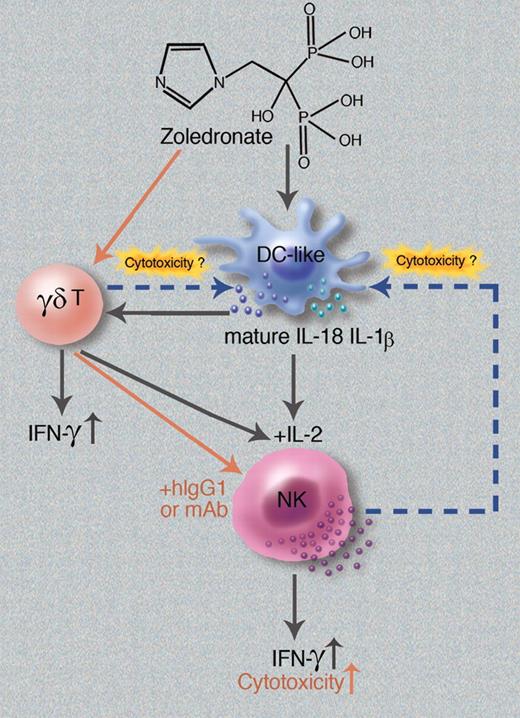
In this issue of Blood, Nussbaumer et al demonstrate that the bisphosphonate zoledronic acid (zoledronate), a US FDA-approved drug for the prevention of osteoporosis and treatment of bone metastases in multiple myeloma and other cancers,1 can enhance IFN-γ production by IL-2–primed human natural killer (NK) cells. This process depends on a CD14+CD56+ dendritic cell (DC)–like population, but occurs in a manner that may be either dependent or independent of γδ T cells (see figure).2
Natural killer cells are a critical component of the innate immune system, and are the first line of defense against both viral infection and tumor cells.3 Consequently, one area of NK-cell research is focused on enhancing NK-cell effector functions, such as IFN-γ production and cytotoxicity. The current study by Nussbaumer and colleagues shows that treatment of CD56+ peripheral blood mononuclear cells (PBMCs) with zoledronate induces IFN-γ production in not only γδ T cells, but also in NK cells (see figure). Depletion of CD14+CD56+ DC-like cells abolishes this induction of IFN-γ in γδ T cells and NK cells, suggesting that stimulation of IFN-γ production by zoledronate depends on a population of DC-like cells. With regard to NK cells, after being primed by IL-2, IFN-γ stimulation by zoledronate can be regulated positively by γδ T cells at the beginning of the treatment, and negatively by these cells at a later time. This is supported by an in vitro culture study showing that NK-cell IFN-γ production was enhanced by zoledronate in the presence of γδ T cells on day 1, suggesting that enhancement of IFN-γ production by NK cells may be attributed to stimulation of NK cells by γδ T cells. On day 2 of culture, DC-like cells were repeatedly eliminated, likely via cytotoxic activity of either γδ T cells or NK cells, resulting in termination of IFN-γ production of NK cells. Furthermore, induction of NK-cell IFN-γ production by zoledronate also occurs in a γδ T cell–independent manner, in which zoledronate may activate caspase-1 through downstream inhibition of isoprenoid formation in the mevalonate pathway. Subsequently, activated caspase-1 cleaves inactive precursors of IL-18 and IL-1β to provide bioactive forms of these cytokines, which activate IL-2–primed NK cells (see figure).
Schematic of zoledronate-induced activation and regulation of NK cells. The current study by Nussbaumer et al2 suggests that zoledronate activates DC-like cells to produce mature IL-18 and IL-1β proteins, which stimulate NK-cell IFN-γ production by acting on IL-2–primed NK cells directly. Alternatively, zoledronate-activated DC-like cells can act on γδ T cells, which in turn costimulate IL-2–primed NK cells to enhance production of IFN-γ indirectly. Later, zoledronate-activated NK cells or γδ T cells may kill DC-like cells, thus terminating NK-cell IFN-γ production. In addition, a separate study by Maniar et al5 shows that zoledronate can activate γδ T cells to subsequently stimulate NK-cell cytotoxcity in the presence of hIgG1 or mAbs (red arrows). Professional illustration by Marie Dauenheimer.
