Abstract
Nitric oxide (NO) stimulates cGMP synthesis by activating its intracellular receptor, soluble guanylyl cyclase (sGC). It is a currently prevailing concept that No and cGMP inhibits platelet function. However, the data supporting the inhibitory role of NO/sGC/cGMP in platelets have been obtained either in vitro or using whole body gene deletion that affects vessel wall function. Here we have generated mice with sGC gene deleted only in megakaryocytes and platelets. Using the megakaryocyte- and platelet-specific sGC-deficient mice, we identify a stimulatory role of sGC in platelet activation and in thrombosis in vivo. Deletion of sGC in platelets abolished cGMP production induced by either NO donors or platelet agonists, caused a marked defect in aggregation and attenuated secretion in response to low doses of collagen or thrombin. Importantly, megakaryocyte- and platelet-specific sGC deficient mice showed prolonged tail-bleeding times and impaired FeCl3-induced carotid artery thrombosis in vivo. Interestingly, the inhibitory effect of the NO donor SNP on platelet activation was sGC-dependent only at micromolar concentrations, but sGC-independent at millimolar concentrations. Together, our data demonstrate important roles of sGC in stimulating platelet activation and in vivo thrombosis and hemostasis, and sGC-dependent and -independent inhibition of platelets by NO donors.
Introduction
The nitric oxide (NO)/cGMP signaling cascade is involved in diverse physiologic and pathophysiologic functions, such as smooth muscle relaxation, vasodilation, neurotransmission, immune responses, and inflammation.1 NO is a short-lived gaseous molecule, synthesized by a family of enzymes known as nitric oxide synthase (NOS). There are 3 known NOS isoforms, neuronal NOS (nNOS, NOS1), inducible NOS (iNOS, NOS2), and endothelial NOS (eNOS, NOS3). Both eNOS and iNOS are expressed and functional in platelets.2-6 The major effect of NO is mediated by its cytosolic receptor, soluble guanylyl cyclase (sGC). The roles of the NO/sGC/cGMP pathway in platelet activation have been investigated for > 3 decades. However, their functions in platelet activation remain controversial. There are 2 major controversies regarding the role of the NO-cGMP pathway in platelets: (1) whether the NO-cGMP pathway plays a stimulatory role, an inhibitory role, or both during platelet activation; and (2) whether the inhibitory effect of NO donors on platelet function is cGMP-dependent or not. Early studies in the mid-1970's showed that during platelet activation by agonists, such as ADP and collagen, intracellular cGMP concentrations were enhanced significantly,7,8 and that exogenous cGMP analogs enhanced platelet aggregation.9 Therefore, a stimulatory role of cGMP in platelet activation was proposed. This view was soon abandoned because nitric oxide donors inhibited platelet activation, and dramatically increased intraplatelet cGMP concentrations. Since then, it has been generally accepted that cGMP plays an inhibitory role in platelet activation. Because the inhibitory effect of NO donors is mainly sGC-dependent,10-13 sGC is believed to play an inhibitory role in platelet activation, and drugs that activate sGC are being developed to prevent thrombotic diseases.14,15
A recent study by Dangel et al reported a dramatically reduced bleeding time in sGC whole body deficient mice, implicating a possible inhibitory role for sGC in hemostasis.16 However, the vascular endothelial-generated NO and smooth muscle sGC-cGMP pathway is known to be important in vasodilation, and thus the whole body sGC-deficiency may cause vaso-constriction and consequently reduce bleeding time. To specifically address the roles of platelet sGC in platelet activation and in thrombosis and hemostasis in vivo, we have developed megakaryocyte- and platelet-specific sGC-deficient mice. Our data show that sGC-deficient platelets have defective granule secretion and aggregation in response to low dose agonists. Importantly, the platelet-specific sGC deficient mice had significantly prolonged tail bleeding times and impaired thrombus formation in the FeCl3-induced thrombosis model compared with wild type littermates, indicating that platelet sGC plays an important stimulatory role in hemostasis and thrombosis in vivo. In addition, we show that NO donors at high concentrations inhibit platelet activation through sGC-dependent and -independent pathways.
