Abstract
The type 1-transmembrane protein LMAN1 (ERGIC-53) forms a complex with the soluble protein MCFD2 and cycles between the endoplasmic reticulum (ER) and the ER-Golgi intermediate compartment (ERGIC). Mutations in either LMAN1 or MCFD2 cause the combined deficiency of factor V (FV) and factor VIII (FVIII; F5F8D), suggesting an ER-to-Golgi cargo receptor function for the LMAN1-MCFD2 complex. Here we report the analysis of LMAN1-deficient mice. Levels of plasma FV and FVIII, and platelet FV, are all reduced to ∼ 50% of wild-type in Lman1−/− mice, compared with the 5%-30% levels typically observed in human F5F8D patients. Despite previous reports identifying cathepsin C, cathepsin Z, and α1-antitrypsin as additional potential cargoes for LMAN1, no differences were observed between wild-type and Lman1−/− mice in the levels of cathepsin C and cathepsin Z in liver lysates or α1-antitrypsin levels in plasma. LMAN1 deficiency had no apparent effect on COPII-coated vesicle formation in an in vitro assay. However, the ER in Lman1−/− hepatocytes is slightly distended, with significant accumulation of α1-antitrypsin and GRP78. An unexpected, partially penetrant, perinatal lethality was observed for Lman1−/− mice, dependent on the specific inbred strain genetic background, suggesting a potential role for other, as yet unidentified LMAN1-dependent cargo proteins.
Introduction
Factor V (FV) and factor VIII (FVIII) are critical cofactors for the coagulation cascade proteases, factor Xa and factor IXa, respectively. Genetic deficiency of FVIII is the cause of hemophilia A, whereas inherited FV deficiency is termed parahemophilia. Combined deficiency of FV and FVIII (F5F8D) is a rare autosomal-recessive bleeding disorder characterized by coordinate reduction of both FV and FVIII to the range of 5%-30% of normal. The majority (70%) of F5F8D patients have mutations in LMAN1,1 with the remaining subset of patients resulting from mutations in MCFD2.2 LMAN1 (also known as ERGIC-53) is a type 1 transmembrane protein with homology to leguminous mannose-binding lectins.3-5 A C-terminal cytoplasmic domain, a diphenylalanine (FF) endoplasmic reticulum (ER) exit motif and a dilysine (KK) ER retrieval signal enable cycling between the ER and the ER-Golgi intermediate compartment (ERGIC).6,7 MCFD2 is a soluble protein with 2 EF-hand domains that colocalizes with LMAN1 to the ERGIC and binds to LMAN1 via a Ca2+-dependent interaction.2,8 More than 30 inactivating mutations in LMAN1 have been described,1,9-12 including 2 missense mutations that either destabilize the protein13 or abolish MCFD2 binding.11 In contrast, 7 of 17 MCFD2 mutations reported to date are missense mutations within the EF-hand domains.2,12-15 Mutagenesis studies indicate that the EF-hand domains of MCFD2 are important for the formation of the LMAN1-MCFD2 complex and interaction of this complex with both FV and FVIII.2,13,16 The carbohydrate recognition domain of LMAN1 interacts with the EF-hand domains of MCFD2 through its N-terminal β-sheet.17-19 The sugar binding activity is required to recognize FV/FVIII.19
Transport between the ER and the Golgi may represent a rate-limiting step in the maturation of many secreted proteins. F5F8D is one of several recently described congenital diseases that are caused by defects in genes that function in the early secretory pathway,1,2,20-22 although why the phenotype appears to be restricted to just FV and FVIII remains unclear. MCFD2 appears to interact with LMAN1 to form a cargo receptor complex required for efficient secretion of FV and FVIII.2,8 Cargo receptors are transmembrane proteins that facilitate the transport of secreted proteins from the ER to the Golgi.23,24 Only a few such cargo receptors have been characterized in detail, primarily in yeast. In mammals, the LMAN1-MCFD2 complex is the only known example of a specific receptor for soluble cargo proteins.23 The requirement for both a transmembrane component and a soluble cofactor is unprecedented in yeast and could represent a paradigm for the organization of other mammalian cargo receptors. In addition to FV and FVIII, cathepsin C (CatC), cathepsin Z (CatZ), and α1-antitrypsin (AAT) have also been reported as potential cargo proteins dependent on LMAN1 for efficient secretion.25-27 LMAN1 has also been suggested to play a role in the polymerization and secretion of IgM.28-30 Of note, knockdown of MCFD2 had no effect on the interaction of CatC and CatZ with LMAN1, suggesting that MCFD2 may not be required for all cargoes.31 However, no protein deficiencies other than FV and FVIII have been documented in human F5F8D patients.
Here we report the characterization of mice with targeted disruption of the Lman1 gene. Analysis of LMAN1-deficient mice reveals secretion defects for FV, FVIII, and A1AT, as well as a strain background-specific partial lethal phenotype.
