Abstract
Memory B cells are involved in long-term maintenance of antibody-dependent immunologic disorders. Therefore, it is essential to understand how the restimulation of FVIII-specific memory B cells in hemophilia A with FVIII inhibitors is regulated. We asked whether concurrent activation of the innate immune system by an agonist for toll-like receptor (TLR) 7 is able to facilitate the differentiation of FVIII-specific memory B cells in the absence of T-cell help. TLR7 recognizes single-stranded RNA as contained in RNA viruses such as influenza, Sendai, and Coxsackie B viruses. Our results indicate that highly purified murine memory B cells do not differentiate into FVIII-specific antibody-secreting cells in the presence of FVIII and the TLR7 agonist when cultured in the absence of CD4+ T cells. However, CD11c+ dendritic cells facilitate the T cell–independent differentiation of FVIII-specific memory B cells but only in the presence of FVIII and the TLR7 agonist. In contrast to T cell–dependent restimulation, the antibody response after T cell–independent restimulation of FVIII-specific memory B cells is skewed toward IgG2a, an antibody subclass that is efficient in activating the complement system and in inducing Fc-receptor–mediated effector functions, both are required for effective immune responses against pathogens.
Introduction
Memory B cells are essential to maintain antibody-dependent immunologic memory that is required for long-lasting protection against invading pathogens such as viruses and bacteria. After encounter with their specific antigen, memory B cells can rapidly proliferate and differentiate into antibody-secreting plasma cells (ASCs), thereby replenishing the pool of plasma cells.1 Moreover, memory B cells act as efficient antigen-presenting cells for the restimulation of CD4+ T cells because they express high-affinity antigen receptors, major histocompatibility complex class II molecules, and costimulatory molecules.2 However, memory B cells are also involved in long-term maintenance of immunopathologic conditions such as chronic antibody-dependent immunologic disorders,3 which would indicate that memory B cells have to be eradicated for successful treatment of such diseases.
We and others have demonstrated the presence of FVIII-specific memory B cells in the circulation of patients with FVIII inhibitors.4-6 Furthermore, several studies have suggested that FVIII-specific memory B cells are down-regulated during successful immune tolerance induction therapy in patients with hemophilia A and FVIII inhibitors.5,6 These results indicate that FVIII-specific memory B cells might be essential for maintaining immunologic memory for antibodies against FVIII in patients. Therefore, it is important to understand the regulation of FVIII-specific memory B cells and to find new approaches to specifically eradicate these cells.
We used a murine model of hemophilia A to study the regulation of FVIII-specific memory B cells in vitro. We demonstrated previously that the restimulation of FVIII-specific memory B cells depends on the dose of FVIII used. Low doses of FVIII (up to physiologic plasma concentrations) restimulate FVIII-specific memory B cells to differentiate into FVIII-specific ASCs, and high doses of FVIII (above physiologic plasma concentrations) prevent the differentiation into ASCs.7 The restimulation of FVIII-specific memory B cells depends on the presence of activated CD4+ T cells and requires either direct cell–cell interactions or very close range interactions between activated CD4+ T cells and memory B cells.8
Recently we showed that the restimulation of FVIII-specific memory B cells is modulated by concurrent activation of the innate immune system by toll-like receptor (TLR) agonists.9 TLR agonists amplify the restimulation and differentiation of memory B cells in the presence of stimulatory FVIII concentrations and counteract the inhibition of memory B-cell differentiation in the presence of inhibitory FVIII concentrations. TLR are conserved pattern recognition receptors that recognize conserved structures of pathogens, so-called pathogen-associated molecular patterns.10 Although TLRs are part of the innate immune system, their activation also modulates adaptive immune responses such as antibody responses or T-cell responses.10-12 So far, 12 different TLRs have been identified in mammals.13 They are differentially expressed on many cells of hematopoietic and nonhematopoietic origin,12-14 either at the cell surface (TLR1, TLR2, TLR4, TLR5, and TLR6) or in endolysosomal compartments (TLR3, TLR7, TLR8, and TLR9). Our recently published data indicate that TLR7 and TLR9 agonists are most effective in modulating FVIII-specific memory responses.9 Based on these data, we now asked whether TLR7 and TLR9 agonists are able to facilitate the differentiation of FVIII-specific memory B cells in the absence of T-cell help, and, if so, whether alternative “helper cells” are required to replace FVIII-specific CD4+ T cells. The answer to this question is very important for the design of new therapeutic strategies for the treatment of patients with FVIII inhibitors, in particular for the design of strategies that specifically target FVIII-specific CD4+ T cells. If FVIII-specific memory B cells could be restimulated to differentiate into FVIII-specific ASCs in the absence of FVIII-specific CD4+ T cells, a therapeutic approach that targets FVIII-specific CD4+ T cells would not be very effective in the situation of an established inhibitor response.
We developed a novel in vitro differentiation microculture system that uses highly purified cell populations obtained from hemophilic mice treated with FVIII. Using this new technology, we were able to study the complex regulation of FVIII-specific memory B-cell differentiation in much more detail than was previously possible.
Methods
Animals
Our colony of fully inbred E17 hemophilic mice (characterized by a targeted disruption of exon 17 of the F8 gene) was established with a breeding pair from the original colony15,16 and crossed into the C57BL/6J background.17 All mice were male and aged 8-10 weeks at the beginning of the experiments. All studies were carried out in accordance with Austrian federal law (Act BG 501/1989) regulating animal experimentation and were also approved by the Baxter BioScience Institutional Animal Care and Use Committee.
Treatment of mice with human FVIII
Mice received 4 intravenous weekly doses of 0.2 μg of recombinant human FVIII (∼ 80 U/kg FVIII), diluted in 200 μL of Dulbecco PBS (Sigma-Aldrich). The recombinant human FVIII used throughout the studies was albumin-free bulk material obtained from Baxter BioScience.
Antibodies and reagents
The following monoclonal anti–mouse antibodies were used for purification procedures and analysis of cells: the biotinylated antibodies anti-IgM (R6-60.2), anti-CD3 (145-2C11), anti-CD49b (DX5), anti-CD138 (281-2), anti-IgA (C10-1), anti-IgG1 (A85-1), anti-IgG2a/2b (R2-40), and anti-IgG3 (R40-82); the FITC-labeled antibodies anti-IgM (R6-60.2), anti–Ly-6C (AL-21), anti-CD8 (53-6.7), and anti-CD49b (DX5); PE-labeled anti-CD138 (281-2); the peridinin chlorophyll protein complex (PerCP)–labeled antibodies anti-CD4 (RM4-5) and anti-CD8 (53-6.7); and phycoerythrin-cyanin-7 (PE-Cy7)–labeled anti-CD19 (1D3; all obtained from BD Biosciences Pharmingen; purified anti-CD16/32 (93); biotinylated anti-IgD (11-26c); the FITC-labeled antibodies anti-F4/80 (BM8), anti-IgD (11-26c), and anti–Ly-6G (RB6-8C5); and allophycocyanin (APC)–labeled anti-CD11c (N418; all obtained from eBioscience); and PE-labeled anti–mPDCA-1 (JF05-1C2.4.1; Miltenyi Biotec). FITC-avidin and PE-streptavidin were obtained from BD Biosciences Pharmingen. Imiquimod (R837), agonist for TLR7, and CpG-oligodeoxynucleotide ([ODN]; CpG-B, ODN1826, sequence 5′-tcc atg acg ttc ctg acg tt-3′), agonist for TLR9, were obtained from InvivoGen.
