Abstract
Increases in serum and liver copper content are noted during iron deficiency in mammals, suggesting that copper-dependent processes participate during iron deprivation. One point of intersection between the 2 metals is the liver-derived, multicopper ferroxidase ceruloplasmin (Cp) that is important for iron release from certain tissues. The current study sought to explore Cp expression and activity during physiologic states in which hepatic copper loading occurs (eg, iron deficiency). Weanling rats were fed control or low iron diets containing low, normal, or high copper for ∼ 5 weeks, and parameters of iron homeostasis were measured. Liver copper increased in control and iron-deficient rats fed extra copper. Hepatic Cp mRNA levels did not change; however, serum Cp protein was higher during iron deprivation and with higher copper consumption. In-gel and spectrophotometric ferroxidase and amine oxidase assays demonstrated that Cp activity was enhanced when hepatic copper loading occurred. Interestingly, liver copper levels strongly correlated with Cp protein expression and activity. These observations support the possibility that liver copper loading increases metallation of the Cp protein, leading to increased production of the holo enzyme. Moreover, this phenomenon may play an important role in the compensatory response to maintain iron homeostasis during iron deficiency.
Introduction
Iron deficiency enhances absorption of dietary iron in several mammalian species1,2 via a host of genetic and morphologic adaptations intended to maximize extraction of iron from the diet. Several key genes encoding iron transport-related proteins are strongly induced during iron deprivation,3-5 some by posttranscriptional stabilization of mRNA transcripts,6 and others via transcriptional induction mediated at least in part by a hypoxia-responsive trans-acting factor, hypoxia-inducible factor (HIF) 2α.7,8 In addition, genes related to intestinal copper homeostasis are induced during iron deprivation in rats,4,9 suggesting that copper is important in the physiologic response of the intestinal epithelium to iron deficiency. Copper levels are known to increase in the liver and serum of iron-deficient mammals,10-12 further hinting at an important physiologic role for copper during iron deprivation.
The best-known links between iron and copper are the multicopper ferroxidases ceruloplasmin (Cp) and hephaestin (Heph). Cp is a liver-derived circulating protein that is important for iron release from certain tissues, as evidenced by the iron overload phenotype in humans that lack Cp.13 Although Cp contains the predominance of serum copper in many species, the lack of Cp does not lead to perturbations in copper homeostasis; rather, it exerts preferential effects on iron metabolism.14 Heph has a highly similar functional (ferroxidase) domain but also has a single transmembrane segment that presumably anchors it to the plasma membrane of certain cells. Heph is expressed in several tissues, with high expression levels in the basolateral membranes of enterocytes of the proximal small intestine.15 Mice expressing a mutant Heph protein (sex-linked anemia [sla] mice) have reduced intestinal iron absorption and develop moderate iron deficiency, especially during early life.16 Moreover, Cp and Heph activities decrease during dietary copper deprivation, as recent studies in laboratory rodents have demonstrated.17-19
The current investigation was undertaken to further explore the potential influence of copper on homeostatic processes related to dietary iron absorption and to test hypotheses on the mechanism for such an influence. Because previous studies suggested that Heph is not strongly regulated during iron deficiency,20 Cp is the focus of the current study. Since Cp is a copper-dependent enzyme, the design was to determine whether Cp expression or activity increased during conditions in which liver copper loading occurs, such as during iron deficiency or when increased dietary copper is consumed. Weanling rats were thus placed on control or low iron diets containing variable copper levels (high, normal, or low). After ∼ 5 weeks on the diets, animals were killed, and various parameters of iron and copper homeostasis were measured, including Cp mRNA and protein expression, and serum ferroxidase activity. Results showed notable effects of iron deprivation and high copper intake on Cp protein expression and serum ferroxidase activity in the absence of alterations in Cp mRNA levels, implicating a posttranscriptional molecular mechanism.
Methods
Chemicals, reagents, and qPCR primers
Chemicals were obtained from Sigma-Aldrich and Thermo Fisher Scientific and were of analytical grade or high purity. Quantitative (q)PCR primers were from Integrated DNA Technologies. Other sources are mentioned as appropriate.
Animals and diets
All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Florida. Weanling, male Sprague-Dawley rats were purchased from Harlan and were raised in overhanging, wire mesh-bottomed cages under 12-hour dark/light cycle and killed between 9 and 11 am to minimize diurnal variation. Twenty-four animals were used in the first study (4 on each dietary treatment), and a separate 24 animals were used in a subsequent study. For some analyses reported here, the n is < or > 8. When it is lower, insufficient sample quantity was obtained to measure the desired parameter. When n is > 8, additional samples were included that were derived from rats under the exact same dietary protocol and at the exact same ages, otherwise used in an independent investigation that was parallel for the parameter in question. All analyses were done on individual animals. The animals had free access to food and iron- and copper-free water. The experimental design consisted of 6 different AIN-93G–based diets (Dyets). The diets were identical except for having variable iron and copper levels. The diets and the corresponding groups of rats consuming them were designated as follows: control (Ctrl); iron deficient (FeD); copper deficient (CuD); iron and copper deficient (FeDCuD); copper extra (CuE); and iron deficient, copper extra (FeDCuE). All diets with normal iron levels (Ctrl, CuD, and CuE) were modeled after standard rodent chows to contain ∼ 200 ppm iron (added as ferric citrate). Low iron diets (FeD, FeDCuD, and FeDCuE) contained < 9 ppm iron. All normal copper-containing diets (Ctrl and FeD) contained ∼ 6 ppm copper, low copper diets (CuD and FeDCuD) contained ∼ 1 ppm copper, and the extra copper diets (CuE and FeDCuE) contained ∼ 28 ppm copper. Animals were placed on each diet for 32-35 days. At the end of the feeding regime, rats were anesthetized by CO2 exposure and killed by cervical dislocation. Blood was collected by cardiac puncture and transferred to prechilled polypropylene tubes. After 1 hour to allow for clotting, the tubes were centrifuged at 1500g for 15 minutes at 4°C. The supernatants (sera) were separated and stored at 4°C and used for Cp enzyme activity assays within 3 days. Portions of livers were snap-frozen in liquid N2 and then stored at −80°C for RNA isolation or stored at −20°C for mineral analysis.
