Abstract
We retrospectively analyzed the effect of HLA mismatching (HLA-A, -B, -C, -DRB1, -DQB1) with molecular typing on transplantation outcome for 301 patients with acquired severe aplastic anemia (SAA) who received an unrelated BM transplant through the Japan Marrow Donor Program. Additional effect of HLA-DPB1 mismatching was analyzed for 10 of 10 or 9 of 10 HLA allele-matched pairs (n = 169). Of the 301 recipient/donor pairs, 101 (33.6%) were completely matched at 10 of 10 alleles, 69 (23%) were mismatched at 1 allele, and 131 (43.5%) were mismatched at ≥ 2 alleles. Subjects were classified into 5 subgroups: complete match group (group I); single-allele mismatch group (groups II and III); multiple alleles restricted to HLA-C, -DRB1, and -DQB1 mismatch group (group IV); and others (group V). Multivariate analysis indicated that only HLA disparity of group V was a significant risk factor for poor survival and grade II-IV acute GVHD. HLA-DPB1 mismatching was not associated with any clinical outcome. We recommend the use of an HLA 10 of 10 allele-matched unrelated donor. However, if such a donor is not available, any single-allele or multiple-allele (HLA-C, -DRB1, -DQB1) mismatched donor is acceptable as an unrelated donor for patients with severe aplastic anemia.
Introduction
BM transplantation from an unrelated donor (UBMT) is indicated as salvage therapy for patients with severe aplastic anemia (SAA) who fail to respond to immunosuppressive therapy. Early results of UBMT have not been encouraging because of a high incidence of graft failure and GVHD.1-3 The Center for International Blood and Marrow Transplant Research (CIBMTR) reported the outcome of 232 patients with SAA who received an UBM transplant between 1988 and 1998.3 The 5-year probabilities of overall survival (OS) were 39% and 36% after matched unrelated and mismatched unrelated donor transplantations, respectively. We previously reported the outcome of 154 patients with SAA who received an UBM transplant between 1993 and 2000 through the Japan Marrow Donor Program (JMDP).4 The 5-year OS rate was 56% in that study.
In several recent studies, the effect of HLA high-resolution matching on outcome of patients who received an UBM transplant has been elucidated.5-8 However, results have been derived primarily from an analysis of patients with hematologic malignancies. Major obstacles for UBMT are different between patients with hematologic malignancies and patients with SAA. Relapse is a main cause of death for patients with hematologic malignancies, and GVL effect may result in decrease of relapse rate. In contrast, graft failure is the main problem, and GVHD is the only negative effect for patients with SAA. Therefore, optimal HLA matching may be different between these 2 populations. Algorithms for donor selection derived from an analysis of patients with hematologic malignancies might not be useful for patients with SAA. However, a few studies have focused on the clinical significance of HLA-allele compatibility in patients with SAA.2,4,9,10
In a previous study, we analyzed the clinical significance of HLA allele mismatching in 142 patients with SAA, in whom data of high-resolution typing of HLA-A, -B, and -DRB1 were available.4 Mismatching of HLA-A or -B alleles between donor and recipient was a strong risk factor for acute and chronic GVHD and OS, whereas mismatching of the HLA-DRB1 allele did not have a significant effect on patient outcomes. In the study from the National Marrow Donor Program, mismatching of HLA-DRB1 was the most crucial risk factor for OS.2 These results indicate that better donor selection through high-resolution typing might result in improved outcome in patients with SAA who receive an UBM transplant. In fact, several recent studies showed a significantly improved outcome in patients with SAA who received and UBM transplant over time.11,12 In particular, better HLA matching by high-resolution typing has been thought to contribute to these improvements.4,9-11
On the contrary, restricting BMT to donor-recipient pairs perfectly matched at high-resolution typing reduces the chance of undergoing UBMT for many patients. Therefore, strategies for selecting a partially HLA allele mismatched donor are required when a full matched donor cannot be identified. Here, we report a detailed analysis of outcome in 301 patients with SAA who were typed for HLA-A, -B, -C, -DRB1, -DQB1, and -DPB1 by a molecular technique and underwent UBMT through the JMDP.