Methods
Lman1 gene targeting
A search of the BayGenomics gene-trap consortium database identified 3 ES cell clones (XST008, XST010, and PST046) that target the Lman1 locus. These ES cell clones were expanded, and correct targeting was confirmed by reverse-transcribed polymerase chain reaction (RT-PCR) in 2 of the 3 clones. Targeting could not be confirmed for XST008. XST010 and PST046 contain integrations within intron 10 and intron 11 of the Lman1 gene, respectively. These latter 2 cell lines were injected into C57BL/6J blastocysts by the Transgenic Mouse Core at the University of Michigan. Chimeric mice were derived from both cell lines, although only one (XST010) achieved germline transmission. Southern blotting was performed on EcoRV-digested tail DNA using as probe an 803-bp genomic DNA fragment encompassing exons 9 and 10 upstream of the insertion site (primers probe-1 and probe-1a; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Genotyping
Two PCR reactions were used for genotyping. A 3-primer PCR reaction with a 5′ primer (I10F16) located upstream of the insertion site in intron 10 and 2 3′ primers that anneal to intron 10 sequence downstream of the insertion site (I10R11) or to the gene-trap vector sequence (V19), produces PCR products of different sizes from the wild-type (WT; 539 bp) and targeted alleles (420 bp), which are resolved by agarose gel electrophoresis (supplemental Figure 1). A microsatellite marker D18mit184 within the Lman1 gene was also used, differentiating the gene-trapped allele (147 bp) derived from the 129P2/OlaHsd strain (origin of the gene-trapped ES cells) from the WT allele (172 bp) derived from the C57BL/6J strain. Primer sequences are listed in supplemental Table 1.
RT-PCR and real-time PCR
Total RNA was purified from mouse liver using Trizol reagent (Invitrogen), followed by DNase digestion and purification using the RNeasy kit (QIAGEN). RT-PCR was performed using various primer combinations (primer sequences are listed in supplemental Table 1) on first-strand cDNA synthesized using the iScript cDNA Synthesis Kit (Bio-Rad). iQ SYBR Green Supermix (Bio-Rad) was used to perform quantitative RT-PCR in a CFX384 Real-Time Thermalcycler (Bio-Rad). For each primer pair, amplification efficiency was confirmed to be near 100%. Relative gene expression was calculated by the 2−ΔCt method using Gapdh or Atcb as reference genes. Student t test was used to assess the significance of gene expression differences (P < .05 is deemed significant).
Measurement of FV and FVIII levels
To compare FV and FVIII levels in plasma and platelets, blood was collected from WT, heterozygous, and homozygous mice via cardiac puncture using acid citrate-dextrose solution (C3821; Sigma-Aldrich) as an anticoagulant. Plasma and platelets were isolated from platelet-rich plasma as described.32 The platelet fraction was washed twice in CGS buffer (129mM sodium citrate, 33.3mM, glucose, 127mM NaCl, pH 7.0) supplemented with 1μM prostaglandin E1 and resuspended in Tyrode buffer (125mM NaCl, 4.7mM KCl, 1.4mM CaCl2, 20mM NaHCO3, 0.4mM NaH2PO4, 1.0mM MgCl2, 10mM D-glucose, pH 7.4) with ethylenediaminetetraacetic acid-free protease inhibitor cocktail (Roche Diagnostics). Platelet concentrations were determined in an Advia120 whole blood analyzer (Bayer). Platelets were lysed on ice by adding Triton X-100 to a final concentration of 0.2% and used directly or stored at −80°C. Plasma FV activity was measured in 4 repeated blood draws collected at 2-week intervals via retro-orbital bleeding using 4% sodium citrate (9:1) as anticoagulant. Plasma was snap frozen and stored until the time of assay. Prothrombin time-based activity assays were performed on a KC4 δ Coagulation Analyzer (Sigma-Aldrich). FV antigen levels in plasma and platelets were determined by Western blot analysis using an antibody against the heavy chain of murine FV33 and an IRDye-conjugated secondary antibody (LI-COR Biosciences; Figure 2B). The band intensities were quantified using the Odyssey Infrared Imaging System (LI-COR Biosciences). FVIII activity was measured using a chromogenic assay kit (Chromagenix) that detects both human and murine FVIII.34
X-gal staining
To obtain the detailed Lman1 expression pattern, tissues were dissected from 1-month-old mice and fixed for 2 hours on ice in 4% paraformaldehyde before embedding in freezing media. Cryosections (6 μm) were postfixed for 10 minutes and subject to X-gal staining using a commercial kit (Cell & Molecular Technologies). Sections were counterstained with nuclear fast red.
Antibodies
Antibodies used in Western blot and immunofluorescence experiments were: monoclonal antibodies against human β-actin (Santa Cruz Biotechnology) and KDEL (MBL), and KDEL receptor (Enzo); rabbit anti–rat LMAN1 (anti-P58, a gift from R. Petterssen, Stockholm), rabbit anti–human ribophorin I (a gift from T. Rapappot, Harvard University), rabbit anti–human translocon-associated protein-α (TRAPα, a gift from R. Hedge, National Institutes of Health), rabbit anti–human antiamyloid precursor protein (Sigma-Aldrich), rabbit antibodies against human Sec23a (Thermo Scientific), and Sec22b (a gift from J. Hay, University of Montana), goat antibodies against murine CatC and CatZ (R&D Systems), and chicken anti–human AAT. An antibody against MCFD2 was raised in rabbit against a synthetic peptide (ADVHHGSVGLDKSTVHDQEH) from murine MCFD2 and affinity purified using peptide-conjugated Affi-Gel 10/15 (Bio-Rad).