Elemental analyses, and Hb and Hct measurements
Rat livers and serum were submitted to the Diagnostic Center for Population and Animal Health at Michigan State University for mineral analysis. Liver samples were dried and weighed followed by digestion with nitric acid. Then, the digested tissues or diluted serum samples were subjected to inductively coupled plasma-mass spectrometry for analysis. Serum transferrin-bound iron was measured with iron reagent on a chemistry-immuno analyzer (both from Olympus America) according to the manufacturer's instructions. Hemoglobin (Hb) and hematocrit (Hct) were measured in house using a HemoCue hemoglobin analyzer (Hemocue) and a Readacrit hematocrit system (Clay Adams), following the manufacturers' instructions.
Real-time PCR
Total RNA was purified from rat liver and enterocytes by the TRIzol reagent (Invitrogen) method, and qRT-PCR was performed as described previously.21 In brief, 1 μg of RNA was converted to cDNA with the iScript cDNA synthesis kit (Bio-Rad Laboratories), in a 20 μL reaction. One microliter of the cDNA sample was subjected to PCR amplification using 10 μL of SYBR Green master mix (Bio-Rad Laboratories) and 0.75 μL (0.25pM) of each forward and reverse gene-specific primer (sequences listed in supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), in a 20 μL reaction. Primers were designed to span large introns to eliminate amplification from genomic DNA. Reactions were run in 96-well plates on an iCycler C1000 thermal cycler (Bio-Rad Laboratories) with the following cycling parameters: 95°C for 3 minutes and then 39 cycles with 95°C for 10 seconds and 58°C for 30 seconds. A melt curve was routinely performed after 39 cycles of amplification by increasing the temperature from 65-95°C in 0.5°C increments for 5 seconds at each temperature; single amplicons were detected in all cases. Preliminary experiments established the validity of each primer pair in that each set was able to linearly amplify each transcript across a range of template concentrations. Each RT reaction was analyzed in duplicate for 18S rRNA, ankyrin repeat domain containing protein 37 (Ankrd37), Menkes copper ATPase (Atp7a), Wilson's copper ATPase (Atp7b), Cp, copper transporter 1 (Ctr1), hepcidin (Hamp), metallothionein 1 (Mt1a), and vascular endothelial growth factor (Vegf) in each of the individual animals used for this investigation. Then, the average of 18S was subtracted from the experimental gene average to generate the cycle threshold (Ct) value. ΔΔCt values were calculated for experimental genes, for all experimental groups versus the control (Ctrl) group. Mean fold-change = 2−ΔΔCt. One-way ANOVA followed by Tukey multiple comparison test was used to determine significance between means. Statistical significance was set at P < .05.
Western blot analysis
Protein concentrations were determined by 660-nm assay (Pierce Chemical), following the manufacturer's instruction. Absorbance at 660 nm was measured using a SpectraMax M5 multimode microplate reader. Thirty micrograms of serum protein was resolved by electrophoresis through SDS-7.5% polyacrylamide gels, followed by transfer to polyvinylidene difluoride membranes (Millipore) as described previously.22 Blots were subsequently reacted with chicken anti-Cp antibody at a 1:5000-fold dilution followed by peroxidase-coupled secondary antibody at a 1:10 000-fold dilution (both gifts from Sigma-Aldrich). Immunoreactive bands were visualized by enhanced chemiluminescence (reagent A: 0.4mM coumaric acid and 2.5mM luminol in 0.1M Tris-HCl, pH 8.5; reagent B: 0.018% H2O2 in 0.1M Tris-HCl, pH 8.5) and autoradiography. Blots were stained with Ponceau S solution to confirm equal sample loading and efficient transfer. The optical density of immunoreactive bands on film and proteins on stained blots was quantified using the digitizing software UN-SCAN-IT (Silk Scientific), and the average pixel numbers were used for normalization and comparison. The intensity of immunoreactive bands on film was normalized to the intensity of proteins on stained blots.