Methods
Patients
From February 1993 to April 2005, 380 consecutive patients with acquired SAA received an UBM transplant through the JMDP. Patients with inherited AA, such as Fanconi anemia, and patients who received a BM transplant > 2 times were excluded. This study includes 301 patients in whom molecular analysis of HLA-A, -B, -C, DRB1, and -DQB1 were performed by DNA-based methods. HLA-DPB1 was analyzed in 299 of these patients. The previous study included 142 patients in whom molecular typing was performed only for HLA-A, -B, and -DRB1.
Characteristics of the 301 patients and donors are shown in Table 1. Briefly, patients (173 males and 128 females) were between birth and 64 years of age (median, 17 years of age). The median disease duration before BMT was 43 months (range, 4-436 months). All patients failed conventional immunosuppressive therapies and were considered candidates for UBMT. All patients or their guardians gave informed consent for transplantation and submission of the data to the JMDP.
Transplantation procedure
Characteristics of the transplantation procedures are also shown in Table 1. Patients underwent transplantations at individual centers following the local protocols for preconditioning regimens and GVHD prophylaxis. The various preconditioning regimens used by individual centers were classified into 5 categories: TBI or LFI + CY + ATG (n = 128), TBI or LFI + CY (n = 103), TBI or LFI + CY + Flu with or without ATG (n = 39), CY + Flu + ATG (n = 8), and others (n = 23). In 130 patients, CsA and MTX were used for prophylaxis against GVHD; 134 patients received FK instead of CsA. The remaining 35 patients received other GVHD prophylaxis. Ex vivo T-cell depletion was not used for any patient. The median number of infused nucleated marrow cells was 3.1 × 108/kg. One-half (n = 150) of the transplantations were performed before 2000, and 151 were done after 2001.
HLA typing and definition of mismatching
HLA matching between patients and donors was based on HLA serotyping according to the standard technique. Partial HLA-A and -B alleles and complete HLA-DRB1 alleles were identified as confirmatory HLA typing during the coordination process, and HLA-A, -B, -C, -DQB1, and -DPB1 alleles were retrospectively reconfirmed or identified after transplantation. Molecular typing of HLA-A, -B, -C, -DQB1, -DRB1, and -DPB1 alleles was performed by the Luminex microbead method (Luminex 100 system) adjusted for the JMDP and in part by the sequencing-based typing method. Mismatching was defined as the presence of donor antigens or alleles not shared by the recipient (rejection vector) or the presence of recipient antigens or alleles not shared by the donor (GVHD vector).
Definition of transplantation-related events
The day of engraftment was defined as the first day of 3 consecutive days on which neutrophil count exceeded 0.5 × 109/L. Patients who did not reach neutrophil counts > 0.5 × 109/L for 3 consecutive days after transplantation were considered to have primary graft failure. Patients with initial engraftment in whom absolute neutrophil counts declined to < 0.5 × 109/L subsequently were considered to have secondary graft failure. Acute GVHD was evaluated according to standard criteria in patients who achieved engraftment, and chronic GVHD was evaluated according to standard criteria in patients who achieved engraftment and survived > 100 days after transplantation.
Data collection and statistical analysis
Transplantation data were collected with the use of standardized forms provided by the JMDP. Patient baseline information and follow-up reports were submitted at 100 days and annually after transplantation. Analysis of patient outcome was performed with the date of last reported follow-up or date of death. Data were analyzed as of July 1, 2007.