Preparation of spleen cells
Purification of memory B cells, naive B cells, T cells, and dendritic cells
The purity of all purified cell populations was confirmed by flow cytometric single-cell analyses using an FACSAria cell sorter and Diva Version 6.1 software from BD Biosciences as well as FlowJo Version 7.5.5 software (TreeStar).
All staining procedures included an initial resuspension of spleen cells in Dulbecco PBS containing 4% preselected FCS (HyClone Laboratories) and staining with a mixture of anti–mouse CD16 and anti–mouse CD32 antibodies (Fc-Block; BD Biosciences Pharmingen) to block unspecific binding sites.
Purification of memory B cells and CD4+ T cells from spleen cells
Because of the lack of a specific marker for murine memory B cells, memory B cells were defined as switched B cells (IgM−IgD−CD138−CD11c−CD19+ splenocytes). Memory B cells and CD4+ T cells were purified in a 2-step process involving an immunomagnetic step to deplete most unwanted cells and a subsequent flow-cytometric single-cell sorting step to further purify wanted cells. Spleen cells were stained with a mixture of the following monoclonal antibodies: anti-IgD, anti-IgM, anti-F4/80, anti-CD49b, anti-CD8, anti–Ly-6C, and anti–Ly-6G, all labeled with FITC; PE-labeled anti-CD138, PE-labeled anti–mPDCA-1, PerCP-labeled anti-CD4, PE-Cy7–labeled anti-CD19, and APC-labeled anti-CD11c. The cell fraction stained with FITC-labeled antibodies (IgD+-, IgM+-, F4/80+-, CD49b+-, CD8+-, Ly-6C+-, and Ly-6G+-splenocytes) was removed using the EasySep FITC selection kit for mouse cells (StemCell Technologies) according to the manufacturer's protocol. Memory B cells and CD4+ T cells were further purified as indicated in Figure 2A using flow cytometric single-cell sorting. For this purpose, an FACSAria cell sorter and Diva Version 6.1 software were used.
Purification of naive B cells from spleen cells
Naive B cells were defined as IgM+IgD+IgG1−IgG2a/2b−IgG3−IgA−CD138−CD19+ B cells. They were purified from spleen cells of naive E17 hemophilic mice that were not treated with FVIII. Purification was achieved by flow cytometric single-cell sorting. Spleen cells were stained with a mixture of the following monoclonal antibodies: FITC-labeled anti-IgM, FITC-labeled anti-IgD, biotinylated anti-CD138, biotinylated anti-IgA, biotinylated anti-IgG1, biotinylated anti-IgG2a/2b, biotinylated anti-IgG3, PerCP-labeled anti-CD4, PerCP-labeled anti-CD8, PE-Cy7–labeled anti-CD19, and APC-labeled anti-CD11c. In a second step, PE-labeled streptavidin was added. IgM+IgD+IgG1−IgG2a/2b−IgG3−CD138−CD19+ naive B cells were single-cell–sorted by flow cytometry using an FACSAria cell sorter and Diva Version 6.1 software.
Purification of CD11c+ DCs from spleen cells obtained from naive hemophilic mice
CD11c+ dendritic cells (DCs) were purified in a 3-step process involving 2 immunomagnetic steps to deplete most unwanted cells and a subsequent flow cytometric single-cell sorting step to further purify wanted cells. Spleen cells were depleted of T cells using mouse pan-T (Thy 1.2) Dynabeads (Invitrogen). Remaining Thy 1.2− cells were stained with a mixture of the following monoclonal antibodies: anti-IgD, anti-IgM, anti-CD3, and anti-CD49b, all labeled with biotin. The fraction of biotinylated cells was depleted using Biotin Binder Dynabeads according to the manufacturer's protocol (Invitrogen). Remaining cells were stained with FITC-avidin (to exclude remaining biotinylated cells), PE-labeled anti–mPDCA-1, PerCP-labeled anti-CD3, PE-Cy7–labeled anti-CD19, and APC-labeled anti-CD11c. The CD11c+ cells were single-cell–sorted as DCs by flow cytometry using an FACSAria cell sorter and Diva Version 6.1 software as indicated in Figure 2B.
Restimulation of purified memory B cells in vitro
Purified cells were washed and resuspended in RPMI 1640 supplemented with 10% preselected FCS, 2 mM l-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin (all from Invitrogen) and 5 × 10−5 M β-mercaptoethanol (Sigma-Aldrich).
Purified memory B cells only or memory B cells together with purified CD4+ T cells (at a ratio of 4:1) or purified CD11c+ DCs (at a ratio of 2:1) were seeded into round-bottomed 96-well cell culture plates (Corning Life Sciences). Microcultures were cultivated at 1.5 × 105 cells (total cells) in a total volume of 200 μL, either in the absence of FVIII or in the presence of 10 or 20 000 ng/mL human FVIII. Some cultures were supplemented with imiquimod (TLR7 agonist) or CpG-ODN (TLR9 agonist) at 100 ng/mL. After 6 days of culture, newly formed ASCs were detected by ELISPOT assays as described previously.18 In some experiments, memory B cells were replaced by equal numbers of naive B cells.
Cell–cell interaction studies.
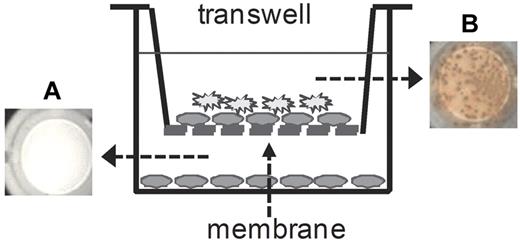
Tissue culture inserts (Nunc, Thermo Fisher Scientific) were used to study direct cell–cell interactions between memory B cells and DCs. These inserts contain an Anopore membrane with a pore size of 0.2 μm that separates cells but is permeable for soluble proteins and fluids. Cultures were set up as indicated in Figure 5 and cultured for 6 days at 37°C and 5% CO2. Newly formed FVIII-specific ASCs were analyzed after 6 days of culture using ELISPOT assays.
Cytokine analysis
Murine IL-4, IL-6, IL-10, IL-12p40, IL-12p70, and IFN-γ were detected in cell culture supernatants using a Bio-Plex Mouse Cytokine 13-Plex assay (Bio-Rad Laboratories). Murine IFN-α was detected in cell culture supernatants using a Verikine Mouse Interferon α ELISA kit from PBL Interferon Source.
Statistical analysis
The unpaired 2-tailed Student t test was used for comparison of means between groups, and the Mann-Whitney test was used for comparison of medians between groups. P <.05 was considered statistically significant.
