidence (risk) of HL was assessed using Poisson regression modeling to calculate RR and 95% confidence intervals (CIs). The crude effect of cART exposure on risk of HL was assessed in a univariate Poisson regression model (Model 0). Potential confounding variables were taken into account in multivariate Poisson regression adjusted for age, sex and exposure group, migration from sub-Saharan Africa, and AIDS stage (Model 1). Model 2 adjusted for the variables in Model 1 plus CD4 cell count. Model 3 adjusted for the variables in Model 2 plus plasma HIV RNA since 1997, the year this assay became available in France. The first cART regimen was added to these models to assess the effect of NNRTI on the risk of HL.
The linearity of the association between CD4 and risk of HL was also assessed by comparing the models with categorical CD4 with the models with linear CD4, each fitted with the same adjustment variables (age, sex and exposure group, migration from sub-Saharan Africa, AIDS stage, and cART duration) using the likelihood ratio test. In sensitivity analyses, models with cubic splines for the CD4 values were used. Mean within-patient changes in CD4 count were described for HL cases that occurred within 3 months of cART initiation.
All analyses were performed using Matlab 7 Version 2009b software (MathWorks).
Results
Characteristics of the study population, as well as the incidence of HL in each stratum of the study population, are given in Table 1. Our study included 64 368 patients, 286 806 person-years (PYs), and 187 HL cases. The proportions of MSM, IDU, other men, and other women were 32%, 14%, 27%, and 27%, respectively. The proportion of migrants from sub-Saharan Africa was 14%. The incidence of HL was not associated with the period: 0.79, 0.60, and 0.64 per 1000 PYs before 1996 (pre-cART era), in 1996-1999, and since 2000, respectively (P = .55). Even with restriction to patients with a history of AIDS or fewer than 200 CD4 cells/mm3, HL risk did not differ in 1996-1999 (P = .39) or since 2000 (P = .41) compared with the pre-cART era.
Overall HL risk did not differ between cART users and nonusers after 1996 (P = .29). However, HL risk was associated with duration of cART (P = .006, Model 0 in Table 2). Specifically, the risk was especially high during the first 3 months of use (n = 15 cases, RR 2.95, CI 1.64-5.31). It also was elevated to 6 months of cART, but this was not statistically significant (n = 8 cases, RR 1.63, CI 0.76-3.46). Similar estimates of HL risk in the first 3 months (RR 3.00) and up to 6 months (RR 1.74) on cART were seen when Model 0 was restricted to people with a history of AIDS. Note that patients may have contributed person-time to multiple strata (for example, before and after starting cART), whereas all patients in the later cART time strata (4-6 months and > 6 months) had also contributed person-time to the strata of ≤ 3 months on cART.
The association with the use and duration of cART remained after adjustment for age, sex and exposure group, migration, and AIDS stage (P = .005, Model 1). HL risk was virtually identical (RR 1.14) in the pre-cART era before 1996 compared with the referent group no cART and year ≥ 1996 (Model 1). The other variables in Model 1 were unrelated to HL risk, except for an elevated risk with AIDS (RR 1.74, CI 1.24-2.44) and a reduced risk, compared with MSM, for IDU, other men, and other women (RR 0.65, CI 0.41-1.01; RR 0.88, CI 0.62-1.24; and RR 0.27, CI 0.16-0.46; respectively). In a sensitivity analysis of individual months in Model 1, HL risk was elevated in the first month on cART (n = 6, RR 3.09, CI 1.31-7.28) and in the third month (n = 7, RR 3.62, CI 1.62-8.08), but not in the second month (n = 2, RR 1.05, CI 0.25-4.33).
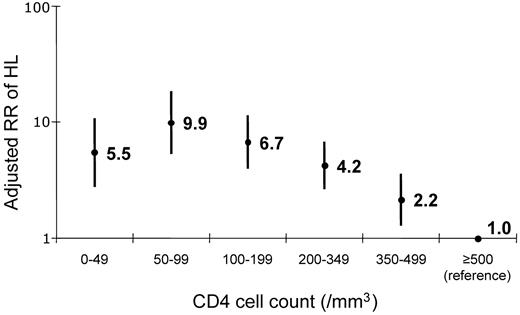
In Model 2, which accounted for the last CD4 cell count before diagnosis, HL risk was elevated, but no longer statistically significant, in the first 3 months on cART (RR 1.42, CI 0.76-2.66). The association between risk of HL and CD4 cell count in Model 2 was highly significant (Figure 1, P < 10−6). HL incidence was increased 9.9-fold at 50-99 CD4 cells/mm3 (Figure 1). Further adjustment for HIV-RNA as well as CD4 count had no effect (Table 2 Model 3). HL risk did not differ by type of first cART regimen (results not shown).
Relative risk of HL. Risk was obtained by last CD4 cell count before diagnosis and was adjusted for age, sex and exposure group, migration from sub-Saharan Africa, AIDS stage, and cART use and duration (Table 2 Model 3).