Probability of OS and 95% confidence interval (95% CI) were estimated from the time of transplantation according to the Kaplan-Meier method. Cumulative incidence of neutrophil engraftment at day 42 was analyzed in the whole of patients by treating deaths until day 42 as a competing risk. Cumulative incidence of acute GVHD at day 100 was analyzed in patients who sustained engraftment by treating deaths until day 100 as a competing risk. Cumulative incidence of chronic GVHD at day 365 was analyzed in patients who sustained engraftment and survived longer than day 100 by treating deaths until day 365 as a competing risk. In univariate analysis, the log-rank test or Gray test was used to assess the significance of HLA allele mismatching on clinical outcomes. The Mann-Whitney U test was used to compare the median days of neutrophil engraftment. The chi-square test or Mann-Whitney U test was used to compare patient characteristics and transplantation procedures between the patient groups. All P values < .05 were considered statistically significant, whereas P values between .05 and .1 were considered as marginally significant.
Multivariate analyses were performed to assess the effect of HLA allele mismatching on the clinical outcome by Cox proportional hazard model (each mismatched group vs fully matched group; hazard risk = 1.0 as a reference group). Factors other than HLA mismatching included in the models were patient age, patient sex, donor age, donor sex, disease duration before BMT, infused cell dose, matching of ABO blood type, GVHD prophylaxis, and preconditioning regimens.
Results
HLA matching by DNA typing
Of the 301 recipient/donor pairs, 101 pairs (33%) were completely matched at HLA-A, -B, -C, -DRB1, and -DQB1 allele; 69 pairs (23%) were mismatched at 1 HLA allele; 59 pairs (20%) were mismatched at 2 HLA alleles; and 72 pairs (24%) were mismatched at ≥ 3 alleles (Table 2). The number and frequency of 1-allele and 2-allele mismatches in either GVHD or rejection vector or both vectors in each HLA allele were 55 (18.3%) and 7 (2.3%) in HLA-A allele, 32 (10.6%) and 2 (0.7%) in HLA-B allele, 130 (43.2%) and 10 (3.3%) in HLA-C allele, 68 (22.6%) and 5 (1.7%) in HLA-DRB1 allele, 80 (26.6%) and 13 (4.3%) in HLA-DQB1 allele, and 179 (59.5%) and 44 (14.6%) in HLA-DPB1 allele, respectively. Because the frequency of mismatching was too high at the DPB1 allele, analysis of DPB1 mismatching was separated from that of other alleles. In addition, because the number of single-allele mismatched pairs of HLA-A, -B, -C, -DRB1, and -DQB1 were too small for separate analyses, HLA-A and -B were grouped into the mismatch of the HLA-A or HLA-B allele (A/B) and HLA-DRB1 and -DQB1 into the mismatch of the HLA-DRB1 or HLA-DQB1 allele (DRB1/DQB1), respectively.
Survival
Of the 301 patients, 202 are alive at the time of analysis with an observation time from 3 to 128 months (median, 44 months) after transplantation. Five-year OS was 66.3% (95% CI, 60.7%-72.5%) in the whole population (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Subgroup analyses were performed in 8 main subgroups (> 15 recipients) as follows: (1) complete match group (n = 101), (2) single locus (A/B) mismatch group (n = 20), (3) single (C) mismatch group (n = 42), (4) 2 loci (A/B + C) mismatch group (n = 20), (5) 2 loci (DRB1/DQB1) mismatch group (n = 19), (6) 3 loci (A/B + C) mismatch group (n = 15), (7) 3 loci (C + DRB1/DQB1) mismatch group (n = 29), and (8) 3 loci (A/B + C + DRB1/DQB1) mismatch group (n = 21). OS was significantly worse in the following groups than in the complete match group (75.2%): 2 loci (A/B + C) mismatch group (49.0%; P = .022), ≥ 3 loci (A/B + C) mismatch group (40.0%; P = .002), and A/B + C + DRB1/DQB1 mismatch group (56.1%; P = .031; supplemental Table 1).