Results
TLR7 and TLR9 agonists facilitate T cell–independent restimulation of FVIII-specific memory B cells
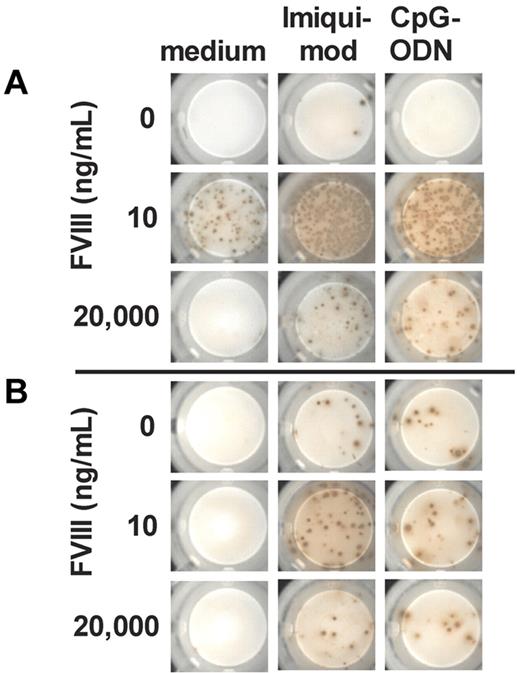
Previously, we demonstrated that in vitro restimulation of FVIII-specific memory B cells depends on both the presence of activated CD4+ T cells and the dose of FVIII.8 We also showed that the presence of TLR7 and TLR9 agonists greatly amplified the restimulation of memory B cells in the presence of stimulatory FVIII doses (10 ng/mL) and counteracted the inhibition in the presence of inhibitory FVIII doses (1000 and 20 000 ng/mL).9 Based on these observations, we now asked whether concomitant activation of the innate immune system by TLR7 and TLR9 agonists facilitate restimulation of memory B cells in the absence of CD4+ T-cell help. We generated CD138− spleen cells from hemophilic mice treated with 4 doses of FVIII, depleted total T cells, and restimulated FVIII-specific memory B cells contained in this cell mixture with different doses of FVIII in the presence of TLR7 or TLR9 agonists. Results of these studies confirm our previously published findings on the ability of TLR7 and TLR9 agonists to amplify restimulation of FVIII-specific memory responses (Figure 1A). Furthermore, results indicate that TLR7 and TLR9 agonists facilitate the restimulation of FVIII-specific memory B cells, even in the absence of CD4+ T cells (Figure 1B). When comparing effects of TLR7 agonist and TLR9 agonist in the T cell–independent setup, the TLR7 agonist was most effective.
TLR7 and TLR9 agonists amplify T cell–dependent and facilitate T cell–independent restimulation of FVIII-specific memory B cells. CD138− (A) and CD138− T− (B) spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and restimulated in vitro with FVIII in the presence of TLR7 agonist imiquimod or TLR9 agonist CpG-ODN. Newly formed FVIII-specific ASCs were detected after 6 days of culture by ELISPOT assay. The concentration of FVIII is indicated. Cells were differentiated in the presence of medium only, TLR7 agonist imiquimod (100 ng/mL) or TLR9 agonist CpG-ODN (100 ng/mL). Representative ELISPOTs are presented.
TLR7 and TLR9 agonists amplify T cell–dependent and facilitate T cell–independent restimulation of FVIII-specific memory B cells. CD138− (A) and CD138− T− (B) spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and restimulated in vitro with FVIII in the presence of TLR7 agonist imiquimod or TLR9 agonist CpG-ODN. Newly formed FVIII-specific ASCs were detected after 6 days of culture by ELISPOT assay. The concentration of FVIII is indicated. Cells were differentiated in the presence of medium only, TLR7 agonist imiquimod (100 ng/mL) or TLR9 agonist CpG-ODN (100 ng/mL). Representative ELISPOTs are presented.
In conclusion, TLR7 and TLR9 agonists facilitate restimulation of memory B cells in the absence of CD4+ T cells.
Restimulation of highly purified memory B cells requires help of CD4+ T cells even in the presence of TLR7 agonists
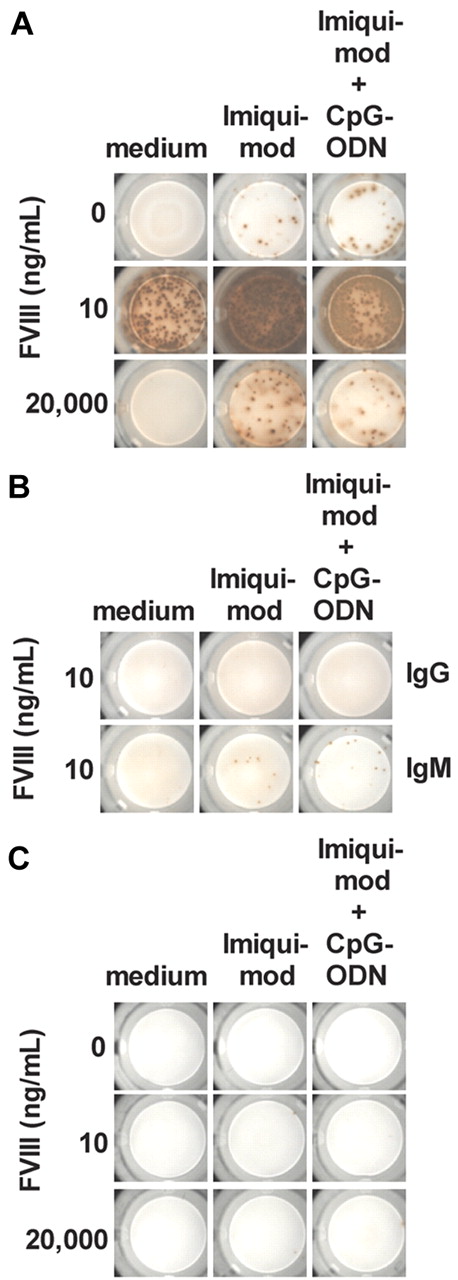
Data shown in Figure 1A and B were generated using our previously described in vitro differentiation culture system of CD138− spleen cells8,9 that contain all spleen cells except CD138+ ASCs and red blood cells. To explain the mechanisms of T cell–dependent and T cell–independent restimulation of FVIII-specific memory B cells in the presence of TLR7 agonist, we established new technology involving highly purified cell populations. We isolated memory B cells and CD4+ T cells from spleens of hemophilic mice treated with 4 doses of FVIII (Figure 2A) and cocultured them in vitro in the presence of different concentrations of FVIII and TLR7 agonist. Results obtained in these studies (Figure 3A) reflect 2 important findings. First, highly purified splenic memory B cells when cocultured with highly purified CD4+ T cells show the same dependence on FVIII concentration for induction and inhibition of differentiation into FVIII-specific ASCs as CD138− total spleen cells do (compare results presented in Figures 1A and 3A). Although concentrations of 10 ng/mL FVIII optimally restimulate the differentiation of memory B cells, concentrations of 20 000 ng/mL inhibit this process. Second, the TLR7 agonist amplifies the restimulation at stimulating FVIII concentrations (10 ng/mL) and counteracts the inhibition at inhibiting concentrations of FVIII (20 000 ng/mL) in the same way as it does in differentiation cultures of CD138− total spleen cells. Importantly, when we used highly purified naive B cells instead of memory B cells, we did not see any differentiation into FVIII-specific IgG-producing ASCs (Figure 3B). We did see, however, some differentiation of naive B cells into FVIII-specific IgM-producing ASCs when naive B cells were cocultured with TLR7 agonist and CD4+ T cells obtained from hemophilic mice treated with 4 doses of FVIII (Figure 3B).
