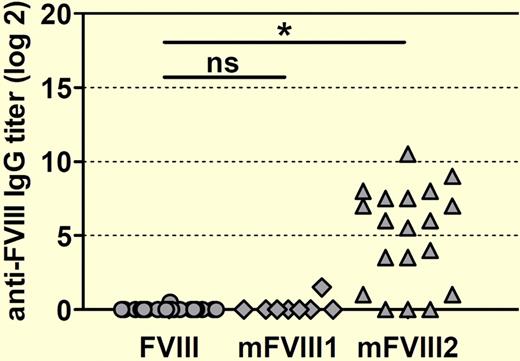
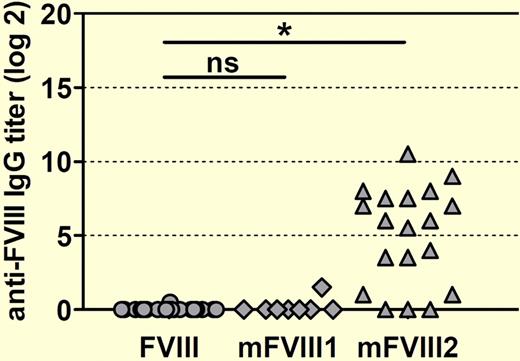
The short circulatory lifetime of factor VIII (fVIII) is a major obstacle to optimal management of patients with hemophilia A. However, any attempt to modify fVIII to increase its circulatory lifetime is attended by the risk of increasing its immunogenicity. In this issue of Blood, van Helden et al describe a transgenic mouse model that may be useful in predicting the immunogenicity of novel factor VIII molecules.1
In the management of hemophilia A, fVIII levels ideally would be therapeutic around the clock. Treatment with fVIII to prevent bleeding (termed prophylaxis) has been shown to decrease the frequency of joint bleeding and other hemorrhages and to prevent chronic joint damage.2 However, the short half-life of fVIII typically requires 3 intravenous infusions of fVIII per week to prevent bleeding. Thus, a major goal in hemophilia research is to develop longer-acting fVIII products. A variety of approaches have been investigated, including modifying fVIII with polyethylene glycol (PEG) and mutation of fVIII to reduce its clearance.
The most significant complication in the management of hemophilia A is the development of inhibitory antibodies (inhibitors) to fVIII. Approximately 25% of previously untreated patients with hemophilia A will develop inhibitors after the first few exposures to fVIII,3 but there are no strong predictors of which patients will do so. Patients who remain inhibitor-free after the first few exposures usually will remain tolerant to fVIII for the rest of their lives. Modified forms of fVIII potentially could result in increased development of inhibitors in previously untreated patients and/or a break in immunologic tolerance in previously treated, inhibitor-free patients. In addition, inhibitors could be directed against both modified fVIII and native fVIII. However, there are no preclinical models available to assess the immunogenicity of modified fVIII proteins.
The immune response to fVIII requires helper T cells.4 The T-cell repertoire in the thymus is shaped during embryonic development, during which T cells that react to self peptides are deleted. Patients with severe hemophilia A develop an immune response to fVIII presumably in part because they synthesize insufficient fVIII to tolerize self-reactive T cells. Conceivably, severe hemophilia A patients who develop tolerance to fVIII express enough fVIII polypeptide during embryonic development to induce tolerance. However, this has not been demonstrated.
van Helden et al produced transgenic mice containing the human F8 gene in a successful attempt to produce immunologic tolerance to human fVIII. Their study was motivated by a model in which transgenic mice express human IFN β that is predictive of the human immunologic response to commercial IFN β products.5 van Helden et al created several transgenic lines containing the human F8 cDNA in an E17 hemophilia A mouse background that has been widely used in hemophilia research.6 They challenged the transgenic mice and conventional E17 hemophilia mice with human fVIII using a dosage and treatment interval that mimics use in humans. This immunologic challenge produces a high titer inhibitor response in conventional E17 hemophilia A mice.7 One of the transgenic lines produced the desired result and did not produce anti-fVIII antibodies after fVIII challenge. The immunologic tolerance in these mice was specific to fVIII because they developed a strong immune response to human von Willebrand factor. Although human fVIII mRNA was identified in the transgenic mice, circulating fVIII was not detected and the mice bled excessively after hemostatic insult. Thus, the transgenic mouse may be analogous to patients with severe hemophilia A that express sufficient fVIII to develop immunologic tolerance.
The authors then challenged conventional and transgenic hemophilia A mice with 2 PEG-fVIII preparations, designated mFVIII1 and mFVIII2, as well as native human fVIII. Native human fVIII and mFVIII1 produced similar titers of anti-fVIII antibodies in conventional hemophilia A mice, whereas mFVIII2 induced significantly higher titers (see figure). Importantly, when challenged with mFVIII2, transgenic hemophilia A mice that were previously tolerant to native human fVIII developed antibodies that recognized both native human fVIII and mFVIII2. If extrapolated to the clinical setting, this outcome would predict that mFVIII2 would produce the extremely undesirable result of inhibitor development in previously tolerant patients, making a manageable situation worse.
Human F8 transgenic mice develop antibodies against human fVIII when treated with a PEGylated human FVIII designated mFVIII2. Mice were treated with 8 weekly doses of either native human fVIII or 1 of 2 PEGylated human FVIII proteins (mFVIII1 and mFVIII2). Anti–human fVIII antibody titers against native human fVIII were determined by ELISA 1 week after the last dose. The asterisk denotes a statistically significant difference at the level P < .001. ns indicates not significant.
Human F8 transgenic mice develop antibodies against human fVIII when treated with a PEGylated human FVIII designated mFVIII2. Mice were treated with 8 weekly doses of either native human fVIII or 1 of 2 PEGylated human FVIII proteins (mFVIII1 and mFVIII2). Anti–human fVIII antibody titers against native human fVIII were determined by ELISA 1 week after the last dose. The asterisk denotes a statistically significant difference at the level P < .001. ns indicates not significant.
The development of commercial fVIII products is an expensive endeavor that requires years of preclinical and clinical effort. The potential benefit of improving lives of patients with hemophilia with a novel fVIII product must be weighed with the risk of product failure because of an adverse event such as inhibitor development and its clinical and economic consequences. The stakes get higher as product development proceeds, forcing a potential fVIII product manufacturer to ask at each stage whether to continue the project. In addition to providing a model that may be useful in the study of mechanisms of loss of immunologic tolerance to fVIII, the transgenic model described by van Helden et al may lead to an important preclinical tool to guide decision making during the development of new fVIII products.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health