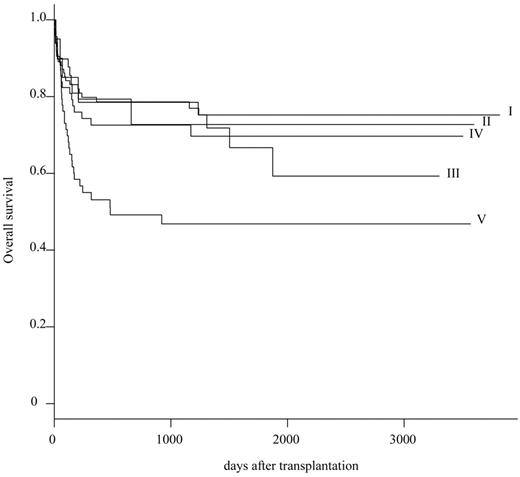
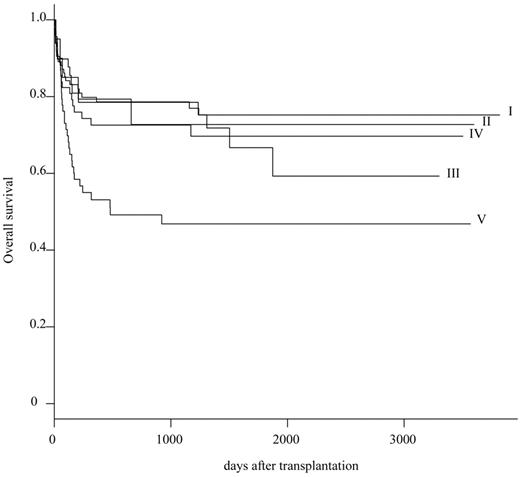
On the basis of these primary results, 301 patients were reclassified into 5 subgroups: HLA complete match group (group I; n = 101), single-allele (A/B) mismatch group (group II; n = 20), single-allele (C or DRB1/DQB1) mismatch group (group III; n = 49), multiple-allele (restricted to C or DRB1/DQB1) mismatch group (group IV; n = 68), and others (group V; n = 63). The probability of OS at 5 years was 75.2% (95% CI, 84.8%-66.7%) in group I, 72.7% (95% CI, 96.7%-54.7%) in group II, 66.7% (95% CI, 85.1%-52.3%) in group III, 69.7% (95% CI, 82.6%-58.8%) in group IV, and 46.8% (95% CI, 61.7%-35.5%) in group V, respectively (Table 3; Figure 1). Survival rate was significantly inferior in group V than in group I (P = .003).
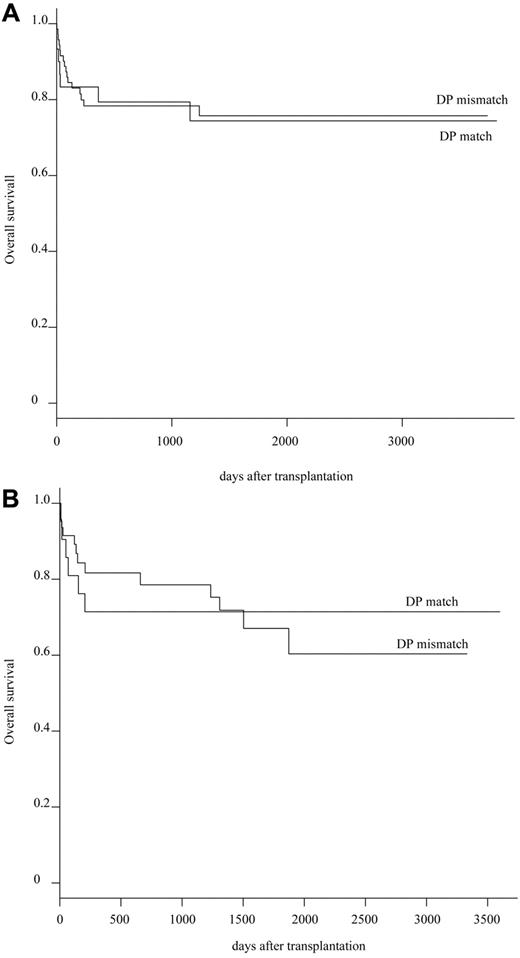
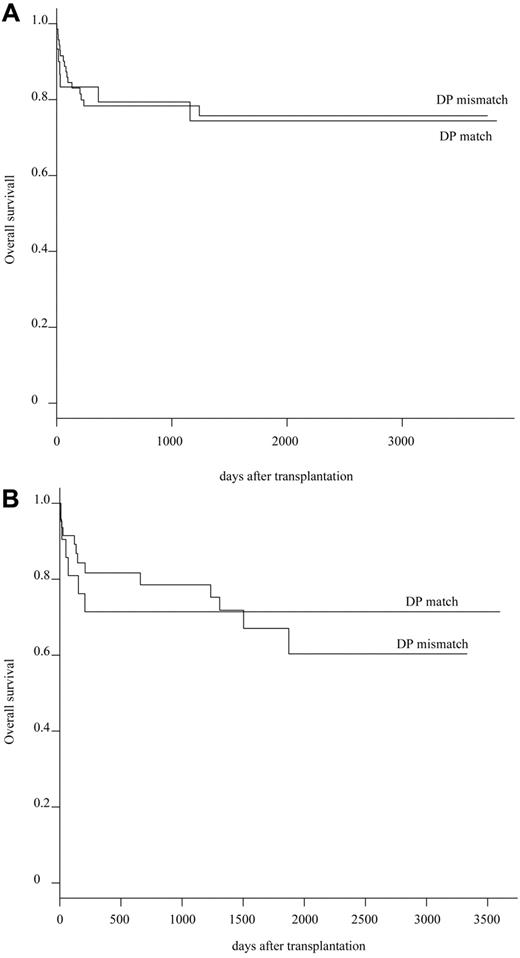
To avoid or minimize the effect of other HLA alleles mismatching, the effect of HLA-DPB1 mismatching was evaluated in group I (n = 101) and groups II + III (n = 69), independently. HLA-DPB1 was matched in 51 recipient/donor pairs (30%) and mismatched in 118 pairs (70%). Patient characteristics and transplantation procedures were not different between HLA-DPB1 matched and mismatched groups (supplemental Table 2). The probability of OS at 5 years in group I was equivalent between the HLA-DPB1 matched group (74.4%; 95% CI, 93.2%-59.4%) and the HLA-DPB1 mismatched group (75.7%; 95% CI, 87.2%-65.8%; P = .894; Table 4; Figure 2A). It was also