Timed mating
Timed mating was performed by intercrossing Lman1+/− mice that had been backcrossed into the C57BL/6J background for 6 or more generations (> N6). Embryos from each cross were harvested at 13.5 or 18.5 days postcoitus (dpc). DNA was prepared from liver of 13.5-dpc embryos or tail biopsies of 18.5-dpc embryos for genotyping. Mouse embryonic fibroblasts (MEFs) were derived from 13.5-dpc embryos using a standard protocol as previously described.26 Three independent MEF lines were generated from Lman1−/− embryos and WT littermate controls.
In vitro budding assay
Immunofluorescence and electron microscopic imaging
WT and Lman1−/− MEFs grown on cover slips were fixed in 4% paraformaldehyde and incubated with relevant first antibodies, followed by AlexaFluor-488– and AlexaFluor-596–conjugated secondary antibodies. Images were viewed on a Leica DMRXE confocal microscope using a 63× oil immersion objective with a 1.40 numeric aperture. Confocal images were aquired using Leica Confocal Software Version 2.61. Images were further processed using Photoshop CS2. Liver samples from Lman1−/− and WT littermate control mice were immediately fixed in 2.5% glutaraldehyde and 4% formaldehyde, pH 7.3, for 24 hours at 4°C. Samples were submitted to the Imaging Core of the Lerner Research Institute for further processing. Images were captured using a Philips CM-12 transmission electron microscope and processed using Photoshop CS2.
Human plasma samples
Peripheral blood samples were obtained in acid citrate-dextrose venous blood vacuum collection tubes from probands and family members after diagnosis of F5F8D, as well as from healthy controls. This family was previously reported as family B21,12 with both probands carrying the homozygous c.1356delC mutation in LMAN1. Both parents are heterozygous for this mutation. Plasma was isolated, snap frozen, and stored at −80°C for later use. The study protocol was approved by the Cleveland Clinic Institutional Review Board on Human Subject Research. Informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Results
Generation of Lman1-deficient mice
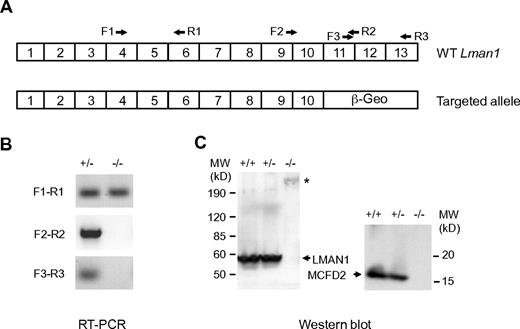
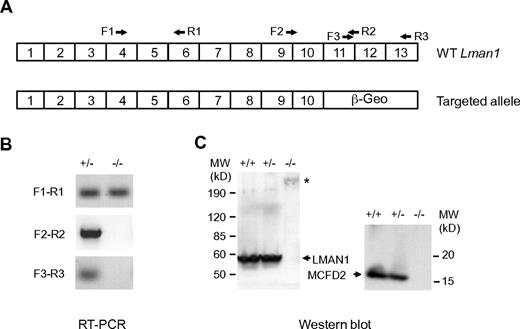
The ES cell clone XST010 contains a gene-trapped vector integration within intron 10 of the Lman1 gene (Figure 1A; supplemental Figure 1), which is predicted to result in splicing of exon 10 into the vector sequence, bypassing the last 3 exons of Lman1. Removal of this C-terminal LMAN1 sequence is expected to generate a loss of function because of the absence of both the transmembrane domain and the ER exit and retention motifs (KKFF), although we cannot rule out the possibility of a weak dominant negative effect of the resulting fusion protein. Consistent with this prediction, a mutation that deletes the C-terminal 4 amino acid residues of LMAN1 results in F5F8D in humans.10 Southern blot analysis confirmed the expected insertion in LMAN1 intron 10 in heterozygous and homozygous mice (supplemental Figure 2). RT-PCR using primers upstream of exon 10 (F1-R1) readily detected Lman1 mRNA in homozygous Lman1−/− mice, whereas no RT-PCR products were detected using primers flanking the exon 10 and exon 11 junction (F2-R2) or primers downstream of exon 10 (F3-R3; Figure 1B). These data indicate a > 1000-fold reduction in full-length Lman1 mRNA in Lman1−/− liver because F2 to R2 detected Lman1 mRNA in control WT liver cDNA diluted up to 1000-fold (supplemental Figure 2). Consistent with this observation, Western blot analysis detected no LMAN1 protein expression in the liver of Lman1−/− mice (Figure 1C). Similar to human LMAN1-deficient cells,2 MCFD2 is also undetectable in liver lysates of Lman1−/− mice by Western blot analysis (Figure 1C).
Characterization of mice carrying the Lman1 targeted allele. (A) Schematics of the cDNA structures of the WT murine Lman1 allele and the targeted allele with the gene-trap vector insertion. Locations of RT-PCR primers are indicated. (B) RT-PCR of total liver RNA isolated from Lman1+/− and Lman1−/− mice using 3 pairs of primers: F1-R1 indicates upstream of exons 10 and 11 junction; F2-R2, cross the exons 10 and 11 junction; and F3-R3, downstream of exons 10 and 11 junction. (C) Western blot analysis of liver lysates from WT (+/+), heterozygous (+/−), and LMAN1-deficient (−/−) mice using antibodies against LMAN1 and MCFD2. *Fusion protein of LMAN1 and β-GEO. MW indicates molecular weight.