FOX enzyme activity assays: principle
Ferroxidase (FOX)–I catalyzes the transfer of electrons from Fe2+ through its T1-T3 Cu2 copper sites, resulting in a 4-electron reduction of O2 to 2 H2O, forming Fe3+. Although a bisubstrate, ping-pong reaction, a high excess of 1 substrate used, either para-phenylenediamine (pPD) or (NH4)2Fe(SO4)2, not only helps drive the reaction forward but also incidentally makes this a kinetically pseudo first-order reaction.
pPD assay: principle.
pPD, a 6-carbon phenyl derivative, on oxidation, is converted to a fused ring aromatic compound with the formula C18H18N6, termed Bandrowski's band, with an absorption maxima at ∼ 530 nm, which can be visualized as a dark band in a gel or quantified by reading A530 in a spectrophotometer.23 Although this is an amine oxidation, because of (NH4)2Fe(SO4)2 and pPD being competing [S], pPD is a surrogate for Fe2+ oxidation.
Ferroxidase–Fz assay: principle.
Ferrozine (Fz), acting as an indicator, complexes with Fe2+, yielding a pink-colored complex, absorbing at ∼ 570 nm, which can be visualized either qualitatively in a gel as a translucent white band (at the site of the enzyme) against a dark-pink background, or quantitatively by reading A570 in a spectrophotometer.24
In-gel assay
One milligram of serum protein per lane was electrophoresed through a native 7.5% polyacrylamide gel at a constant 80 V in native running buffer (0.12M Tris and 0.04M glycine) at 4° C, until the tracking dye just left the gel. For pPD assay, the gel was briefly rinsed in water, incubated in 30 mL of 0.1% pPD in 0.1M CH3COONa-CH3COOH buffer, pH 5.0, for 2-3 hours in the dark with gentle shaking, rinsed in MilliQ water (Millipore), and air-dried overnight in a gel-frame. For Fz assay, the gel was incubated in 200μM Fe(NH4)2(SO4)2(H2O)6 in 0.1M CH3COONa-CH3COOH buffer, pH 5.0, for 2-3 hours in the dark with gentle orbital shaking. After rinsing with MilliQ water, the gel was reacted with 15mM Fz solution, and color development was allowed to proceed.
Spectrophotometric assay
pPD assay.
The reaction mix contained equal quantities of serum protein (either 200 or 500 μg) from each animal, with 0.1% pPD in 0.1M CH3COONa-CH3COOH buffer, pH 5.0, with or without inhibitors, in a 1 mL final volume, which was incubated at 37°C. The volume of serum was kept at 15 to 20 μL (providing up to 500 μg of total protein), to minimize serum-induced variations and higher concentrations of endogenous inhibitors such as Cl−, citrate, or PO43−. After considering the relative apparent Km values of the substrates used, potential backward reaction, the low quantity of sera (hence the enzyme) used and after extensive preliminary trials, a 1-hour reaction time, end point assay was chosen. Previous reports have used longer times, such as 90 minutes and even up to 3 hours, to measure serum Cp activity.23,25 The reaction was stopped by addition of NaN3 to a final concentration of 10mM. The sample was then mixed, and absorbance was read at 530 nm in a DU 640 spectrophotometer (Beckman Coulter). Blank (complete reaction buffer devoid of serum; ie, enzyme source) readings were subtracted from sample readings.
Fz assay.
The reaction conditions were as mentioned in the preceeding paragraph except the reaction was stopped by addition of Fz to a final concentration of 3mM, and then it was mixed, and absorbance was read at 570 nm in a DU 640 spectrophotometer (Beckman Coulter). Blank readings were subtracted from sample readings. For both methods, sera also were assayed in the presence of 10mM NaN3, a well-studied FOX-I inhibitor, to confirm the enzyme's identity (ie, Cp).
Statistical analysis
Each group had a minimum of 8 rats, although in some cases data are reported for different numbers for reasons explained in “Animals and diets.” Data are expressed as means ± SD. Outliers in each group were determined by GraphPad outlier calculator (http://www.graphpad.com/quickcalcs/Grubbs1.cfm; GraphPad Software) and eliminated from subsequent analyses. The data were analyzed by descriptive statistics, 2-way ANOVA followed by Tukey multiple comparison test and Pearson correlation coefficient using SPSS Version 19.0 (SPSS) and Prism 4 for Macintosh Version 4.0c software (GraphPad Software). P < .05 was considered statistically significant. Liver copper was normalized by log transformation before Pearson correlation coefficient analysis to correct for skewed distribution. In all bar graphs, data with different letters are statistically different from one another, and those that share a letter are not statistically different from each other.
Results
Dietary feeding strategy
The dietary feeding regime used in this study was developed over several years, a time period when numerous dietary iron and copper deprivation studies were performed in Sprague-Dawley rats.3,4,21 These studies showed that diets containing < 10 ppm iron lead to severe iron deficiency anemia and that diets containing ∼ 1 ppm copper resulted in notable symptoms of copper deficiency. Moreover, we found that feeding weanling rats (∼ 21 days old) these deficient diets for a minimum of 30 days led to maximal effects on various iron- and copper-related parameters. The dietary feeding regime used for this investigation was successful at meeting the following goals: (1) rats consuming iron-deficient diets were severely iron deficient, (2) rats consuming low copper diets were severely copper deficient, and (3) rats consuming extra copper-containing diets showed increased body copper content. The data supporting these statements are delineated throughout “Results.”