equivalent in groups II + III (71.4%; 95% CI, 93.6%-54.5% in the HLA-DPB1 matched group and in the HLA-DPB1 mismatched group (67.1%; 95% CI, 85.6%-52.5%; P = .826; Table 4; Figure 2B). Multivariate analysis identified significant unfavorable variables as follows: recipient age (0-10 years: relative risk [RR] = 1.0; 11-20 years: RR = 4.092, P = .002; 21-40 years: RR = 3.970, P = .004; > 41 years: RR = 5.241, P = .003), conditioning regimen (Flu + CY + TBI/LFI ± ATG: RR = 1.0; CY + TBI/LFI: RR = 4.074, P = .058; others: RR = 6.895, P = .013), HLA mismatching (group I: RR = 1.0; group V: RR = 1.967, P = .023), donor sex (female: RR = 1.0; male: RR = 1.850, P = .016), and GVHD prophylaxis (FK + MTX: RR = 1.0; other: RR = 1.754, P = .024), blood type (ABO match or minor mismatch: RR = 1.0; major mismatch or bidirection: RR = 1.948, P = .005), and disease duration (< 7 years: RR = 1.0; > 7 years: RR = 1.540, P = .084; Table 5).
OS between HLA-DPB1 matched group and HLA-DPB1 mismatched group. (A) Difference of OS between HLA-DPB1 matched group and HLA-DPB1 mismatched group in 10 of 10 HLA allele matched pairs. (B) Difference of OS between HLA-DPB1 matched group and HLA-DPB1 mismatched group in 9 of 10 HLA allele matched pairs.
OS between HLA-DPB1 matched group and HLA-DPB1 mismatched group. (A) Difference of OS between HLA-DPB1 matched group and HLA-DPB1 mismatched group in 10 of 10 HLA allele matched pairs. (B) Difference of OS between HLA-DPB1 matched group and HLA-DPB1 mismatched group in 9 of 10 HLA allele matched pairs.