Characterization of mice carrying the Lman1 targeted allele. (A) Schematics of the cDNA structures of the WT murine Lman1 allele and the targeted allele with the gene-trap vector insertion. Locations of RT-PCR primers are indicated. (B) RT-PCR of total liver RNA isolated from Lman1+/− and Lman1−/− mice using 3 pairs of primers: F1-R1 indicates upstream of exons 10 and 11 junction; F2-R2, cross the exons 10 and 11 junction; and F3-R3, downstream of exons 10 and 11 junction. (C) Western blot analysis of liver lysates from WT (+/+), heterozygous (+/−), and LMAN1-deficient (−/−) mice using antibodies against LMAN1 and MCFD2. *Fusion protein of LMAN1 and β-GEO. MW indicates molecular weight.
LMAN1 and MCFD2 expression profiles
The relative expression levels of Lman1 and Mcfd2 were compared by quantitative RT-PCR using total RNA isolated from various organs of newborn mice. Lman1 and Mcfd2 are both widely expressed, although at variable levels, with the highest expression observed in professional secretory organs, such as pancreas and salivary gland, with lower levels in tissues with low secretion activities, such as heart, muscle, and brain (supplemental Figure 3). Overall, the levels of Lman1 and Mcfd2 expression appear highly correlated, consistent with the known complex formation and 1:1 stoichiometry. The Lman1 mutant allele is expected to direct expression of a fusion protein of an LMAN1 fragment encoded by the first 10 exons followed by the β-Geo (β-galactosidase and neomycin phosphotransferase fusion gene) coding sequence. Indeed, a protein corresponding to the expected size of such a fusion protein is detected in Lman1−/− liver by an anti-LMAN1 antibody, although at significantly reduced amounts (Figure 1C). Expression of the LMAN1/β-GEO fusion protein should mirror the transcription program of the endogenous Lman1 gene, providing a convenient marker for Lman1 expression by staining for β-galactosidase activity. Staining in tissues of 1-month-old Lman1+/− mice reveals striking variations in expression levels among different cells within the same organ, although generally with the highest levels localized to secretory cell types (supplemental Figure 4).
FV and FVIII levels in LMAN1-deficient mice
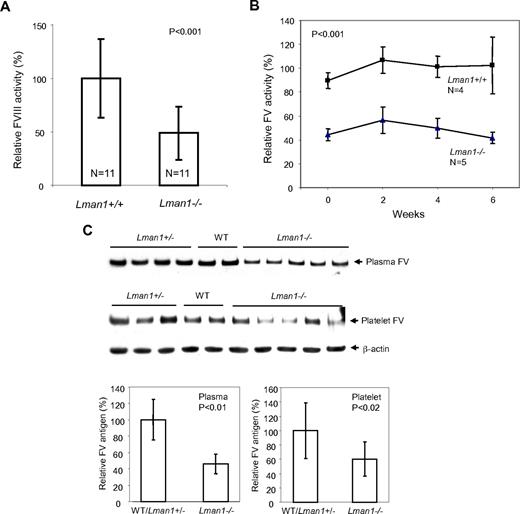
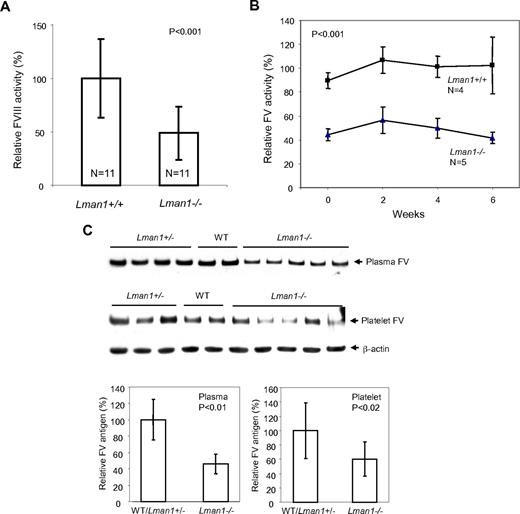
FVIII activity, as measured by a 2-stage chromogenic assay, showed considerable variation among individual mice of both WT and Lman1−/− genotypes. However, the mean FVIII activity in Lman1−/− mice was ∼ 50% of the WT level (P < .001, Figure 2A). To monitor fluctuations of FV activities in individual mice over time, blood was collected via a retro-orbital bleed from the same cohort of 6-month-old mice every 2 weeks over a 6-week period and FV activity measured by a prothrombin time-based assay. FV activity was ∼ 50% lower in Lman1−/− mice than in WT controls, with no consistent change over the 6-week period (P < .001; Figure 2B). FV antigen levels were measured by Western blot analysis. Plasma FV antigen in Lman1−/− mice (measured by Western blot analysis) is also decreased to an average of 50% of the WT level (P < .01), with no significant difference observed between WT and Lman1+/− mice (Figure 2C). FV antigen is also significantly reduced in platelets of Lman1−/− mice compared with WT and Lman1+/− mice (P < .02; Figure 2C), although considerable variation was observed among individual mice. Thus, LMAN1-deficient mice exhibit reductions of both FV and FVIII levels, albeit to a lesser extent than those observed in human F5F8D patients.12
FV and FVIII levels in Lman1 WT (+/+), heterozygote (+/−), and homozygous (−/−) mice. (A) Comparison of FVIII activities between WT and Lman1−/− mice. FVIII activity was determined at ∼ 6 months of age by a 2-stage chromogenic assay. (B) Variation in FV activity for individual mice over a 6-week period. Blood was collected from each individual mouse at 2-week intervals, beginning at ∼ 6 months of age. FV activity was determined by a one-stage clotting assay. The group averages of each time points were plotted, with the 6-week average of FV levels in WT mice designated as 100%. (C) Relative FV antigen (%) in plasma (equal volume) and in platelets (equal number) as determined by Western blot analysis. The β-actin level in platelets serves as a loading control. The relative levels in different individual mice at ∼ 6 months of age were quantified and normalized against β-actin. Vertical bars in all panels represent SD.