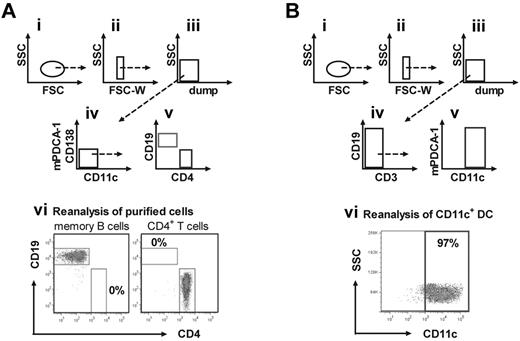
Multistep purification process provides highly purified memory B cells, CD4+ T cells, and CD11c+ DCs. (A) Highly purified memory B cells and CD4+ T cells were obtained from spleens of hemophilic mice treated with 4 weekly doses of 200 ng FVIII. After initial depletion of unwanted cells by magnetic cell separation, wanted cells were purified by flow cytometric single-cell sorting. Presented is the gating strategy (i-iv) for the purification of IgM−IgD−CD138−CD11c−CD19+ memory B cells and CD4+ T cells (dump: remaining unwanted FITC+ cells after initial magnetic cell separation). A representative result of the purity of sorted cell populations (gated on viable cells) is shown in panel vi. (B) Highly purified CD11c+ DCs were obtained from spleens of naive hemophilic mice. After initial depletion of total T cells and other unwanted cell populations using a 2-step magnetic cell separation, CD11c+ DCs were further purified by flow cytometric single-cell sorting. Shown is the gating strategy (i-v) for the purification of CD11c+ DCs (dump: remaining unwanted cells after initial magnetic depletion). A representative result of the purity of sorted CD11c+ DCs (gated on viable cells) is shown in panel vi.
Multistep purification process provides highly purified memory B cells, CD4+ T cells, and CD11c+ DCs. (A) Highly purified memory B cells and CD4+ T cells were obtained from spleens of hemophilic mice treated with 4 weekly doses of 200 ng FVIII. After initial depletion of unwanted cells by magnetic cell separation, wanted cells were purified by flow cytometric single-cell sorting. Presented is the gating strategy (i-iv) for the purification of IgM−IgD−CD138−CD11c−CD19+ memory B cells and CD4+ T cells (dump: remaining unwanted FITC+ cells after initial magnetic cell separation). A representative result of the purity of sorted cell populations (gated on viable cells) is shown in panel vi. (B) Highly purified CD11c+ DCs were obtained from spleens of naive hemophilic mice. After initial depletion of total T cells and other unwanted cell populations using a 2-step magnetic cell separation, CD11c+ DCs were further purified by flow cytometric single-cell sorting. Shown is the gating strategy (i-v) for the purification of CD11c+ DCs (dump: remaining unwanted cells after initial magnetic depletion). A representative result of the purity of sorted CD11c+ DCs (gated on viable cells) is shown in panel vi.
Highly purified memory B cells require CD4+ T-cell help for differentiation into FVIII-specific ASCs. (A) Purified memory B cells and CD4+ T cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. Cells were cultured in the presence of FVIII only, FVIII and TLR7 agonist imiquimod (100 ng/mL), or FVIII and a mixture of TLR7 agonist imiquimod (100 ng/mL) and TLR9 agonist CpG-ODN (100 ng/mL). The concentration of FVIII is indicated. Newly differentiated FVIII-specific ASCs were analyzed after 6 days of culture. Representative ELISPOTs are presented. (B) Purified naive B cells were obtained from untreated hemophilic mice, and CD4+ T cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. Naive B cells together with CD4+ T cells were cultured in the presence of FVIII only, FVIII and TLR7 agonist imiquimod (100 ng/mL), or FVIII and a mixture of TLR7 agonist Imiquimod (100 ng/mL) and TLR9 agonist CpG-ODN (100 ng/mL). FVIII was used at a concentration of 10 ng/mL. Newly differentiated FVIII-specific IgG ASCs and IgM ASCs were analyzed after 6 days of culture. Representative ELISPOTs are presented. (C) Purified memory B cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. Cells were cultured in the presence of FVIII and TLR7 agonist imiquimod or FVIII and a mixture of TLR7 agonist imiquimod (100 ng/mL) and TLR9 agonist CpG-ODN (100 ng/mL). The concentration of FVIII is indicated. Newly differentiated FVIII-specific ASCs were analyzed after 6 days of culture. Representative ELISPOTs are presented.
Highly purified memory B cells require CD4+ T-cell help for differentiation into FVIII-specific ASCs. (A) Purified memory B cells and CD4+ T cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. Cells were cultured in the presence of FVIII only, FVIII and TLR7 agonist imiquimod (100 ng/mL), or FVIII and a mixture of TLR7 agonist imiquimod (100 ng/mL) and TLR9 agonist CpG-ODN (100 ng/mL). The concentration of FVIII is indicated. Newly differentiated FVIII-specific ASCs were analyzed after 6 days of culture. Representative ELISPOTs are presented. (B) Purified naive B cells were obtained from untreated hemophilic mice, and CD4+ T cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. Naive B cells together with CD4+ T cells were cultured in the presence of FVIII only, FVIII and TLR7 agonist imiquimod (100 ng/mL), or FVIII and a mixture of TLR7 agonist Imiquimod (100 ng/mL) and TLR9 agonist CpG-ODN (100 ng/mL). FVIII was used at a concentration of 10 ng/mL. Newly differentiated FVIII-specific IgG ASCs and IgM ASCs were analyzed after 6 days of culture. Representative ELISPOTs are presented. (C) Purified memory B cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. Cells were cultured in the presence of FVIII and TLR7 agonist imiquimod or FVIII and a mixture of TLR7 agonist imiquimod (100 ng/mL) and TLR9 agonist CpG-ODN (100 ng/mL). The concentration of FVIII is indicated. Newly differentiated FVIII-specific ASCs were analyzed after 6 days of culture. Representative ELISPOTs are presented.
Next, we asked whether the addition of a TLR9 agonist could amplify the stimulating activities of TLR7 on B-cell differentiation. We compared the effect of TLR7 agonist with a mixture of TLR7 and TLR9 agonists. Our results indicate that the addition of TLR9 agonist does not amplify the effect of TLR7 agonist (Figure 3A-B).