Engraftment
The cumulative incidence of neutrophil engraftment at day 42 was evaluated in 300 patients. It was 90.3% (95% CI, 93.7%-86.9%) in the whole population. Subgroup analyses showed that it was 93.0% (95% CI, 98.2%-87.8%) in group I, 90.0% (95% CI, 100%-74.6%) in group II, 89.8% (95% CI, 98.9%-80.7%) in group III, 92.6% (95% CI, 99.2%-86.0%) in group IV, and 84.1% (95% CI, 93.4%-74.8%) in group V (P = .185; Table 3). The median time to engraftment was 17 days in group I; 18 days in groups II, III, and IV; and 19 days in group V. Engraftment was marginally delayed in group V compared with group I (P = .053). Additional HLA-DPB1 mismatching did not affect the cumulative incidence of engraftment in the 10 of 10 and 9 of 10 matched groups, respectively (Table 4). In multivariate analysis, blood type (ABO match or minor mismatch: RR = 1.0; major mismatch or bidirection pair: RR = 5.102, P = .039) and HLA mismatching (group I: RR = 1.0; group V: RR = 4.906, P = .035) were significant risk factors for engraftment.
Acute GVHD
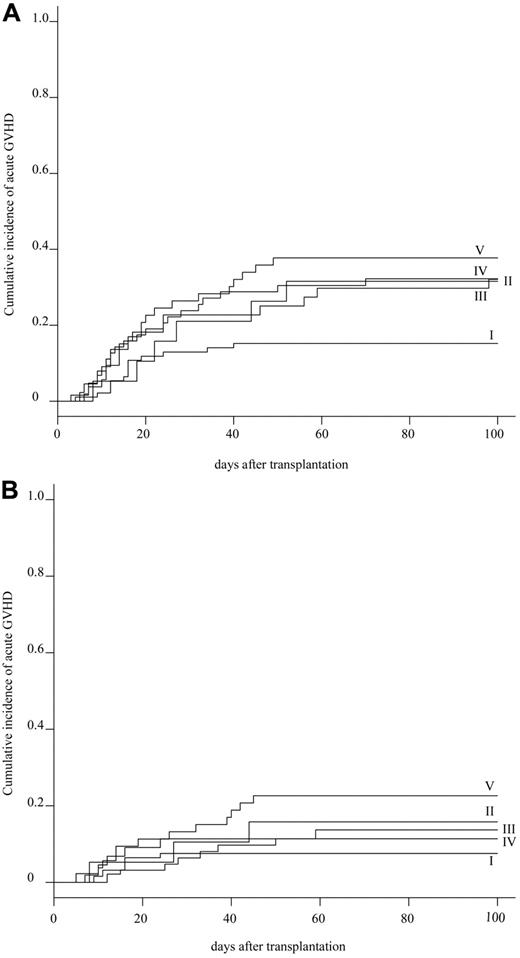
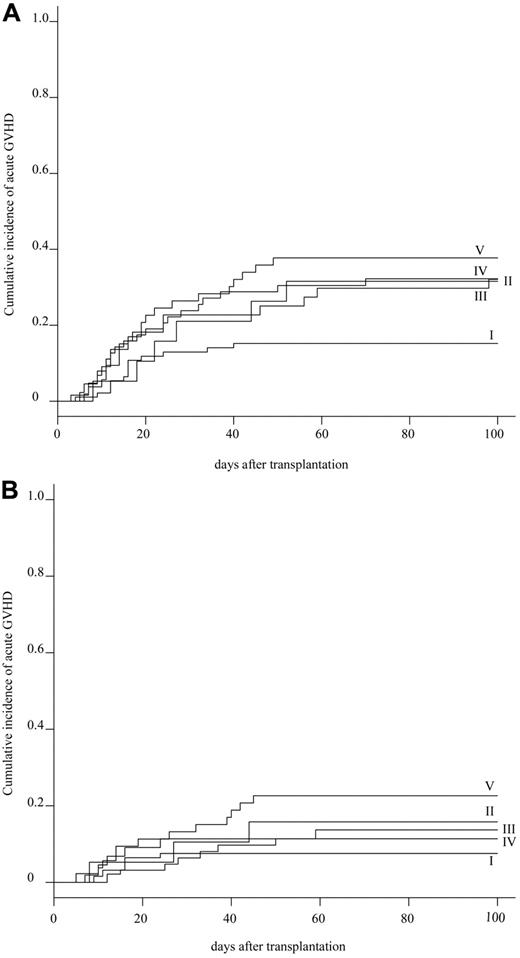
The cumulative incidence of acute GVHD at day 100 was evaluated in 272 patients. The cumulative incidence of grade II-IV and grade III-IV acute GVHD was 27.2% (95% CI, 32.5%-21.9%) and 12.9% (95% CI, 16.9%-8.9%) in the whole population, respectively (supplemental Figure 2). Subgroup analyses showed that the cumulative incidence of grades II-IV acute GVHD was statistically lower in group I (15.1%; 95% CI, 22.4%-7.8%) than in group V (37.7%; 95% CI, 50.9%-24.5%; P = .037), and marginally lower than in group III (31.8%; 95% CI, 45.8%-17.8%) and group IV (31.7%; 95% CI, 43.3%-20.1%; Table 3; Figure 3A). Whereas the cumulative incidence of grade III-IV acute GVHD was not significantly different