FV and FVIII levels in Lman1 WT (+/+), heterozygote (+/−), and homozygous (−/−) mice. (A) Comparison of FVIII activities between WT and Lman1−/− mice. FVIII activity was determined at ∼ 6 months of age by a 2-stage chromogenic assay. (B) Variation in FV activity for individual mice over a 6-week period. Blood was collected from each individual mouse at 2-week intervals, beginning at ∼ 6 months of age. FV activity was determined by a one-stage clotting assay. The group averages of each time points were plotted, with the 6-week average of FV levels in WT mice designated as 100%. (C) Relative FV antigen (%) in plasma (equal volume) and in platelets (equal number) as determined by Western blot analysis. The β-actin level in platelets serves as a loading control. The relative levels in different individual mice at ∼ 6 months of age were quantified and normalized against β-actin. Vertical bars in all panels represent SD.
Strain-specific lethality in Lman1−/− mice
Crosses between Lman1+/− mice in a mixed C57BL6/J and 129p2 genetic background produced offspring of the expected genotype distribution, indicating that LMAN1 is not required for murine development or survival (Table 1). No spontaneous bleeding was observed in Lman1−/− mice, and bleeding time from tail-vein transection37,38 was indistinguishable between Lman1−/− mice and WT controls (data not shown). No other obvious abnormalities were observed on routine autopsy and histologic examination. However, an intercross of Lman1+/− mice on a C57BL6/J genetic background (N6-N8) revealed an unexpected loss of Lman1−/− mice, with only approximately one-third of the expected homozygous mice present at the time of weaning (Table 1). Analysis at embryonic day 18.5 demonstrated the expected number of Lman1−/− embryos (Table 1), indicating that the Lman1−/− lethality is occurring perinatally. Surviving null animals appear grossly normal and exhibit normal fertility and survival. To investigate whether the partial lethal phenotype is specific to the C57BL6/J background, the Lman1 knockout allele was also backcrossed into 129/SvImJ (N8-N11), DBA/2J (N8-N10), and A/J (N10) mice. Similar loss of Lman1−/− mice was observed in the 129/SvImJ and DBA/2J backgrounds. In contrast, normal survival for the expected number of Lman1−/− offspring was observed in crosses between Lman1+/− mice N6 in the A/J background (Table 1).
Budding of COPII-coated vesicles is not affected by Lman1 deletion
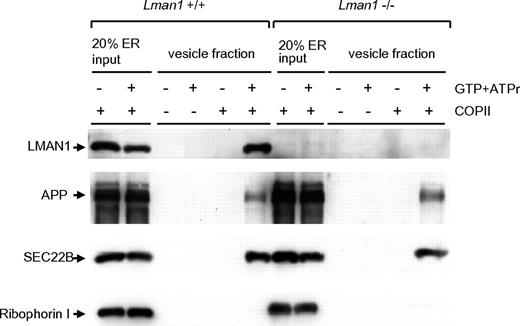
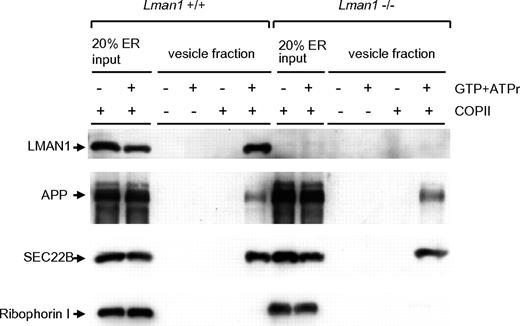
An in vitro assay was used to analyze the content of cargo-containing vesicles budded from the ER.35 In this assay, donor ER membranes were prepared from WT and Lman1−/− MEFs. ER membranes were incubated with COPII coat proteins supplied in rat liver cytosol. Budding of COPII-coated vesicles was initiated by the addition of adenosine triphosphate regeneration mix as an energy source and guanosine triphosphate as a source for nucleotide exchange. The vesicles were purified by differential centrifugation and analyzed by immunoblotting. As shown in Figure 3, LMAN1 is detected in the high-speed fraction from WT ER membranes (lane 6), indicating that it is packaged into vesicles. In contrast, ribophorin I, an ER resident protein, remains in the ER and was not detected in the vesicle fractions (lanes 6 and 12). As expected, no LMAN1 was detected in vesicles isolated from Lman1−/− ER membranes. SEC22B and APP (amyloid precursor protein), 2 transmembrane cargo proteins expected to be independent of LMAN1 for ER exit, were packaged into vesicles with a similar efficiency from either WT or Lman1-deficient ER membranes (lanes 6 and 12). These results suggest that LMAN1 deficiency does not cause a general defect in COPII-dependent ER exit.