Relative risk of HL. Risk was obtained by last CD4 cell count before diagnosis and was adjusted for age, sex and exposure group, migration from sub-Saharan Africa, AIDS stage, and cART use and duration (Table 2 Model 3).
Adjusted for age, exposure group, origin, and AIDS stage, categorical CD4 fit the HL risk data significantly better than linear CD4 (likelihood ratio test P = .04). Categorical CD4 also tended to fit better than linear CD4 when the models were further adjusted for cART use and duration (likelihood ratio test P = .08). When using spline models for the relationship between CD4 and risk of HL, results were very similar to those observed for categorical CD4: the highest risk for HL was observed in the 50-99 CD4 cell-count category (results not shown).
The mean change in CD4 count in those who developed HL within 3 months of cART initiation resembled the CD4 increase observed in the cohort overall.21 Specifically, of the 9 HL cases with available data, CD4 count increased by a mean of 30 cells/mm3 over a mean of 50 days on cART before HL diagnosis. There was, however, heterogeneity: CD4 count increased steeply (9.8 cells/mm3/d over 14-20 days) before diagnosis in 2 HL cases; less steeply in 2 other HL cases (1.6 cells/mm3/d over 57-71 days); and little or none in the remaining 5 HL cases (−0.8 cells/mm3/d over 20-78 days).
Discussion
In this study of more than 60 000 HIV-infected people, we found that the risk of HL increased significantly by 2.6-fold during the first 3 months of cART. This increase was largely explained by the concurrent CD4 cell count, because this association with duration on cART was greatly attenuated when we included the CD4 count in the same model. Our results also highlight the importance of immunosuppression in the development of HL because, compared with HIV-infected people with a CD4 count of at least 500 cells/mm3, HL risk was increased nearly 10-fold in those with a CD4 cell count of 50-99 cells/mm3.
This is the first study to investigate the possibility that initiation of cART may precipitate the development of HL, and it may help to reconcile conflicting reports in the literature.5,7,8 Previous studies have not considered the effects of the rapid clearance of HIV RNA and the more gradual recovery of CD4 cells with cART initiation. The rate of immune reconstitution varies widely among patients. In general, after starting cART, HIV RNA decreases very rapidly for the first 2-4 weeks,22 with the rate of RNA clearance significantly correlated with increasing CD4 counts over the first 8 weeks.22,23 Thereafter, HIV RNA clearance and especially recovery of CD4 counts and other immunologic abnormalities are more gradual.22,23 Among the HL cases in our cohort that occurred during the first 3 months of cART, mean within-patient changes in CD4 count were similar to changes in the cohort overall.21 We observed individual cases who had very rapid increases in CD4 count leading up to HL diagnosis, but our data are too sparse to assert that these events contributed to HL. Changes within individuals have not been evaluated to corroborate the shift reported from a low CD4/CD8 ratio to total lymphocytopenia during the 1-2 years before HL diagnosis.8
HL in the general population that is unrelated to HIV is associated with elevated circulating levels of immunoregulatory T cells,24-26 as well as with T-cell and total lymphopenia,27 perhaps the result of chemoattraction of T cells from the blood to tumor-associated malignant Hodgkin/Reed-Sternberg cells.28-30 Chemokines CCL22 and especially CCL17 have been specifically implicated,28,29,31 but other chemokines may also be involved.31 To our knowledge, chemokine levels in HIV-associated HL have not been evaluated by immunohistochemistry or serology.29,31 Investigation into the effect of cART initiation on circulating serum levels of CCL17, CCL22, and perhaps other chemokines31 and the relationship of these to HIV RNA clearance and CD4 recovery would be of considerable interest.
HIV-infected people have a substantial excess risk of HL that is not merely misdiagnosis of nonHodgkin lymphoma (NHL).3 Whereas roughly half of systemic AIDS NHL tumors contain EBV, the majority of HIV-associated HL tumors have clonal infection with EBV and express high levels of a classic EBV-transforming protein, latency membrane protein 1 (LMP-1).9 The possibility that HL could be triggered by immune reconstitution has not been established, but this is implied by the elevated risk of HL observed in the 3 months after cART initiation, which is the typical interval for IRIS to manifest.15,16,32 Assessment of changes in EBV infection during immune reconstitution, such as increased expression of LMP-1 in germinal-center or circulating B cells, would support this possibility.