Schematic of zoledronate-induced activation and regulation of NK cells. The current study by Nussbaumer et al2 suggests that zoledronate activates DC-like cells to produce mature IL-18 and IL-1β proteins, which stimulate NK-cell IFN-γ production by acting on IL-2–primed NK cells directly. Alternatively, zoledronate-activated DC-like cells can act on γδ T cells, which in turn costimulate IL-2–primed NK cells to enhance production of IFN-γ indirectly. Later, zoledronate-activated NK cells or γδ T cells may kill DC-like cells, thus terminating NK-cell IFN-γ production. In addition, a separate study by Maniar et al5 shows that zoledronate can activate γδ T cells to subsequently stimulate NK-cell cytotoxcity in the presence of hIgG1 or mAbs (red arrows). Professional illustration by Marie Dauenheimer.
A previous study by the same group also showed that statins, another class of inhibitors of the mevalonate pathway, act cooperatively with IL-2 to induce IFN-γ production in CD56dim NK cells.4 The authors of the current study also demonstrate that natural cytotoxicity of NK cells was moderately increased by the zoledronate treatment. Consistent with this, a recent study by Maniar et al demonstrates that zoledronate augments both direct (natural) cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) of NK cells (see red arrows in figure) in a separate experimental system.5 It will be interesting to determine whether the increase in cytotoxicity induced by zoledronate can also be positively regulated by CD14+CD56+ DC-like cells, and whether IFN-γ production is also induced in the system used by Maniar et al.
Because the enhancement of NK-cell activation by zoledronate is complicated and can involve the interaction of several cell types, it is unclear whether this enhancement will occur in vivo, which should be determined in future studies. It also remains unclear whether zoledronate preferentially stimulates IFN-γ production in CD56dim NK cells rather than CD56bright NK cells, reflecting patterns previously shown for statins.4 This study by Nussbaumer et al reports an interesting observation, in which DC-like cells were eliminated by zoledronate stimulation during culture of CD56+ PBMCs. However, it is unclear whether these cells were eliminated by zoledronate-activated NK cells, by zoledronate-activated γδ T cells, or simply by zoledronate-induced apoptosis of DC-like cells. Although activated NK cells have the capacity to kill immature DCs,6 the evidence in the current study seems to not necessarily support the possibility that DC-like cells were killed by NK cells, because DC-like cells remain present when γδ T cells, but not NK cells, were depleted. In addition, in their study of zoledronate-mediated activation of NK cells, Nussbaumer and colleagues used CD56+ PBMCs and focused on accessory cells, CD14+CD56+ DC-like cells, and γδ T cells. Because their current study is limited to analysis of CD56+ PBMCs rather than total PBMCs, the role of other cell types, such as CD14+CD56− cells, in zoledronate-mediated activation of NK cells has not been determined. Finally, as Nussbaumer et al discuss, it remains unknown whether zoledronate-mediated NK-cell IFN-γ production contributes to the development of osteonecrosis of the jaw, a side effect of zoledronate, which involves substantial leukocyte infiltration.
Although further studies are required to address the above questions, the current study by Nussbaumer and colleagues adds a novel approach for activation of NK cells and advances our understanding of cell-cell interaction during NK-cell activation. In addition, some of the authors' findings may have translational potential for clinical applications. Osteoporosis represents a condition predominantly found in elderly populations. Because NK-cell cytotoxicity and IFN-γ production both decrease during the process of aging,7 it might be assumed that zoledronate treatment could have therapeutic potential for the elderly, not only in the treatment of osteoporosis, but also in enhancing NK-cell effector functions to prevent or treat certain cancers and infections. On the other hand, zoledronate has also been shown to inhibit mevalonate metabolism, likely resulting in an over-abundance of active caspase-1 and bioactive IL-1β and IL-18, which may contribute to the progression of inflammatory disease. An increase of inflammatory gene expression because of the inhibition of mevalonate pathway has recently been observed in patients with mevalonate kinase deficiency (MKD), a rare hereditary autoinflammatory syndrome.8
Conflict-of-interest disclosure: The author declares no competing financial interests. ■