Methods
Generation and characterization of sGC β1−/− mice
All animal experiments were performed in compliance with relevant laws and were approved by the Institutional Animal Care and Use Committee of the University of Kentucky. We engineered a targeting vector with 3 loxP sites in the sGC β1 allele that flanked exons 7 and 8 and an inserted Neo cassette. Mouse genomic DNA from a 129 P2 mouse liver was used as a template to generate a sGC β1 targeting construct. A PGK-1 promoter-driven herpes simplex virus–thymidine kinase (HSV-TK) gene was placed outside the sGC β1 gene homology region for negative selection of nonhomologous recombination during targeted embryonic stem (ES) cell selection using ganciclovir. The sGC targeting vector was electroporated into E14 ES cells (a gift of Dr Zuoming Sun, Beckman Research Institute of the City of Hope), which were propagated on mouse embryo fibroblasts feeder cells and selected for neo (G418) and HSV-TK (ganciclovir) resistance. ES cells heterozygous for sGC gene deficiency with a normal karyotype were microinjected into C57BL/6J blastocysts, which were then implanted into pseudopregnant mice. Chimeras among the offspring were bred with C57BL/6J mice. Mice containing the sGC fl targeted allele were determined by PCR amplification with primers flanking the loxP sequence located in the eighth intron (Figure 1). These sGC primers included the sense 5′-taagagcagtcagctttcact-3′ and the antisense 5′-ttctgcttccacatctacagct-3′. Chimeric mice were bred with C57BL/6J wild type mice to produce sGC β1fl/+ mice, which were backcrossed to generate viable sGC β1fl/fl mice. sGC β1fl/fl mice were backcrossed into the C57BL/6J background for 10 generations. Megakaryocyte-specific deletion of the sGC β1fl/fl allele was accomplished through breeding with the Pf4-Cre recombinase C57BL/6J transgenic mice, and verified by Western blot with a rabbit polyclonal anti-sGC β1 antibody.
Methods
Luciferin/luciferase reagent and collagen were purchased from Chronolog. Human α-thrombin was from Enzyme Research Laboratories. Fura-2/AM and Pluronic F-127 were from Invitrogen. A rabbit polyclonal antibody against sGC was purchased from FabGennix International Inc. A rabbit polyclonal antibody against a recombinant human Akt 1 fragment (amino acid residues 345-480), a rabbit polyclonal antibody against Src, and mouse monoclonal antibodies against the phosphorylated Ser239 site of VASP, 16C2, and against the phosphorylated Ser157 site of VASP, 5C6, were purchased from Santa Cruz Biotechnology Inc. Rabbit monoclonal antibodies against phosphorylated Ser473 residue of Akt was from Cell Signaling Technology. A rabbit monoclonal antibody against β-actin, and NO donor SNP were from Sigma-Aldrich. NO donor DETA NONOate was from Cayman Chemical Company. NO donor, L-penicillaminamide-[N-(β-D-glucopyranosyl)-N2-acetyl-S-nitroso-D (SNAP1), and forskolin were from Calbiochem. cGMP ELISA kit was from Amersham Biosciences.
Preparation of mouse washed platelets
Eight- to 10-week-old mice of either sex were anesthetized with isofluorane, and blood was collected from the abdominal aorta using 1/7 volume of ACD (85mM trisodium citrate, 83mM dextrose, and 21mM citric acid) as anticoagulant.17 Blood from 5-6 mice of either genotype was pooled, and platelets were isolated by differential centrifugation as described previously. Platelets were washed once with CGS buffer (0.12M sodium chloride, 0.0129M trisodium citrate, and 0.03M D-glucose, pH 6.5), resuspended in modified Tyrode's buffer18 and allowed to rest for at least 1 hour at room temperature before use.
Measurement of cGMP levels
Washed platelets (3 × 108/mL) in Tyrode's buffer were incubated with selected concentrations of NO donors at 37°C for 5 minutes, or stirred at 37°C after addition of agonists, thrombin or collagen, for 5 minutes. Reactions were stopped by addition of equal volume of ice-cold 12% (wt/vol) trichloroacetic acid. Samples were mixed and centrifuged at 2000g for 15 minutes at 4°C. Supernatants were removed and washed 4 times with 5 volumes of water-saturated diethyl ether and then lyophilized. cGMP concentrations were measured using a cGMP enzyme immunoassay kit from Amersham-Pharmacia.