among 5 groups: 7.5% (95% CI, 24.6%-0%) in group I, 15.8% (95% CI, 32.7%-0%) in group II, 13.6% (95% CI, 23.9%-3.3%) in group III, 11.1% (95% CI, 18.9%-3.3%) in group IV, and 22.6% (95% CI, 34.0%-11.2%) in group V (P = .139; Table 3; Figure 3B). Additional HLA-DPB1 mismatching evaluated in 155 patients did not affect the cumulative incidence of grade II-IV acute GVHD in the 10 of 10 and 9 of 10 matched groups, respectively (Table 4). Multivariate analysis showed that a significantly higher incidence of grade II-IV acute GVHD was associated with HLA mismatching (group I: RR = 1.0; group III: RR = 3.975, P = .002; group IV: RR = 3.334, P = .004; group V: RR = 3.665, P = .002). Other significant risk factors were the preconditioning regimen (Flu +CY + TBI/LFI ± ATG: RR = 1.0; TBI/LFI + CY: RR = 5.224, P = .003), and donor sex (female: RR = 1.0; male: RR = 1.844, P = .034; supplemental Table 3).
Cumulative incidence of acute GVHD. (A) Cumulative incidence of grade II-IV acute GVHD in 5 HLA groups. (B) Cumulative incidence of grade III-IV acute GVHD in 5 HLA groups.
Cumulative incidence of acute GVHD. (A) Cumulative incidence of grade II-IV acute GVHD in 5 HLA groups. (B) Cumulative incidence of grade III-IV acute GVHD in 5 HLA groups.
Chronic GVHD
The cumulative incidence of chronic GVHD at day 365 was evaluated in 232 patients. It was 24.5% (95% CI, 30.3%-18.7%) in the whole population. Subgroup analyses showed that it was comparable among the 5 HLA groups: 19.8% (95% CI, 28.8%-10.8%) in group I, 26.3% (95% CI, 49.3%-3.3%) in group II, 28.2% (95% CI, 43.3%-13.1%) in group III, 26.9% (95% CI, 39.2%-14.6%) in group IV, and 27.3% (95% CI, 42.1%-12.5%) in group V (P = .922; Table 3; supplemental Figure 3). HLA-DPB1 mismatching did not affect the cumulative incidence of chronic GVHD (Table 4).