Iron and copper levels in rat sera and liver
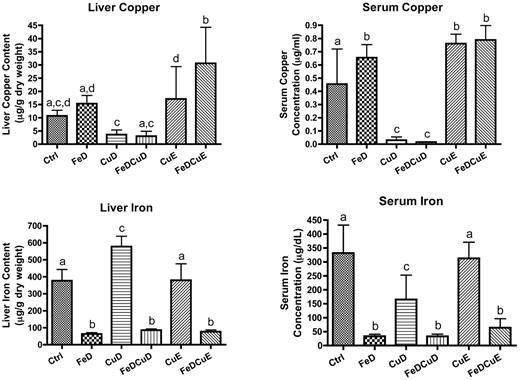
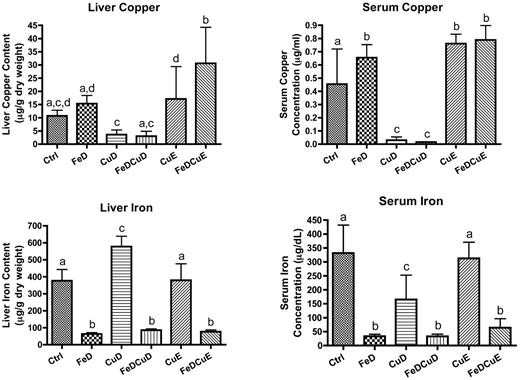
Hepatic and serum iron levels were reduced 80%-90% in the 3 groups consuming iron-deficient chows (P < .05 compared with control values; Figure 1). Hepatic iron increased in the CuD group (53%; P < .001), whereas it was not different from controls in the CuE group. Serum iron decreased in the CuD group (51%; P < .001), but no change was noted in the CuE group. Serum copper levels were significantly decreased in rats consuming the low copper diets (∼ 95%; P < .001), whereas although hepatic copper levels were decreased by ∼ 70% in the same dietary groups, the difference did not reach statistical significance (P > .05). Furthermore, a trend toward increased liver copper was apparent in the FeD and CuE groups, but statistical significance was not achieved because of large variation between individual rats. Serum copper was notably higher in the rats consuming higher copper diets (CuE and FeDCuE groups; 65% to 70%, P < .001) and in the FeD group (∼ 40%; P < .05) compared with controls.
Iron and copper levels in rat liver and serum. Means ± SD are shown. Liver samples were dried and wet-ashed before inductively coupled plasma-mass spectrometry analysis; data are presented as micrograms per gram dry weight. Serum samples were diluted and analyzed similarly; data are presented as micrograms per milliliter for copper or micrograms per deciliter for iron. Bars with different letters on top are statistically different from one another (P < .05; n = 7-9/group).
Iron and copper levels in rat liver and serum. Means ± SD are shown. Liver samples were dried and wet-ashed before inductively coupled plasma-mass spectrometry analysis; data are presented as micrograms per gram dry weight. Serum samples were diluted and analyzed similarly; data are presented as micrograms per milliliter for copper or micrograms per deciliter for iron. Bars with different letters on top are statistically different from one another (P < .05; n = 7-9/group).
Hematologic status as a function of diet
The effects of varying the iron and copper content of the diets were even more apparent when hematologic parameters were considered (Table 1). Hb in the FeD and FeDCuD groups was decreased ∼ 85% (P < .001), whereas Hb was also significantly decreased in the FeDCuE group (∼ 50%; P < .001), compared with controls. Hb in the CuD group was reduced ∼ 60% (P < .001), whereas the CuE diet had no effect on Hb levels (both compared with controls). Hct levels followed a similar trend with significant decreases seen in the FeD and FeDCuD groups (∼ 70%; P < .001), with a less severe decrease noted in the FeDCuE group (32%; P < .001). Hct was reduced less in the CuD group compared with the reduction seen in the FeD group (51%; P < .001), and no change was noted in the CuE group (compared with controls).
Expression of copper homeostasis-related genes in liver
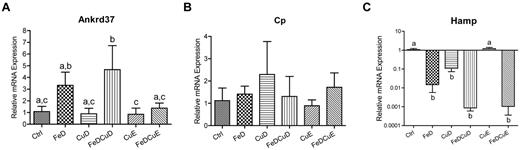
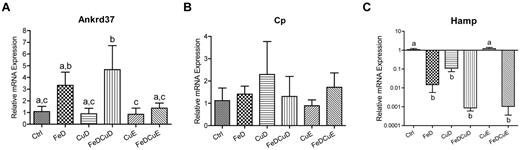
Because hepatic iron and copper levels are modulated by various transporters and metal-binding proteins, we considered that changes in expression of the genes encoding these proteins could reveal mechanistic insight into the inverse relationship between iron and copper during states of deficiency. qRT-PCR analysis of hepatic genes related to copper (and iron) homeostasis was thus done in all rats from the 6 dietary groups. No differences were noted among the dietary groups for the following genes: Atp7a, Atp7b, Ctr1, Mt1a, and Vegf (data not shown), or Cp (Figure 2). Hamp and Ankrd37 did, however, show significant differences between groups (Figure 2). Hamp (hepcidin) mRNA expression was significantly reduced in the FeD, CuD, FeDCuD, and FeDCuE groups compared with controls, whereas Ankrd37 was increased in the FeD and FeDCuD groups.