Platelet aggregation and secretion
Platelet aggregation was measured in a lumiaggregometer model 700 (Chronolog) at 37°C with stirring (1000 rpm). Platelet secretion was monitored in parallel with platelet aggregation as ATP release with the addition of luciferin-luciferase reagent to platelet suspension. Quantification was performed using ATP standards. Experiments were repeated at least 3 times. Statistical significance was examined using a paired t test and data from 3 or more experiments.
Western blot analysis of VASP phosphorylation
Washed platelets from wild-type or platelet-specific sGC-deficient mice were resuspended in Tyrode's solution (3 × 108/mL) and incubated with SNP (100μM), SNAP1 (100μM), or forskolin (10μM) in the aggregometer at 37°C for 5 minutes and solubilized in SDS-PAGE sample buffer. Platelet lysates were analyzed by SDS-PAGE on 4%-15% gradient gel and electrotransfered to polyvinylidenefluoride membranes. Phosphorylation of VASP was detected as described previously19 by immunoblotting with monoclonal antibody 16C2, specific for phosphorylated Ser239 site of VASP or 5C6, specific for the phosphorylated Ser157 site of VASP.
Bleeding time
Seven- to 8-week-old mice were anesthetized with intraperitoneal injection of pentobarbital. The distal portion of the tail (5 mm) was amputated with a scalpel, and the tail was immersed in 0.15M NaCl at 37°C as previously described.20 Time to stable cessation of the bleeding was defined as the time where no rebleeding for longer than 60 seconds was recorded. Statistical analysis was performed using the Mann-Whitney test.
In vivo thrombosis
An in vivo thrombosis model was performed as described previously.5 Briefly, 7- to 8-week-old mice were anesthetized with intraperitoneal injection of pentobarbital. Left carotid arteries were isolated from surrounding tissues.21 MA-0.5PSB nanoprobe (Transonic Systems) was hooked to arteries, and blood flow was monitored with a TS420 flowmeter (Transonic Systems). After stabilization, 1.2 μL of 5% FeCl3 was applied to a filter paper disc (1-mm diameter) that was immediately placed on top of the artery for 3 minutes. After removing the filter paper, blood flow was monitored continuously until 5 minutes after occlusion. Time to occlusion was calculated as a difference in time between the removal of the filter paper and stable occlusion (no blood flow for 1 minute). Statistical analysis was performed using the Mann-Whitney test for the evaluation of differences in median occlusion time.
Western blot analysis of Akt and ERK phosphorylation in platelets
Washed platelets from sGC deficient and wild type mice were resuspended in modified Tyrode's buffer (3 × 108/mL), and stimulated with various concentrations of thrombin or collagen in a platelet aggregometer at 37° for 5 minutes and then solubilized in SDS-PAGE sample buffer. Platelet lysates were analyzed by SDS-PAGE on 4%-15% gradient gels and immunoblotted using rabbit monoclonal antibodies specific for phosphorylated p42/44 MAPK residues Thr202/Tyr204 and Akt residue Ser473 (Cell Signaling Technologies).22 Enhanced chemiluminescence (GE Healthcare) was used for visualization of antibody reactions.
Calcium mobilization
Intraplatelet calcium was measured using Fura-2/AM as described previously.23 Briefly, washed mouse platelets were incubated with 12.5μM Fura-2/AM/0.2% Pluronic F-127 for 45 minutes at 37°C. After washing with CGS once more, platelets were resuspended to 3 × 108/mL in Tyrode's solution. Continuous fluorescent measurements were analyzed by excitation at 340 nm and 380 nm, and emission was measured at 509 nm using a model LS55 Luminescence Spectrometer (Perkin-Elmer Cetus). The intracellular Ca2+ level was expressed as relative fluorescence, calculated based on the ratio of emissions simultaneously using FL WinLab 4.0 software (Perkin-Elmer Cetus).