Budding of COPII-coated vesicles is not affected by LMAN1 deficiency. Semi-intact WT and Lman1−/− MEFs were permeabilized with digitonin and served as sources of the ER membranes. COPII proteins were supplied in rat liver cytosol. COPII-coated vesicles bud from the ER membrane with the addition of guanosine triphosphate and the adenosine triphosphate regeneration mix. The vesicles were purified by differential centrifugation and analyzed by Western blotting using the indicated antibodies.
Budding of COPII-coated vesicles is not affected by LMAN1 deficiency. Semi-intact WT and Lman1−/− MEFs were permeabilized with digitonin and served as sources of the ER membranes. COPII proteins were supplied in rat liver cytosol. COPII-coated vesicles bud from the ER membrane with the addition of guanosine triphosphate and the adenosine triphosphate regeneration mix. The vesicles were purified by differential centrifugation and analyzed by Western blotting using the indicated antibodies.
Limited cellular phenotype associated with LMAN1 deficiency
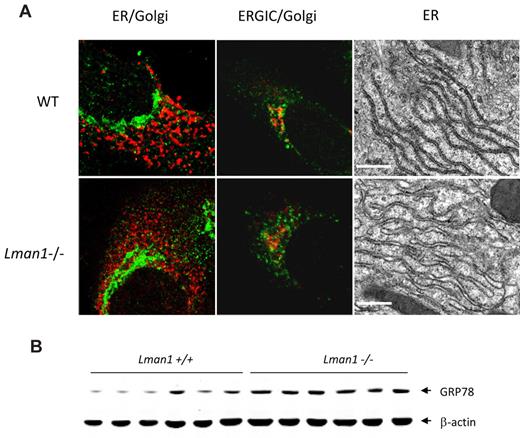
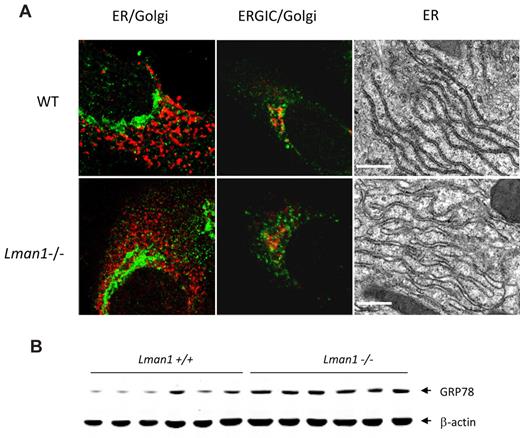
The general cell morphology of MEFs appeared indistinguishable in the presence or absence of LMAN1. Morphology of the early secretory organelles was first examined by immunofluorescence staining for the ER marker TRAPα, the ERGIC marker KDEL receptor, and the cis-Golgi marker giantin in WT and Lman1−/− MEFs. No obvious abnormalities were observed in Lman1−/− cells with any of these markers (Figure 4A). The ultrastructure of the ER was further examined by electron microscopy in sections prepared from WT and Lman1−/− mouse liver. A subtle but consistent difference in ER morphology was observed between WT and Lman1−/− hepatocytes (Figure 4A). The Lman1−/− ER is slightly dilated compared with the ER of WT cells, particularly at the ends of the ER tubules. Although no significant induction of ER stress and unfolded protein response genes (Grp78, Grp94, Xbp1, Chop, Atf4, Gadd34, and Trb3) were detected in Lman1−/− liver (supplemental Figure 5A), the protein level of the ER chaperone GRP78 is increased by ∼ 2-fold in Lman1−/− liver (Figure 4B; supplemental Figure 5B).
ER morphology and expression of UPR markers in WT (+/+) and Lman1−/− cells. (A) Color panels: Immunofluorescence staining of MEFs derived from WT and Lman1−/− embryos. MEFs were fixed in 4% paraformaldehyde, incubated with polyclonal antibodies against ER TRAPα for the ER (red), KDEL receptor for the ERGIC (green), and giantin for the cis-Golgi (green in the first panel, red in the second panel). Black-and-white panels: Morphology of rough ER was visualized by electron microscopy in WT and Lman1−/− hepatocytes. Scale bars represent 0.5μM. (B) Western blot analysis of GRP78 in liver lysates from WT and Lman1−/− mice using anti-KDEL antibody. Each lane contains a sample from a different individual mouse.
ER morphology and expression of UPR markers in WT (+/+) and Lman1−/− cells. (A) Color panels: Immunofluorescence staining of MEFs derived from WT and Lman1−/− embryos. MEFs were fixed in 4% paraformaldehyde, incubated with polyclonal antibodies against ER TRAPα for the ER (red), KDEL receptor for the ERGIC (green), and giantin for the cis-Golgi (green in the first panel, red in the second panel). Black-and-white panels: Morphology of rough ER was visualized by electron microscopy in WT and Lman1−/− hepatocytes. Scale bars represent 0.5μM. (B) Western blot analysis of GRP78 in liver lysates from WT and Lman1−/− mice using anti-KDEL antibody. Each lane contains a sample from a different individual mouse.