Finally, we wanted to know whether FVIII-specific memory B cells are restimulated to differentiate into FVIII-specific ASCs in the presence of FVIII and TLR7 agonist or a mixture of TLR7 and TLR9 agonists without additional CD4+ T-cell help. To address this question, we cultured highly purified memory B cells in the presence of different concentrations of FVIII and TLR7 agonist or a mixture of TLR7 and TLR9 agonists and analyzed newly formed FVIII-specific ASC after 6 days. We did not see any restimulation and differentiation of memory B cells into FVIII-specific ASCs (Figure 3C), which indicates that highly purified memory B cells cannot differentiate into FVIII-specific ASCs in the absence of CD4+ T-cell help.
In summary, highly purified memory B cells obtained from hemophilic mice treated with 4 doses of FVIII differentiate into FVIII-specific ASCs in the presence of stimulating concentrations of FVIII (10 ng/mL) in vitro when cocultured with highly purified CD4+ T cells obtained from the same population of hemophilic mice. Furthermore, highly purified memory B cells in the presence of FVIII or FVIII together with TLR7 agonist or a mixture of TLR7 and TLR9 agonists do not differentiate into FVIII-specific ASC when cultured in the absence of CD4+ T-cell help.
Restimulation of highly purified memory B cells in the absence of CD4+ T cells requires “alternative” helper cells
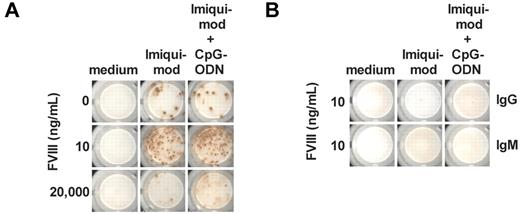
Results shown in Figures 1B and 3C suggest that cells other then CD4+ T cells could provide help to memory B cells and trigger their differentiation into FVIII-specific ASCs. Whereas the cell culture system used for the generation of results shown in Figure 3C contained highly purified memory B cells, the culture system used for the generation of results shown in Figure 1B contained CD138− total spleen cells that are composed of several other cell populations in addition to memory B cells. Several recent studies indicated that DCs are able to modulate B-cell function.19-21 Therefore, we asked whether DCs could facilitate the differentiation of FVIII-specific memory B cells in the absence of CD4+ T cells. We generated highly purified CD11c+ DCs from spleens of naive E-17 hemophilic mice (Figure 2B) and cocultured them with highly purified memory B cells in the presence of different concentrations of FVIII with or without TLR7 agonist or a mixture of TLR7 and TLR9 agonists. We analyzed newly formed FVIII-specific ASCs after 6 days of culture. We did not find any differentiation of memory B cells into FVIII-specific ASCs in the presence of CD11c+ DCs and FVIII only (Figure 4A). However, when we added TLR7 agonist or a mixture of TLR7 and TLR9 agonists, we observed differentiation into FVIII-specific ASCs that depended on the concentration of FVIII present in the system (Figure 4A). FVIII concentrations previously found to be stimulatory (10 ng/mL) stimulated differentiation and concentrations that were shown previously to be inhibitory (20 000 ng/mL) inhibited differentiation. For comparison, naive B cells were not stimulated to differentiate into FVIII-specific ASCs (Figure 4B).
Purified CD11c+ DCs in the presence of TLR7 agonist facilitate T cell–independent restimulation of FVIII-specific memory B cells. Memory B cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. DCs and naive B cells were obtained from untreated hemophilic mice. (A) Purified memory B cells and purified CD11c+ DCs were cultured in the presence of FVIII only, FVIII and TLR7 agonist imiquimod (100 ng/mL), or FVIII and a mixture of TLR7 agonist imiquimod (100 ng/mL) and TLR9 agonist CpG-ODN (100 ng/mL). The concentration of FVIII is indicated. Newly differentiated FVIII-specific ASCs were analyzed after 6 days of culture. Representative ELISPOTs are presented. (B) Purified naive B cells and purified CD11c+ DCs were cultured in the presence of FVIII only, FVIII and TLR7 agonist imiquimod (100 ng/mL), or FVIII and a mixture of TLR7 agonist imiquimod (100 ng/mL) and TLR9 agonist CpG-ODN (100 ng/mL). The concentration of FVIII was 10 ng/mL. Newly differentiated FVIII-specific IgG ASCs and IgM ASCs were analyzed after 6 days of culture. Representative ELISPOTs are presented.
Purified CD11c+ DCs in the presence of TLR7 agonist facilitate T cell–independent restimulation of FVIII-specific memory B cells. Memory B cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. DCs and naive B cells were obtained from untreated hemophilic mice. (A) Purified memory B cells and purified CD11c+ DCs were cultured in the presence of FVIII only, FVIII and TLR7 agonist imiquimod (100 ng/mL), or FVIII and a mixture of TLR7 agonist imiquimod (100 ng/mL) and TLR9 agonist CpG-ODN (100 ng/mL). The concentration of FVIII is indicated. Newly differentiated FVIII-specific ASCs were analyzed after 6 days of culture. Representative ELISPOTs are presented. (B) Purified naive B cells and purified CD11c+ DCs were cultured in the presence of FVIII only, FVIII and TLR7 agonist imiquimod (100 ng/mL), or FVIII and a mixture of TLR7 agonist imiquimod (100 ng/mL) and TLR9 agonist CpG-ODN (100 ng/mL). The concentration of FVIII was 10 ng/mL. Newly differentiated FVIII-specific IgG ASCs and IgM ASCs were analyzed after 6 days of culture. Representative ELISPOTs are presented.
In conclusion, our data indicate that CD11c+ DCs in the presence of stimulatory concentrations of FVIII (10 ng/mL) and TLR7 agonist can provide help to FVIII-specific memory B cells to differentiate into FVIII-specific ASCs.
T cell–independent restimulation of FVIII-specific memory B cells in the presence of CD11c+ DCs and TLR7 agonist requires cell–cell interactions between memory B cells and DCs
Next, we asked whether T cell–independent restimulation of FVIII-specific memory B cells in the presence of naive CD11c+ DC and TLR7 agonist is mediated by soluble factors released from DCs or requires direct cell–cell interactions. To address this question, we used a 2-chamber transwell system (Figure 5). The transwell that formed the top chamber was loaded with a mixture of highly purified memory B cells and CD11c+ DCs, and the bottom chamber was filled with memory B cells only. The transwell had a semipermeable membrane that allowed exchange of soluble proteins such as chemokines and cytokines between both chambers but retained cells in the top compartment. Both compartments were restimulated with stimulating concentrations of FVIII (10 ng/mL) and TLR7 agonist. After 6 days of culture, newly formed FVIII-specific ASCs were detected by ELISPOT assays. FVIII-specific ASCs were detectable in the top compartment but not in the bottom compartment of the 2-chamber system (Figure 5). These results indicate that differentiation of memory B cells supported by CD11c+ DCs in the presence of TLR7 agonist requires direct cell-cell interactions between memory B cells and DCs.