Volume pressure recording
Systolic blood pressure was measured by volume pressure recording of the tail, using the CODA noninvasive blood pressure system on 5 consecutive days (Kent Scientific).24
Results
Generation of megakaryocyte and platelet specific sGC β1 deficient mice
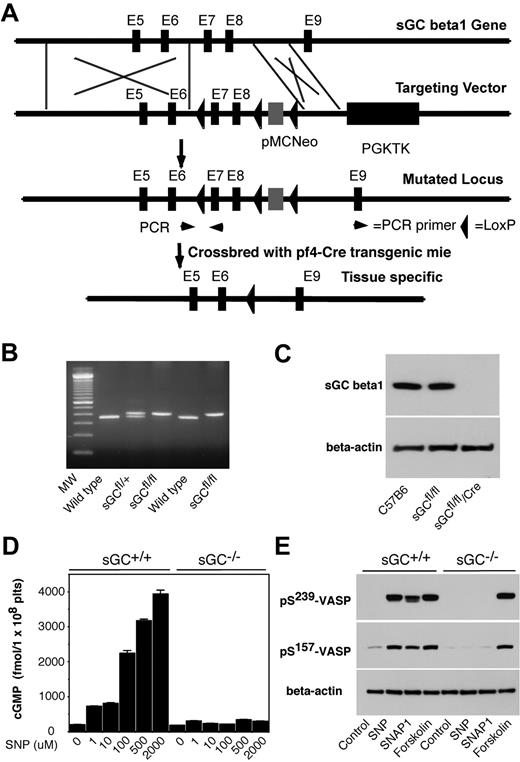
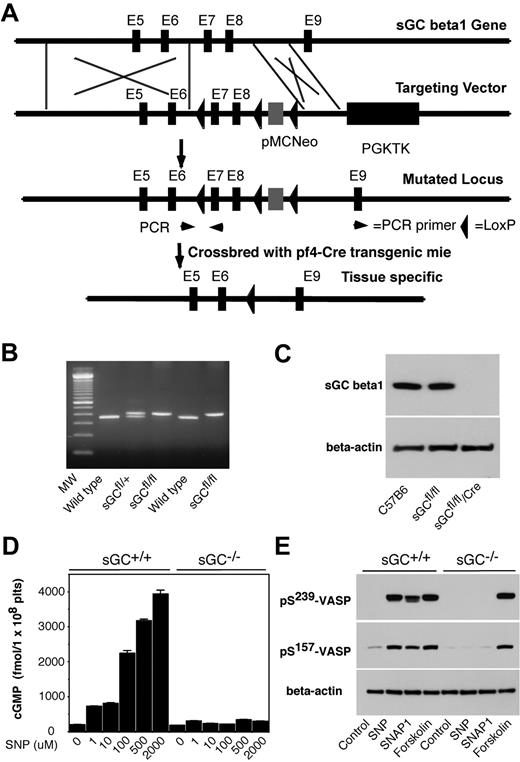
sGC is a heterodimer consisting of 2 different subunits, α and β. Heterodimerization of α and β subunits is required for the sGC activity.25-27 In mammals, 2 isoforms of sGC, designated as α1/β1, α2/β1, have been identified.28 Therefore, disrupting sGC β1 subunit gene of sGC should abolish the activity of all sGC isoforms. To establish a role of platelet sGC in thrombosis, we generated mice harboring a “floxed” sGC β1 allele and then used Cre-mediated recombination to generate platelet-specific sGC deficient mice. In “floxed” sGC β1 mice (sGC β1fl/fl), the exons 7 and 8 of sGC β1 gene and an inserted Neo cassette were flanked with 3 LoxP sites (Figure 1 A-B). sGC β1fl/fl mice were viable and fertile. Immunoblot analysis with a polyclonal antibody that recognizes sGC β1 protein revealed that sGC β1 abundance in platelets from the sGC β1fl/fl mice is comparable with that in platelets from C57BL/6J mice (Figure 1C). Megakaryocyte and platelet specific deletion of the floxed region was accomplished through breeding of the sGC β1fl/fl mice with Pf4-Cre recombinase transgenic mice. Immunoblotting demonstrated the complete absence of the protein in sGC β1fl/fl/Cre (sGC β1 deficient) platelets (Figure 1C). Mice lacking sGC β1 in platelets appeared to develop normally and had normal blood counts, including platelets (data not shown).