qRT-PCR analysis of hepatic gene expression. The expression of several genes was measured in individual rats from the 6 dietary treatment groups. Shown are the 2 genes that showed significant changes in expression among the groups (A,C) and the data for Cp (which showed no significant differences; B). In all panels, bars with different letters atop or beneath error bars are statistically different from one other (P < .05). Hamp, hepcidin; Ankrd37, ankyrin repeat domain protein 37. n = 7 for CuD group and n = 8 for all other groups. Data are expressed as means ± SD except for data in panel C, which are expressed as means ± SEM (because a log scale is used).
qRT-PCR analysis of hepatic gene expression. The expression of several genes was measured in individual rats from the 6 dietary treatment groups. Shown are the 2 genes that showed significant changes in expression among the groups (A,C) and the data for Cp (which showed no significant differences; B). In all panels, bars with different letters atop or beneath error bars are statistically different from one other (P < .05). Hamp, hepcidin; Ankrd37, ankyrin repeat domain protein 37. n = 7 for CuD group and n = 8 for all other groups. Data are expressed as means ± SD except for data in panel C, which are expressed as means ± SEM (because a log scale is used).
Quantification of immunoreactive Cp protein levels
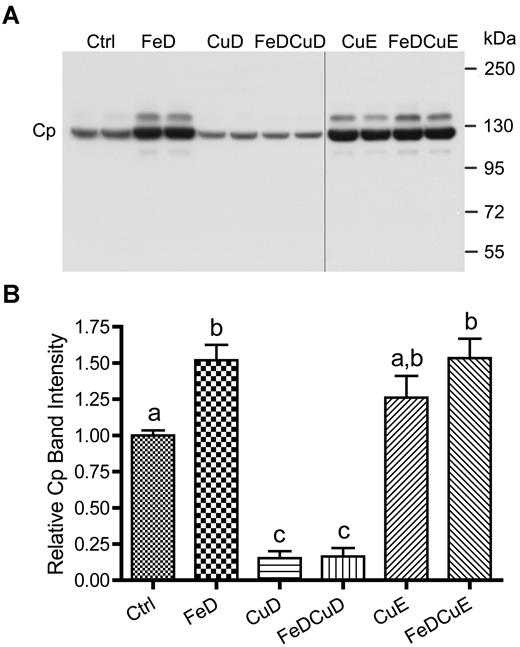
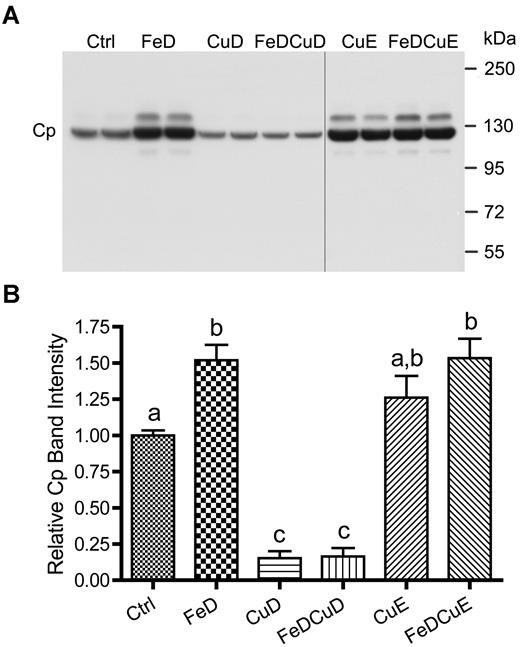
Given the striking changes in serum copper (Figure 1) and the high proportion of serum copper that Cp represents, we asked whether Cp reflected serum copper changes. Serum Cp levels were thus quantified from individual rats in the different dietary treatment groups. Results from a representative experiment are shown in Figure 3, along with quantitative data from all animals studied. Cp was detected in all 6 experimental groups, but both groups consuming low copper (CuD and FeDCuD) showed a significant decrease compared with controls (∼ 70% reduction; P < .001). Dietary iron deprivation with and without supplemental copper (FeD and FeDCuE groups) led to an increase in Cp expression (50%-60%; P < .001). Moreover, Cp expression in rats consuming the CuE diet was moderately increased (∼ 27%), but this increase did not reach statistical significance.
Cp protein expression in rat serum. A representative Western blot is shown (top panel), representing 2 rats per dietary treatment group. Quantitative data from all rats is also given (n = 8-12 rats/group; bottom panel). Bars with different letters atop error bars are statistically different (P < .05) from each other. Data are expressed as means ± SD. The image is of one blot from a single x-ray film; the vertical black line indicates where a blank lane with nonchemiluminescent molecular weight markers was removed from the image.
Cp protein expression in rat serum. A representative Western blot is shown (top panel), representing 2 rats per dietary treatment group. Quantitative data from all rats is also given (n = 8-12 rats/group; bottom panel). Bars with different letters atop error bars are statistically different (P < .05) from each other. Data are expressed as means ± SD. The image is of one blot from a single x-ray film; the vertical black line indicates where a blank lane with nonchemiluminescent molecular weight markers was removed from the image.