T cell–independent restimulation of FVIII-specific memory B cells requires cell–cell interactions between memory B cells and CD11c+ DCs. Purified memory B cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. CD11c+ DCs were obtained from untreated hemophilic mice. (A) Purified memory B cells only were seeded in the lower compartment. (B) Purified memory B cells and CD11c+ DCs were seeded in the upper compartment. Both compartments were restimulated with FVIII (10 ng/mL) together with TLR7 agonist imiquimod (100 ng/mL). Newly differentiated FVIII-specific ASCs were analyzed after 6 days of culture. Representative ELISPOTs are presented, each represents a pool of 4 wells.
T cell–independent restimulation of FVIII-specific memory B cells requires cell–cell interactions between memory B cells and CD11c+ DCs. Purified memory B cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. CD11c+ DCs were obtained from untreated hemophilic mice. (A) Purified memory B cells only were seeded in the lower compartment. (B) Purified memory B cells and CD11c+ DCs were seeded in the upper compartment. Both compartments were restimulated with FVIII (10 ng/mL) together with TLR7 agonist imiquimod (100 ng/mL). Newly differentiated FVIII-specific ASCs were analyzed after 6 days of culture. Representative ELISPOTs are presented, each represents a pool of 4 wells.
T cell–dependent and T cell–independent restimulation of FVIII-specific memory B cells differ in the IgG subclass distribution of newly formed FVIII-specific ASCs
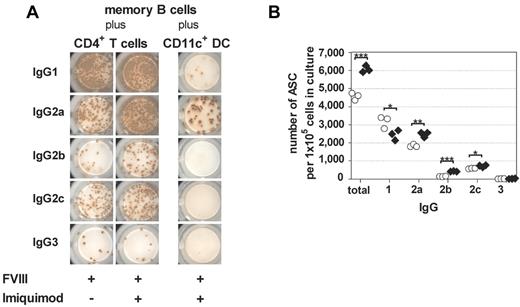
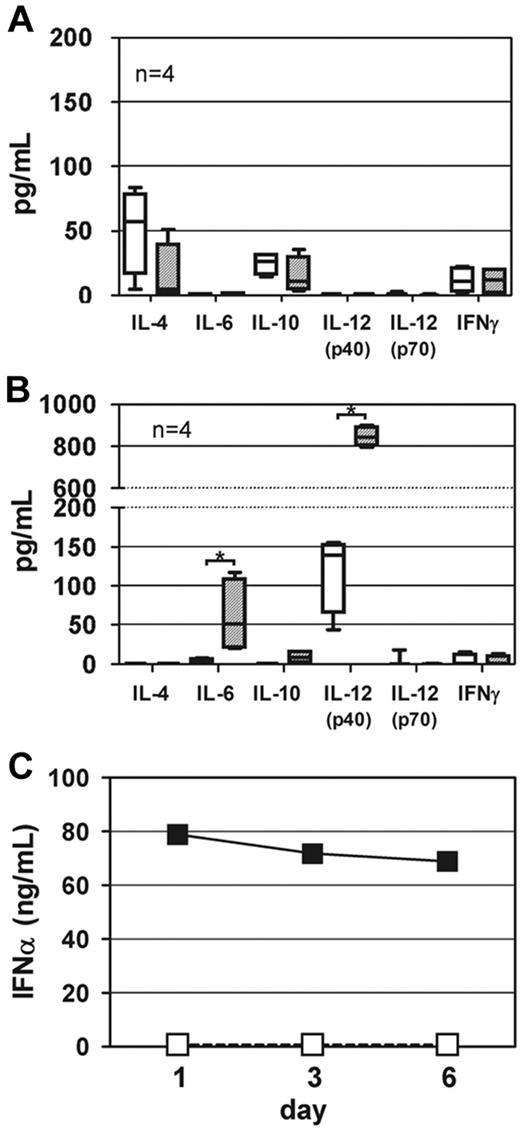
Previously, we reported that the presence of TLR9 agonist favored a T-helper (Th)1–driven restimulation of FVIII-specific memory B cells.9 Based on these data, we now asked whether the addition of TLR7 agonist to the culture system consisting of highly purified memory B cells and CD4+ T cells modifies the IgG subclass distribution of newly differentiated FVIII-specific ASCs. Furthermore, we asked whether the IgG subclass distribution of newly differentiated FVIII-specific ASCs differs between T cell–dependent and T cell–independent restimulation of FVIII-specific memory B cells. To address these questions, we analyzed the IgG subclasses of FVIII-specific ASCs differentiated under different culture conditions. Results obtained in these studies demonstrate 2 things. First, the presence of TLR7 agonist during T cell–dependent restimulation of FVIII-specific memory B cells preferentially amplifies the differentiation of FVIII-specific ASC of the IgG2a, IgG2b, and IgG2c subclasses (Figure 6A-B), which is indicative of a Th1-driven response and confirms previous results that we obtained in the CD138− spleen cell culture system.9 These results were supported by a decrease in IL-4 release during the culture (Figure 7A). Second, our results indicate a striking difference in the IgG subclass distribution of FVIII-specific ASCs after T cell–dependent and T cell–independent restimulation of FVIII-specific memory B cells. Whereas T cell–dependent restimulation induces the differentiation into FVIII-specific ASCs of all IgG subclasses with a dominance of IgG1 and IgG2a, T cell–independent restimulation almost exclusively induces differentiation into FVIII-specific ASCs of the IgG2a subclass (Figure 6A). This differentiation is associated with a release of IL-6 and IL-12p40, 2 major DC cytokines, into cell culture supernatants (Figure 7B). Furthermore, addition of TLR7 agonist to CD11c+ DCs induces an amplification of the release of IFN-α (Figure 7C) during the 6-day culture period.
T cell–dependent and T cell–independent restimulation of FVIII-specific memory B cells differ in the IgG subclass distribution of newly formed FVIII-specific ASCs. Purified memory B cells and purified CD4+ T cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. CD11c+ DCs were obtained from untreated hemophilic mice. Purified memory B cells were restimulated with FVIII (10 ng/mL) or FVIII (10 ng/mL) together with TLR7 agonist imiquimod (100 ng/mL), in the presence of either purified CD4+ T cells or purified CD11c+ DCs. Newly differentiated FVIII-specific ASCs of different IgG subclasses were analyzed after 6 days of culture. Note: The control cultures of memory B cells together with CD11c+ DCs in the absence of TLR7 agonist did not induce any differentiation into FVIII-specific ASCs (see Figure 4A). Therefore, we did not include these data in Figure 6A. (A) Representative ELISPOTs are presented. (B) Quantification of results for T cell–dependent restimulation of memory B cells presented in (A). Results of individual ELISPOT analyses are presented. *P < .05, **P < .01, ***P < .001 for comparison of means. ○ indicates FVIII only; and ♢, FVIII + TLR7 agonist imiquimod.