Unlike the prototypical AIDS-defining malignancies NHL and Kaposi sarcoma, which increase in risk with the most severe immunosuppression,11,12 in our study the lowest CD4 count category did not convey the highest risk of HL. The effect of competing risk by mortality or severe clinical AIDS-defining conditions with fewer than 50 CD4 cells/mm3 cannot be ruled out.11,33 Complementing the study of Biggar et al,4 who studied CD4 count measured at AIDS diagnosis, we showed that current CD4 count had a nonlinear association with HL risk. The CD4 count at which HL risk is highest has not been precisely defined, but Biggar et al indicated that the nonlinearity of risk with CD4 count appeared to hold across histologic subtypes of HL. Although we lacked data on HL histologic subtypes, it is likely that the distribution in our HIV-infected population would be similar to that reported in the United States, with 54% mixed cellularity, 37% nodular sclerosis, and 7% lymphocyte depleted.4 We did not have an expert pathology panel review all of our HL cases, but 100% of HIV-associated HL and systemic NHL cases were confirmed by histologic review in a pilot study conducted by the FHDH and other participants in the COHERE consortium (D. Costagliola, unpublished data).34,35 This replicates findings from an American study in which expert review confirmed all 16 cases of HIV-associated HL that had been diagnosed by local pathologists.3 In addition, the nonlinear association with CD4 count (Figure 1) suggests little contamination of our HL case series by NHL, which is characterized by a clear inverse correlation with CD4 count.11,12
Evaluating short-term effects in an epidemiologic study is challenging. Our study included strata totalling more than 280 000 PYs, from which we ascertained 187 HL cases. Only 15 of these cases occurred in the first 3 months after cART initiation. We cannot exclude the possibility that indication bias led to accelerated diagnosis of HL during the first month on cART. However, the lack of elevation in month 2 on cART (when patients experiencing symptoms, but not most patients, are evaluated), the persistent elevation in month 3 on cART (when all patients are evaluated) and the lack of a compensatory deficit of HL in months 4-6 on cART (Table 2) suggest that indication bias and accelerated diagnosis had little effect. Nonetheless, our data in the first 3 months were very sparse, yielding risk estimates that are imprecise. The possibility of a mere chance association is reduced, because we tested a specific hypothesis: that HL risk would be elevated soon after cART initiation and that this risk would be mediated by CD4 count.
Defining the appropriate reference category for no cART exposure is critically important. We defined unexposed cART-era patients as our reference category. Medications for cART became available in March 1996. Our definition ensured that the reference group could have received cART and it also enabled us to evaluate whether HL incidence changed in calendar time. We found no difference in HL incidence during 1992-1995 (0.79 of 1000 PYs), 1996-1999 (0.60 of 1000 PYs), or 2000-2007 (0.79 of 1000 PYs), even with restriction to patients with a history of AIDS. Moreover, with inclusion of the covariates, we found no difference in HL risk between the reference group and the pre-cART-era patients (Table 2). With both pre-cART-era and unexposed cART-era patients, we could examine the possibility and magnitude of an indication bias in which cART would be administered soonest to the patients who may have had the highest risk of HL. Using the pre-cART era as the reference category (results not shown), the elevated risk of HL during the first 3 months after cART initiation was similar to that presented in Models 1 and 2 (Table 2). Although this is reassuring, we cannot exclude some impact by indication bias. Perhaps because we carefully controlled for cART duration, CD4 count, and confounding factors, we found no difference in HL risk with use of NNRTI.5
Successful treatment with cART markedly improves survival and reduces AIDS-defining NHL and Kaposi sarcoma incidence.11,12 We did not observe an overall increased risk of HL in cART users, so we emphasize that our results certainly should not dissuade the initiation and use of cART. Instead, we suggest that the increase in HL incidence during the first 3 months on cART implies that clinicians should be alert to the possibility of HL during cART-induced immune reconstitution. Corroboration and basic understanding of changes in CD4 counts, EBV, and chemokines during immune reconstitution may provide insight into the pathogenesis of HL.
Presented orally at the 12th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies (ICMAOI), April 26-27, 2010, National Institutes of Health, Bethesda, MD, and as a poster at the XVIII International AIDS Conference, July 18-23, 2010, Vienna, Austria.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to all the participants and research assistants of the French Hospital Database on HIV.
The French Hospital Database on HIV is supported by Agence Nationale de Recherches sur le SIDA et les hépatites (ANRS), INSERM, and the French Ministry of Health. This work was also supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (Bethesda, MD).
National Institutes of Health
Authorship
Contribution: J.J.G., E.L., D.C., E.A.E., and P.S.R. designed and conceived the study; D.C. and J.J.G. obtained financial support; F.F., A.-S.L., V.M., M.P., I.P.-M., and E.R. provided essential study materials and patients; E.L., D.C., F.F., A.S.L., V.M., M.P., I.P.-M., and E.R. collected and assembled the data; E.L., P.S.R., E.A.E., D.C., and J.J.G. analyzed the data; E.L. and J.J.G. drafted the manuscript; and all authors critically appraised and gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of FHDH-ANRS CO4 members, see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: James J. Goedert, MD, Infections and Immunoepidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, 6120 Executive Blvd, Rm 7068, Rockville MD 20852; e-mail: goedertj@mail.nih.gov.