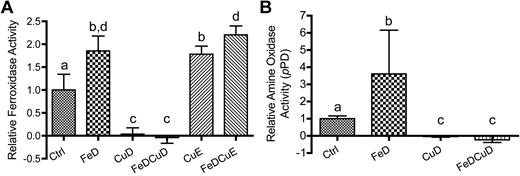
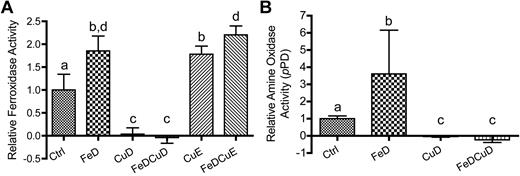
In-gel serum ferroxidase and amine oxidase activity assays
Figure 4A depicts the product of the reaction catalyzed by serum protein using pPD as a substrate. Activity in the FeD, CuE, and FeDCuE groups was noticeably higher than that of controls, whereas no activity was noted in samples derived from animals consuming the low copper diets (CuD and FeDCuD). Figure 4B shows Fe2+ to Fe3+ oxidation catalyzed by serum ferroxidase. Translucent whitish bands represent the absence of the Fe2+–Fz complex at the site of the immobilized ferroxidase, because the latter has oxidized from Fe2+ to Fe3+. Results were almost identical to pPD assay results, with higher activity detected in the FeD, CuE, and FeDCuE groups, and no activity being detected in the CuD and FeDCuD groups.
In-gel Cp enzyme activity assays. Serum samples were separated by gel electrophoresis and subsequently reacted with different substrates (pPD or Fz) to estimate enzyme activity levels. In both panels, each treatment group is represented by 2 samples derived from 2 individual rats (except for 3 rats for FeDCuD).
In-gel Cp enzyme activity assays. Serum samples were separated by gel electrophoresis and subsequently reacted with different substrates (pPD or Fz) to estimate enzyme activity levels. In both panels, each treatment group is represented by 2 samples derived from 2 individual rats (except for 3 rats for FeDCuD).
Spectrophotometric serum ferroxidase and amine oxidase activity assays
As a complementary approach to in-gel assays, spectrophotometric Cp assays were developed, because quantification of data are more precise and different reaction times and conditions can be used. Extensive preliminary experiments were performed to optimize reaction conditions and to determine the stability of the enzyme under our purification and storage conditions. Pilot studies determined that Cp enzyme activity was relatively stable for up to 3 days when serum was processed on ice and stored at 4°C. As such, all assays were run by the end of the third day after serum was collected, with the majority of assays actually being run within 48 hours. Results from serum ferroxidase assays using Fz are depicted in Figure 5A. Ferroxidase activity was significantly increased in the FeD, CuE, and FeDCuE groups compared with controls (∼ 90%, 80%, and 120%, respectively; P < .001). Activity was essentially absent in the rats consuming the low copper diets (CuD and FeDCuD groups; P < .001), whereas activity was abolished in all samples tested using NaN3 as an inhibitor (data not shown). Note that NaN3 is a known inhibitor of FOX-I (ie, Cp). To confirm the increase in Cp activity noted in the FeD group and using the CuD and FeDCuD groups as controls, experiments were also performed spectrophotometrically using pPD as the substrate; serum amine oxidase activity, probably mediated by Cp, was thus measured. Figure 5B depicts the results obtained. As is evident, activity was increased in the FeD group (∼ 3.6-fold; P < .05) and activity was again absent with dietary copper deprivation (values < 0 in the CuD and FeDCuD groups). Enzyme activity in the Ctrl and FeD groups was once again abolished by NaN3 (data not shown).
Spectrophotometric Cp enzyme activity assays. (A) Fz assays. (B) pPD assays. For both panels, bars with different letters atop error bars are statistically different (P < .05) from each other. Data are expressed as means ± SD. For panel A, Ctrl, n = 12; FeD and FeDCuE, n = 11; FeDCuD and CuE, n = 8; and CuD, n = 7. For panel B, Ctrl and FeD, n = 7; CuD, n = 2; and FeDCuD, n = 4. Note that for Fz assay, we included additional samples derived from rats that were from a different study in which the dietary protocol and animal ages were identical.
Spectrophotometric Cp enzyme activity assays. (A) Fz assays. (B) pPD assays. For both panels, bars with different letters atop error bars are statistically different (P < .05) from each other. Data are expressed as means ± SD. For panel A, Ctrl, n = 12; FeD and FeDCuE, n = 11; FeDCuD and CuE, n = 8; and CuD, n = 7. For panel B, Ctrl and FeD, n = 7; CuD, n = 2; and FeDCuD, n = 4. Note that for Fz assay, we included additional samples derived from rats that were from a different study in which the dietary protocol and animal ages were identical.
Discussion
Results described herein provide evidence of increased serum ferroxidase activity during iron deficiency anemia. This observation concurs with scientific findings that were documented several decades ago.26 A seminal observation was made more than 80 years ago when it was demonstrated that anemia induced in birds by bloodletting resulted in increased protein bound copper in plasma.27 Additional investigations in the 1930s and 1940s documented increased serum copper in humans suffering from anemia associated with dietary iron deficiency, massive hemorrhage,26 pregnancy,28 and infection or inflammation.29 Once it was recognized that most copper in the blood is bound to Cp,30 noted increases in serum copper were shown to be because of elevated Cp in most of these and other pathologic conditions (eg, sickle cell disease and renal failure). The induction of Cp activity during infection and inflammation, and pregnancy, is probably the result of cytokine and hormonal stimulation of Cp expression. However, increased Cp activity during dietary iron deficiency, an observation that has been reported in the literature twice,31,32 probably reflects a distinct regulatory pathway and exemplifies the relationship between Cp activity and body iron levels.