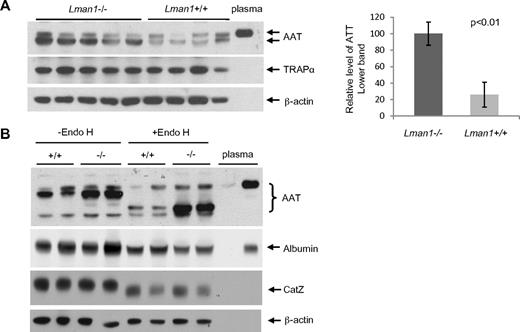
Levels of CatC, CatZ, and AAT in WT and Lman1−/−mice
Previous cell biologic studies identified 2 lysosomal glycoproteins, CatC and CatZ, as potential cargo proteins for LMAN1.25,27 Recently, a protein fragment complementation assay screen also identified AAT as another potential cargo protein for LMAN1.26 To test the significance of these findings in vivo, the levels of CatC, CatZ, and AAT were measured in LMAN1-deficient mice by Western blot analysis. No significant differences were observed in the levels of CatC or CatZ in liver extracts prepared from Lman1−/− and WT controls (supplemental Figure 6A). The plasma levels of AAT, one of the most abundant proteins in plasma, were also indistinguishable between Lman1−/− and WT mice, or between 2 human F5F8D patient siblings, their obligate carrier parents, and 2 normal controls (supplemental Figure 6B).
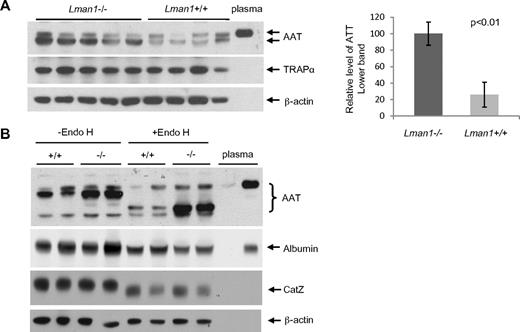
Despite the plasma AAT level findings, higher levels of intracellular AAT were observed in Lman1−/− liver extracts compared with Lman1+/+ controls (Figure 5A). No increase was observed in Lman1−/− liver for the control ER marker protein, TRAPα. To test whether more AAT accumulates in the ER of Lman1−/− hepatocytes, we treated the liver lysates with endoglycosidase H (endo H), which specifically cleaves the high mannose oligosaccharide chain of glycoproteins in the ER.6,7 Glycoproteins in the Golgi are resistant to endo H digestion. A doublet band was detected by the anti-AAT antibody in liver extracts, with the lower, more intense band sensitive to endo H digestion, consistent with retention in the ER (Figure 5B). The upper band, similar in size to plasma AAT, is resistant to endo H digestion and probably represents the postER fraction of intracellular AAT. The unglycosylated albumin is not affected by endo H digestion (Figure 5B). Although the lysosome-localized CatZ remains sensitive to endo H digestion as previously reported,39 its level is not altered in Lman1−/− cells (Figure 5B). We conclude that more AAT accumulates in the ER of hepatocytes in Lman1−/− mice than in WT mice.
Plasma and liver AAT levels. (A) AAT antigen is increased in Lman1−/− mouse liver lysates. Liver lysates were prepared from WT and Lman1−/− mice and analyzed for AAT, TRAPα, and β-actin by immunoblotting. Relative levels of the lower band of AAT were quantified and normalized to β-actin. (B) AAT accumulates in the ER of Lman1−/− mouse liver. Liver lysates from WT and Lman1−/− mice were digested with endo H and analyzed for AAT, albumin, CatZ, and β-actin by immunoblotting. Each lane contains a sample from a different individual mouse.
Plasma and liver AAT levels. (A) AAT antigen is increased in Lman1−/− mouse liver lysates. Liver lysates were prepared from WT and Lman1−/− mice and analyzed for AAT, TRAPα, and β-actin by immunoblotting. Relative levels of the lower band of AAT were quantified and normalized to β-actin. (B) AAT accumulates in the ER of Lman1−/− mouse liver. Liver lysates from WT and Lman1−/− mice were digested with endo H and analyzed for AAT, albumin, CatZ, and β-actin by immunoblotting. Each lane contains a sample from a different individual mouse.
Discussion
Human F5F8D patients and Lman1−/− mice both exhibit parallel reductions in plasma and platelet FV12 (Figure 2B), despite the distinct origins of the platelet FV pool in humans and mice.38,40-42 Our results suggest that ER-to-Golgi transport is a rate-limiting step in the biosynthesis of FV in both megakaryocytes and hepatocytes, as well as for plasma FVIII biosynthesis, presumably in hepatic endothelial cells.43 The incomplete block to FV/FVIII secretion could be the result of functional overlap of cargo receptor-mediated transport or a significant contribution from bulk-flow mechanisms. The less marked reduction in plasma FV and FVIII in Lman1-deficient mice (30%-50% of the WT levels) compared with human F5F8D patients (10%-30% of normal) could account for the lack of a significant bleeding phenotype in Lman1−/− mice. Of note, human heterozygous carriers of FVIII deficiency (hemophilia A) or FV deficiency (with ∼ 50% levels of FVIII and FV, respectively) are generally asymptomatic. Mice with plasma FV levels ranging from 6%-46% exhibit near-normal bleeding times and no spontaneous hemorrhage.38 The lower FV and FVIII levels in LMAN1-deficient humans compared with mice could be the result of differences in the synthetic rates or stabilities of FV/FVIII, their interactions with LMAN1 and MCFD2, or functional overlap with other cargo receptors. Although the normally spliced Lman1 mRNA level is undetectable (< 0.1% of the WT level; Figure 1B; supplemental Figure 2B), we cannot exclude the existence of a trace amount of functional LMAN1 in Lman1−/− mice.