T cell–dependent and T cell–independent restimulation of FVIII-specific memory B cells differ in the IgG subclass distribution of newly formed FVIII-specific ASCs. Purified memory B cells and purified CD4+ T cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. CD11c+ DCs were obtained from untreated hemophilic mice. Purified memory B cells were restimulated with FVIII (10 ng/mL) or FVIII (10 ng/mL) together with TLR7 agonist imiquimod (100 ng/mL), in the presence of either purified CD4+ T cells or purified CD11c+ DCs. Newly differentiated FVIII-specific ASCs of different IgG subclasses were analyzed after 6 days of culture. Note: The control cultures of memory B cells together with CD11c+ DCs in the absence of TLR7 agonist did not induce any differentiation into FVIII-specific ASCs (see Figure 4A). Therefore, we did not include these data in Figure 6A. (A) Representative ELISPOTs are presented. (B) Quantification of results for T cell–dependent restimulation of memory B cells presented in (A). Results of individual ELISPOT analyses are presented. *P < .05, **P < .01, ***P < .001 for comparison of means. ○ indicates FVIII only; and ♢, FVIII + TLR7 agonist imiquimod.
Cytokine release into culture supernatants. Purified memory B cells and purified CD4+ T cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. CD11c+ DCs were obtained from untreated hemophilic mice. Purified memory B cells were restimulated with FVIII (10 ng/mL) or FVIII (10 ng/mL) together with TLR7 agonist imiquimod (100 ng/mL) in the presence of either purified CD4+ T cells (A) or purified CD11c+ DCs (B). Cytokines released into culture supernatants were analyzed after 6 days of culture. Boxplots including median, minimum, maximum, 25% percentile, and 75% percentile of 4 independent experiments are presented. *P < .05. □ indicates FVIII only; and ▨, FVIII + TLR7 agonist imiquimod; (C) Purified CD11c+ DCs were incubated with FVIII (10 ng/mL) or FVIII (10 ng/mL) together with TLR7 agonist Imiquimod (100 ng/mL). INF-α released into culture supernatants was analyzed after 1, 3, and 6 days of culture. Values below detection limit (36 ng/mL) were set to zero. □ indicates FVIII only; and ■, FVIII + TLR7 agonist imiquimod.
Cytokine release into culture supernatants. Purified memory B cells and purified CD4+ T cells were obtained from hemophilic mice treated with 4 weekly doses of FVIII. CD11c+ DCs were obtained from untreated hemophilic mice. Purified memory B cells were restimulated with FVIII (10 ng/mL) or FVIII (10 ng/mL) together with TLR7 agonist imiquimod (100 ng/mL) in the presence of either purified CD4+ T cells (A) or purified CD11c+ DCs (B). Cytokines released into culture supernatants were analyzed after 6 days of culture. Boxplots including median, minimum, maximum, 25% percentile, and 75% percentile of 4 independent experiments are presented. *P < .05. □ indicates FVIII only; and ▨, FVIII + TLR7 agonist imiquimod; (C) Purified CD11c+ DCs were incubated with FVIII (10 ng/mL) or FVIII (10 ng/mL) together with TLR7 agonist Imiquimod (100 ng/mL). INF-α released into culture supernatants was analyzed after 1, 3, and 6 days of culture. Values below detection limit (36 ng/mL) were set to zero. □ indicates FVIII only; and ■, FVIII + TLR7 agonist imiquimod.
In conclusion, T cell–dependent and T cell–independent restimulation of FVIII-specific memory B cells induce a different IgG subclass distribution of newly formed FVIII-specific ASCs.
Discussion
Previously, we demonstrated that TLR agonists modulate FVIII-specific memory responses. In particular, they amplify restimulation and differentiation of memory B cells in the presence of stimulatory FVIII concentrations and counteract the inhibition of memory B-cell differentiation in the presence of inhibitory FVIII concentrations. TLR7 and TLR9 agonists were most effective.9 Based on these data, we now asked whether TLR7 and TLR9 agonists facilitate the differentiation of FVIII-specific memory B cells in the absence of T-cell help and, if so, whether alternative “helper cells” are required to replace CD4+ T cells. The answer to this question is very important because it will provide some indication as to which immune cells should be targeted in the design of future therapies for the prevention of anamnestic antibody responses. Recent studies indicated that DC activated by TLR agonists modulate B-cell function and can induce B-cell differentiation to ASCs in the absence of T cells.19-21 Therefore, DCs were a good candidate to study.
Initially, we used our previously described in vitro differentiation culture system that consisted of total spleen cells that were depleted of CD138+ ASCs and red blood cells. Differentiation of FVIII-specific memory B cells in this system was dependent on the presence of CD4+ T cells, which confirmed our previously published data.8 However, addition of TLR7 and TLR9 agonists facilitated memory B-cell differentiation even in the absence of T cells with TLR7 agonist being more effective than TLR9 agonist. TLR7 recognizes single-stranded RNA as contained in RNA viruses such as influenza, Sendai, and Coxsackie B viruses.22 Thus, our results suggest that infection with these viruses could facilitate T cell–independent restimulation of FVIII-specific memory B cells. If these findings were translatable to hemophilia patients with inhibitors, they would imply that T cell–directed therapies for the prevention of anamnestic responses in patients with established FVIII inhibitors might be of limited value in the situation of a concomitant infection with single-stranded RNA viruses. The situation would be similar for TLR9 agonists as found in CpG-containing DNA sequences expressed in DNA viruses such as herpes viruses and in many bacteria.23,24
The question arose whether TLR7 agonists in the presence of stimulatory concentrations of FVIII could directly restimulate FVIII-specific memory B cells or if additional cells that express TLR7 would be required to provide help to memory B cells in the absence of CD4+ T-cell help. Expression of TLR7 was shown for a range of different murine spleen cell populations.25 Therefore, our spleen cell culture system that contained all spleen cells except ASCs and red blood cells was not suitable to address this question. We established new technology that works with highly purified memory B cells. Because of the lack of a specific marker for murine memory B cells, we worked with switched B cells that contain all IgG and IgA memory B cells but exclude IgM memory B cells. When we cocultured highly purified memory B cells with highly purified CD4+ T cells, we obtained results that were similar to those obtained with the crude spleen cell system. We observed the same dependence on FVIII concentrations for restimulation and inhibition of FVIII-specific memory B differentiation and the same dependence on the presence of CD4+ T cells. Furthermore, when we added a TLR7 agonist, we observed an amplification of the activity of stimulatory concentrations of FVIII and a counteraction against the activity of inhibitory concentrations of FVIII. These results confirm the suitability of highly purified switched B cells as a source of FVIII-specific memory B cells. When we replaced memory B cells with naive B cells obtained from untreated hemophilic mice, we did not see any differentiation into FVIII-specific IgG-producing ASCs. However, when we cocultured naive B cells with CD4+ T cells and FVIII in the presence of TLR7 agonist, we observed some differentiation into FVIII-specific IgM-producing ASCs. This confirms previous reports demonstrating that activation of naive B cells in the presence of TLR7 or TLR9 agonists induces differentiation into ASCs.26,27 Importantly, we only observed differentiation of naive B cells into IgM-producing ASCs in the presence of both CD4+ T cells and TLR7 or a mixture of TLR7 and TLR9 agonists.