The current investigation provides evidence that dietary iron deficiency in rats leads to notable increases in serum copper and Cp activity. Extensive molecular analysis of tissue and serum samples allowed comparison of functional changes in circulating Cp to the Cp gene expression pathway, permitting inference to be drawn regarding the molecular mechanism of Cp induction. Moreover, tissue mineral analyses reported here allowed consideration of potential relationships between the different physiologic parameters analyzed. The comprehensive nature of the current investigation has thus revealed novel aspects of this phenomenon that were not appreciated previously, including the observation that Cp expression and activity are enhanced in response to liver copper loading and that the underlying molecular mechanism is probably related to increased metallation of the Cp protein leading to higher circulating levels of the holo (ie, functional) form of the enzyme.
The biologic purpose for enhanced Cp activity probably relates to the role of Cp in iron homeostasis. During dietary iron deficiency, Cp induction would increase iron mobilization from storage depots, such as from the liver, thereby maximizing iron delivery to the bone marrow for erythropoiesis.33 It is also possible that Cp plays a role in enhancing iron extraction from the diet during states when body iron stores are depleted. Several investigations have examined this possibility, with mixed results. Some studies do not support a role for Cp in intestinal iron absorption.34,35 Another investigation, however, provides strong evidence of a positive effect of Cp in this process.36 Of particular note is the observation that bleeding of mice combined with low dietary iron intake resulted in a dramatic shift of Cp into the lamina propria of the intestinal villus.37 A model was proposed in which Cp was present in enterocytes and then released into the lamina propria in response to iron deficiency, where it presumably enhances iron release from enterocytes. Furthermore, although Cp knockout mice did not exhibit notable defects in intestinal iron transport,38 the role of Cp during states of low iron-related stress was not determined. Also important to consider is the role of Heph in intestinal iron transport. Although Heph plays an undisputed role in iron absorption, the phenotype of sex-linked anemia (sla) mice (which lack functional Heph), suggests that another, redundant ferroxidase exists, as the anemia seen in these mutant mice resolves with age.16 Additional studies are thus clearly warranted to further consider the physiologic role of Cp in iron absorption, particularly during states of low iron stress.
A previous investigation demonstrated that the induction of Cp in HepG2 cells (a human hepatoma cell line) by iron chelation or hypoxia was transcriptionally mediated via activation of the Cp promoter by HIF1α.39 The current observations, however, are not consistent with a hypoxia-responsive transcription factor mediating the induction of Cp, because no change in Cp mRNA expression was noted. The livers of the experimental animals in the FeD groups were probably hypoxic as hemoglobin levels were significantly decreased. Moreover, there was a significant induction of hepatic Ankrd37 mRNA. Ankrd37 is a sensitive hypoxia-responsive gene and a proven HIF1α target40 ; in fact, it was shown previously to be the most strongly up-regulated gene (∼ 15-fold) in intestinal epithelial cells treated with an iron chelator that mimics hypoxia.41 The current investigation thus demonstrates that the induction of Cp during dietary iron deficiency in this in vivo rodent model is mediated by a posttranscriptional mechanism; findings that lead to speculation that increased production of the active, copper-containing form of the enzyme occurs in the copper-loaded livers of these experimental animals.
Cp is synthesized in the liver in the apo (devoid of copper) and holo (replete with copper) forms in more or less equal quantities.42 The holo form, which is functional Cp enzyme, has a half-life of ∼ 5 days,43 whereas that of the apo form is estimated to be < 6 hours.44 The predominance of Cp in the circulation is thus holo-Cp. Serum Cp activity is drastically reduced during copper deprivation,45 probably because most Cp enzyme synthesized during low copper conditions is apo-Cp that is rapidly catabolized. Given this role for copper in the synthesis of the functional Cp enzyme, it was important to also quantify Cp expression and activity when liver copper levels increase (eg, during iron deficiency). This is particularly relevant given the fact that scant experimental evidence documenting increases in serum ferroxidase activity because of iron deficiency anemia exists,31,32 and no plausible insight as to a potential mechanism has been put forth. Interestingly, one of these reports that describes increased serum Cp activity in iron-deficient patients concludes with the statement that “The question as to what initiates and maintains the increase in circulating ceruloplasmin in this clinical condition [iron deficiency anemia] is baffling and needs further study.”31
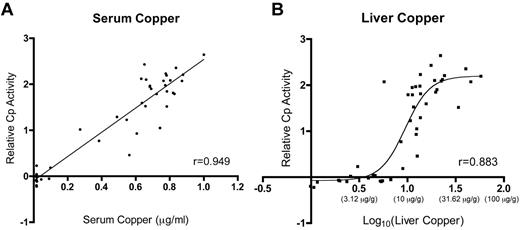
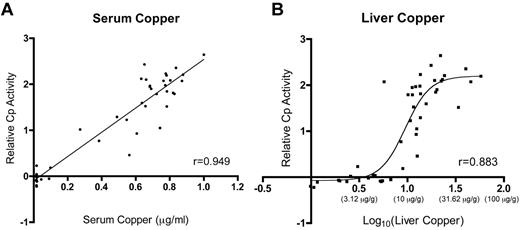