The partial perinatal lethality observed in Lman1−/− mice on the C57BL/6J, 129sv, and DBA backgrounds was unexpected and is unlikely to be the result of bleeding, given the normal perinatal survival of FVIII null mice44,45 and F5−/− mice carrying an FV transgene expressing < 0.1% of the normal level of FV.38 The lethal phenotype could be the result of a further drop in the level of one or more LMAN1-dependent protein(s) below a critical threshold or a strain-specific difference in another cargo receptor whose function overlaps with LMAN1. However, we cannot rule out the possibility that the partial lethality is the result of another effect of the gene trap insertion, or a “passenger mutation” at another gene closely linked to the Lman1 locus.46 The cause of death in null pups on the C57BL6/J background is not evident from gross and histologic examination of E18.5 null embryos.
The availability of Lman1−/− mice makes it possible to study the effect of LMAN1 deficiency on other potential LMAN1 cargo proteins, including CatC, CatZ, and AAT.25-27 CatC and CatZ belong to the family of lysosomal cysteine cathepsins. They are synthesized in the ER as pro-cathepsins, and the processed proteins are stored in lysosomes.39,47 The observation of normal CatC and CatZ levels in cell lysates from Lman1−/− mice should mainly reflect the amount of these proteins stored in lysosomes. These results suggest that ER-to-Golgi transport is not rate-limiting for the lysosomal accumulation of CatC and CatZ, consistent with a previous report that overexpression of a dominant negative form of LMAN1 causes delayed CatC secretion but does not affect the steady-state level in conditioned media.27 In contrast to CatC and CatZ, the cellular content of AAT should primarily represent AAT in transit in the ER. The observation that AAT accumulates in the ER of Lman1−/− cells (Figure 5) is consistent with the previous report of a kinetic delay in ER exit.26 However, the steady-state plasma level of AAT is not affected in either human F5F8D patients or Lman1−/− mice. Of note, FV and FVIII are much larger proteins and are more heavily glycosylated than CatC, CatZ, and AAT, potentially making them more dependent on a cargo receptor to exit the ER. Although FV/FVIII exhibit significant sequence similarity to each other, no homology to CatC, CatZ, or AAT is evident, suggesting a unique cargo dependence for FV and FVIII. Consistent with this notion, MCFD2 is required for FV and FVIII transport but appears dispensable for other putative cargo proteins.26,31
LMAN1 is not required for the general budding of COPII vesicles (Figure 3), consistent with the limited set of proteins that are transported by LMAN1. The apparent accumulation of AAT in the hepatic ER of Lman1−/− mice (Figure 5) is associated with mild dilation of the ER lumen and the accumulation of the major ER chaperone GRP78 (Figure 4). AAT-Z, the variant protein responsible for the most common form of human AAT deficiency, is known to accumulate in the ER and is associated with significant liver disease.48 Our results suggest that variations in LMAN1 function could potentially be a significant modifier of this latter complication or of overall plasma AAT levels in these patients. The appearance of dilated ER and accumulation of GRP78 suggest ER stress in Lman1−/− hepatocytes. However, most ER stress and unfolded protein response genes are not significantly induced (supplemental Figure 5), suggesting that these cells may have adapted to the low level of chronic ER stress.49
In conclusion, we have shown that Lman1 knockout mice duplicate the F5F8D phenotype in humans, albeit with a milder presentation. Our results suggest that the in vitro effect of LMAN1 on the previously reported putative cargo proteins CatC, CatZ, and AAT may not be physiologically relevant in vivo. The partial lethal phenotype of Lman1 knockout mice in certain genetic backgrounds and the existence of LMAN1 orthologs in lower eukaryotes suggest functions of LMAN1 beyond the blood coagulation system. However, the identity of specific protein cargos other than FV and FVIII is not readily evident from the analysis of LMAN1-deficient mice or humans, under physiologic conditions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the March of Dimes Foundation (Basil O'Connor Starter Scholar Research Award grant 5-FY08-06) and the National Institutes of Health (grant RO1 HL094505, B.Z.; grant PO1 HL057346, D.G. and R.J.K.; grant R37 HL039693, D.G.; and grant HL052173, R.J.K.). D.G., R.J.K., and R.S. are investigators of the Howard Hughes Medical Institute. C.Z. is the recipient of a postdoctoral fellowship from the American Heart Association. Support for the Transgenic Animal Model Core of the University of Michigan was provided by the National Institutes of Health (grants AR20557 and P30AG013283) and the University of Michigan Center for Organogenesis.
National Institutes of Health
Authorship
Contribution: B.Z. and D.G. designed the research; B.Z., C.Z., M.Z., J.T., M.P.V., and A.B. performed the experiments; J.K., R.J.K., and R.S. reviewed the data and provided critical conceptual insight; B.Z., C.Z., and D.G. wrote the manuscript; and all authors commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bin Zhang, Genomic Medicine Institute, Lerner Research Institute of Cleveland Clinic, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: zhangb@ccf.org.
References
Author notes
B.Z. and C.Z. contributed equally to this study.