Discussion
The survival rate in UBMT has increased substantially over the past 10 years in patients with SAA.8-15 A 5-year survival rate of 90% has been reported in a small series of children.16,17 A recent meta-analysis showed that detailed HLA-matching facilitated by DNA-based typing has contributed to the improved survival rate in patients with SAA who received an UBM transplant.18 However, many patients with SAA who need hematopoietic stem cell transplantation do not have an HLA-complete matched donor. Our multivariate analysis indicated that among 4 HLA-mismatched groups, only HLA disparity of group V was a statistically significant unfavorable variable. We conclude that any type of HLA single-allele mismatch or multiple-allele mismatch within HLA-C and HLA class II (DRB1 or DQB1) is acceptable as an unrelated donor when an HLA complete match donor is unavailable.
We previously reported that HLA class I allele mismatching (HLA-A or -B) but not class II allele (HLA-DRB1) mismatching was a significant risk factor for survival when 6 alleles were analyzed.4 HLA-A or -B mismatching pairs in the previous study were separated into 2 groups in the current study in which 10 alleles were analyzed. One group was a true single-allele mismatching pair of HLA-A or -B alleles (group II), and another was a multiple-allele mismatching pair of HLA-A or -B plus HLA-C and/or class II HLA alleles (group V). Because HLA-C and -DQB1 alleles were not typed, this type of multiple-allele mismatching might be mistaken as a single-allele mismatching pair, which was the reason for the inferior outcome of HLA-class I mismatching pairs in our previous study.
As the same in our previous study, mismatching of HLA-DRB1 did not provide a significant impact on clinical outcome. An HLA-DRB1 mismatching pair was also classified into a true single-allele mismatching of HLA-DRB1 (group III) and HLA-DRB1 plus HLA-C and/or HLA-DQB1 mismatching pairs (group IV). Interestingly, multiple mismatching of group IV was not associated with increased mortality, which may explain why mismatching of HLA-DRB1 did not have a deleterious effect in the previous study.
The effect of HLA-DPB1 mismatching was also evaluated in HLA complete matched pairs (n = 101) and single-allele mismatched pairs (n = 69). The importance of DPB1 matching in the UBMT setting has been mainly discussed in patients with hematologic malignancies. Although results were controversial in early reports, recent studies support a significant effect of DPB1 mismatching on the incidence of acute GVHD, disease relapse, and OS.19-22 In a large dataset of the International Histocompatibility Working Group, there was a statistically significant higher risk of both grade II-IV and grade III-IV acute GVHD.19 The increased risk of acute GVHD was accompanied by a statistically significant decrease in disease relapse, probably because of the GVL effect, which offset the deleterious effect of acute GVHD. Survival rate was significantly better in DPB1-matched transplantations in patients with standard-risk leukemia but not in advanced leukemia. Conversely, in the HLA-mismatched group, there was a significant survival advantage in DPB1 mismatched pairs.
We expected that DPB1 matching might be beneficial for patients with AA who do not need the GVL effect. However, clinical outcomes, including incidence of acute GVHD, were not affected by DPB1 mismatching. HLA-DPB1 typing may not be essential to the donor selection algorithm for patients with SAA. Indeed, HLA-DPB1 mismatching was observed in 74% of recipient/donor pairs, and it may be practically difficult to find HLA 12 of 12 matched donors.
In conclusion, this retrospective study confirms the importance of HLA matching between recipients and donors to improve the outcome of UBMT for patients with SAA patients. However, this study showed that only 33% of patients received transplants from an HLA 10 of 10 matched donor. The availability of unrelated hematopoietic stem cell transplants can be increased through the judicious selection of donors with HLA mismatches that do not substantially lower survival.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H. Yagasaki analyzed the data and wrote the paper; S. Kojima designed the research and analyzed the data; and H. Yabe, K.K., H.K., H.S., M.T., S. Kato, T.K., Y.M., and Y.K performed and supervised the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Seiji Kojima, Department of Pediatrics, Nagoya University Graduate, School of Medicine, Tsurumai-cho 65, Showa-ku, Nagoya 466-8550, Japan; e-mail: kojimas@med.nagoya-u.ac.jp.