This investigation documents increased Cp protein levels and serum ferroxidase activity in iron-deficient rats and in those consuming higher copper levels. Statistical comparisons of the biologic parameters analyzed here reveal strong correlations between Cp activity and serum and liver copper (Table 2; Figure 6), allowing a reasonable conclusion to be drawn. It is hypothesized that increases in liver copper result in enhanced metallation of the Cp protein, leading to a higher proportion of the holo form of the enzyme. This supposition is supported by the observation that Cp protein was increased and that serum ferroxidase and amine oxidase activities were also significantly higher in rats with higher liver copper levels. Moreover, because copper is a cofactor that is inserted cotranslationally into the Cp protein in an energy-independent manner, it is reasonable to predict Cu (content) to be rate limiting. A recent in vitro study in fact provides support for this hypothesis, because it was demonstrated that Cp protein expression increased in copper-loaded HepG2 cells with no corresponding changes in mRNA expression.46 Whether such a phenomenon occurs in vivo in other mammalian species is unknown, but the authors note a published report that documented increased Cp activity in a human patient suffering from acute copper toxicosis.47 Perhaps the most common clinical condition in which patients present with liver copper loading is Wilson disease,48 in which the ATP7B copper exporter is dysfunctional. These individuals are unable to synthesis functional Cp enzyme, though, as the ability of ATP7B to deliver copper to the secretory pathway is abolished.
Relative Cp activity as a function of liver and serum copper levels. Plots depict the relationship between serum (A) and liver (B) copper and Cp activity. Lines or curves fitting the data were derived by linear regression for Cp activity versus serum copper and by nonlinear regression for Cp activity versus liver copper. Data for liver copper were log transformed for reasons addressed under “Statistical analysis”; actual values for liver copper (in micrograms per gram dry weight) are shown below indicates the y-axis in parentheses. In panel A, P < .0001. r indicates Pearson correlation coefficient.
Relative Cp activity as a function of liver and serum copper levels. Plots depict the relationship between serum (A) and liver (B) copper and Cp activity. Lines or curves fitting the data were derived by linear regression for Cp activity versus serum copper and by nonlinear regression for Cp activity versus liver copper. Data for liver copper were log transformed for reasons addressed under “Statistical analysis”; actual values for liver copper (in micrograms per gram dry weight) are shown below indicates the y-axis in parentheses. In panel A, P < .0001. r indicates Pearson correlation coefficient.
It is also worthwhile to consider the body's handling of copper and to briefly consider how this handling could relate to the current observations. Dietary cupric copper must first be reduced by a brush-border reductase (perhaps Cybrd1) followed by transport into enterocytes via Ctr1.49 Once in enterocytes, copper is bound to various chaperones that deliver it to different cellular locations. Atox1 delivers copper to the trans-Golgi where it is taken up by the Menkes copper ATPase (Atp7a), allowing synthesis of cuproenzymes as part of the secretory pathway. Under conditions of copper excess, Atp7a traffics to the basolateral membrane of cells where it mediates copper export.50 Interestingly, under low iron conditions in rats, Atp7a expression is strongly induced3,4 and the protein is present on the basolateral membrane of enterocytes, suggesting enhanced copper uptake.9 After export of copper from enterocytes, binding to albumin or other serum proteins occurs for delivery and transport into hepatocytes by Ctr1. It is tempting to speculate that the induction of Atp7a (and presumed increased copper export from enterocytes) increases copper delivery to hepatocytes, secondarily leading to increased holo-Cp production. Whether this is the case or whether decreased copper excretion into the bile is responsible for liver copper loading during iron deficiency is currently not known.
In summary, data presented here support the possibility that Cp protein expression and activity are determined by liver copper levels. It was noted previously that this relationship existed in the negative direction whereby decreased hepatic copper content led to the production of nonfunctional Cp enzyme, but the current investigation now suggests that such a relationship also exists in the positive direction. Moreover, observations described here provide evidence of a posttranscriptional mechanism underlying the induction of Cp protein and activity levels during iron deficiency anemia. A clear focus for future studies relates to recapitulating these observations in vitro so as to be able to precisely define the molecular mechanism underlying the induction of Cp protein and activity during hepatic copper loading.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Michael and Laura Garrick (University at Buffalo) for critical review of this manuscript and for helpful discussion.
This study was supported by National Institutes of Health grant R01 DK074867 (J.F.C.).
National Institutes of Health
Authorship
Contribution: P.N.R. designed experiments, carried out studies, and assisted with drafting the manuscript; Y.L. designed and carried out experiments, performed statistical analysis, and designed figures and tables; L.J. designed and performed experiments, produced figures, and performed statistical analysis; C.K. designed and performed experiments and assisted with animal handling, sacrifice, and tissue harvest; and J.F.C. oversaw all aspects of this study, including experimental and intellectual design and drafting of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James F. Collins, Department of Food Science & Human Nutrition, FSHN Bldg, #441, PO Box 110370, Newell Dr, University of Florida, Gainesville, FL 32611; e-mail: jfcollins@ufl.edu.