In contrast to the results that we obtained with the crude spleen cell culture system, neither the TLR7 agonist nor the mixture of TLR7 and TLR9 agonists was able to overcome the requirement for CD4+ T-cell help in the differentiation of purified memory B cells into FVIII-specific IgG ASCs. These results indicate that another cell population that is present in the crude spleen cell culture system had contributed to the restimulation of FVIII-specific memory B cells in the absence of CD4+ T-cell help. Several recent studies indicated that DCs are able to modulate B-cell function.19-21 Therefore, we asked whether DC found in the spleen could contribute to the T cell–independent restimulation of memory B cells in the presence of FVIII and the TLR7 agonist. We prepared highly purified naive CD11c+ DCs and cocultured them with memory B cells in the absence of CD4+ T cells. Our results demonstrate that CD11c+ DCs in the presence of the TLR7 agonist facilitate the restimulation and differentiation of FVIII-specific memory B cells in the presence of stimulatory concentrations of FVIII. In this way, DCs in the presence of TLR7 agonist can replace CD4+ T-cell help. Importantly, the help by DCs requires direct cell–cell interactions with memory B cells as shown in the transwell experiment. This indicates that DCs might provide costimulatory molecules to memory B cells. Moreover, the TLR7 agonist stimulates DCs to release IL-12p40, IL-6, and IFN-α. IL-12, IL-6, and IFN-α were previously shown to amplify antibody responses.28-30 Importantly, TLR7 induced large amounts of IL-12p40 but no detectable IL-12p70. This finding supports the model proposed by Abdi.31 Abdi suggested that the induction of IL-12p40 is a T cell–independent response of DCs to early host–pathogen interactions and IL-12p70 is a late product, whose induction requires interaction of DCs with T cells. Consequently, TLR7 would induce IL-12p40 but no IL-12p70 in DCs in the absence of T cells.
Based on our data, we conclude that both costimulatory molecules expressed on activated DCs as well as cytokines released by activated DCs contribute to the differentiation of FVIII-specific memory B cells in the absence of CD4+ T-cell help.
Interestingly, high concentrations of FVIII that inhibit T cell–dependent memory B-cell differentiation also inhibit differentiation of memory B cells in the presence of activated DCs. This finding confirms our previous observation that the inhibition of FVIII-specific memory B-cell differentiation induced by high concentrations of FVIII is not mediated by CD4+ T cells, but is due rather to a direct action on memory B cells.7
Previously, we demonstrated that the presence of TLR9 agonists skews the T cell–dependent differentiation of FVIII-specific memory B cells toward a Th1-type response associated with a down-regulation of IL-4 and an up-regulation of IFN-γ in cell culture supernatants.9 We observed a similar effect when we added the TLR7 agonist to the coculture of purified memory B cells and CD4+ T cells in the presence of stimulatory concentrations of FVIII. IL-4 in cell culture supernatants was down-regulated, which was associated with a preferential amplification of FVIII-specific memory B-cell differentiation into FVIII-specific ASCs of the Th1-dependent IgG subclasses IgG2a, 2b, and 2c. In contrast, the T cell–independent differentiation in the presence of DCs and TLR7 agonist was very restricted and resulted almost exclusively in the generation of FVIII-specific ASCs of the IgG2a subclass. This could indicate that the differentiation was mainly driven by IL-12, which was previously shown to skew the antibody response to IgG2a.30,32 The fact that DCs activated with TLR7 agonist can skew an already existing unrestricted memory B-cell response is surprising and might provide a mechanism for changes in the IgG subclass distribution of anti-FVIII antibodies in patients over time. If translatable to patients, such a strong skewing of FVIII-specific memory responses could also trigger unexpected adverse events after treatment with FVIII. DCs activated with TLR7 agonist seem to polarize the antibody response toward an IgG subclass that is well suited to deal with certain infections. IgG2a is efficient in activating the complement system and in inducing Fc-receptor–mediated effector functions, both are required for effective immune responses against pathogens.33,34 However, in the context of therapies with therapeutic proteins such as FVIII, IgG subclasses that efficiently activate the complement system and trigger Fc-receptor–mediated effector functions could induce undesired adverse events in patients. There are only few papers that present data on IgG subclass distribution in hemophilia A patients with FVIII inhibitors.35-38 IgG1 and IgG4 seem to be the predominant subclasses, but all IgG subclasses are found in patients. There have not been any extensive longitudinal studies published that would demonstrate the development of isotypes and IgG subclasses of antibodies against FVIII in patients over time. Reding et al37 followed 3 patients with hemophilia A and FVIII inhibitors for 4 or 6 months. However, this period is very short and does not include the early events in the course of treatment with FVIII products. Therefore, we believe that it is too early to draw final conclusions on how the data on T cell–independent restimulation of FVIII-specific memory B cells described here relate to the situation in patients with hemophilia A. There are, however, sporadic cases of anaphylactoid reactions after application of FVIII in patients. These reactions could be mediated by IgE or by complement-activating IgG subclasses.39,40 The latter would functionally correspond to the IgG2a subclass in mice that we found in T cell–independent restimulation of FVIII-specific memory B cells.
Summarizing our data, we conclude that DCs activated with TLR7 agonist facilitate T cell–independent restimulation of FVIII-specific memory responses. This activity of DCs is associated with a release of IL-12p40, IFN-α, and IL-6. Furthermore, it requires direct cell–cell interactions of DCs with memory B cells. The T cell–independent memory response facilitated by DCs and TLR7 agonists is restricted to the IgG2a subclass, which reflects a skewing of the existing unrestricted FVIII-specific memory response. Based on our data, we believe that DCs would be a good target for the design of future therapies for the prevention of anamnestic antibody responses in patients. “Silencing” of DCs would possibly prevent both T cell–dependent and T cell–independent restimulation of FVIII-specific memory antibody responses. Moreover, it might also prevent the de novo induction of antibody responses.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Elisabeth Hopfner, Josenato Ilas, Christian Lubich, Thomas Prenninger, Katharina Steinitz, Nidha Abrar, and Monika Grewal for technical assistance. They also thank Elise Langdon-Neuner for editing the manuscript.
This study was supported by Baxter BioScience.
Authorship
Contribution: A.G.P. designed research, did most in vitro analysis, analyzed and interpreted data, and wrote the paper; C.K.B. and P.A. designed research and analyzed and interpreted data; R.U.A., M.W., and A.N.S. designed, supervised, and performed animal experiments; H.P.S. interpreted data; and B.M.R. designed research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: A.G.P., P.A., R.U.A., M.W., A.N.S., H.P.S., and B.M.R. are employees of Baxter BioScience. The remaining authors declare no competing financial interests.
The current affiliation for C.K.B. is BloodCenter of Wisconsin, Blood Research Institute, Milwaukee, WI.
Correspondence: Birgit M. Reipert, Baxter BioScience, Industriestrasse 72, A-1220 Vienna, Austria; e-mail: birgit_reipert@baxter.com.