Generation of platelet-specific sGC β1 deficient mice. (A) Targeted disruption of sGC β1 gene. Top: structure of sGC β1 gene. Top middle: sGC β1 gene targeting vector, containing a pMC-Neo gene that is flanked with sGC β1 genomic sequence, and PGK-TK that is cloned 3′ to the sGC β1 sequence. Bottom middle: the mutated locus showing the site of PCR used for genotyping. Exons 7 and 8 of sGC were flanked with LoxP sites. Bottom: pMC-Neo gene and exons 7 and 8 were removed by crossbreeding of the sGC conditional mice with specific Cre transgenic mice. (B) PCR genotyping of DNA from offsprings of sGC LoxP chimeras. PCR amplifies a 362-bp fragment from the wild-type allele and a 402-bp fragment from heterozygotes carrying a loxP site (sGCfl/fl) in the sGC β1 allele. (C) Detection of sGC β1 subunit in mouse platelets by Western blot. Washed platelets from C57BL/6J, sGC β1fl/fl, and sGC β1fl/fl/Pf4-Cre mice were solublized in 1 × SDS sample buffer. sGC β1 in platelet lysates was detected by Western blot with a polyclonal antibody against sGC β1. β-actin was used as a loading control. (D) Washed platelets were incubated with selected concentrations of SNP at 37°C for 10 minutes. The reaction was stopped by adding an equal volume of ice-cold 12% (wt/vol) trichloroacetic acid, and cGMP concentrations were determined using a cGMP enzyme immunoassay kit. Means and standard deviations of triplicates are shown from one representative experiment of 3. (E) Washed platelets were incubated with SNAP1 (100μM), SNP (100μM), or forskolin (10μM) at 37°C for 10 minutes. VASP phosphorylation was analyzed by Western blot with monoclonal antibodies specifically recognizing phosphorylated VASP at residues Ser239 or Ser157. Data shown are representative of 3 independent experiments.
Generation of platelet-specific sGC β1 deficient mice. (A) Targeted disruption of sGC β1 gene. Top: structure of sGC β1 gene. Top middle: sGC β1 gene targeting vector, containing a pMC-Neo gene that is flanked with sGC β1 genomic sequence, and PGK-TK that is cloned 3′ to the sGC β1 sequence. Bottom middle: the mutated locus showing the site of PCR used for genotyping. Exons 7 and 8 of sGC were flanked with LoxP sites. Bottom: pMC-Neo gene and exons 7 and 8 were removed by crossbreeding of the sGC conditional mice with specific Cre transgenic mice. (B) PCR genotyping of DNA from offsprings of sGC LoxP chimeras. PCR amplifies a 362-bp fragment from the wild-type allele and a 402-bp fragment from heterozygotes carrying a loxP site (sGCfl/fl) in the sGC β1 allele. (C) Detection of sGC β1 subunit in mouse platelets by Western blot. Washed platelets from C57BL/6J, sGC β1fl/fl, and sGC β1fl/fl/Pf4-Cre mice were solublized in 1 × SDS sample buffer. sGC β1 in platelet lysates was detected by Western blot with a polyclonal antibody against sGC β1. β-actin was used as a loading control. (D) Washed platelets were incubated with selected concentrations of SNP at 37°C for 10 minutes. The reaction was stopped by adding an equal volume of ice-cold 12% (wt/vol) trichloroacetic acid, and cGMP concentrations were determined using a cGMP enzyme immunoassay kit. Means and standard deviations of triplicates are shown from one representative experiment of 3. (E) Washed platelets were incubated with SNAP1 (100μM), SNP (100μM), or forskolin (10μM) at 37°C for 10 minutes. VASP phosphorylation was analyzed by Western blot with monoclonal antibodies specifically recognizing phosphorylated VASP at residues Ser239 or Ser157. Data shown are representative of 3 independent experiments.
NO-induced cGMP production was abolished in sGC β1 deficient platelets
To determine whether sGC activity was abolished in sGC β1 deficient platelets, washed platelets from sGC β1 deficient (sGC β1fl/fl/Cre) mice or wild type littermates (sGC β1fl/fl) were incubated with selected concentrations of a NO donor SNP, and intracellular cGMP concentrations were measured. As shown in Figure 1D, SNP concentration-dependently induced elevation in intracellular cGMP levels in platelets from sGC β1fl/fl mice, but not in platelets from sGC β1fl/fl/Cre mice, indicating that NO-induced cGMP synthesis was abolished in sGC β1fl/fl/Cre platelets. VASP is a well-known substrate for both cAMP-dependent protein kinase (PKA) and cGMP-dependent protein kinase (PKG). Accordingly, VASP phosphorylation induced by NO donors, SNP and SNAP1, respectively, was abolished in sGC β1fl/fl/Cre platelets (Figure 1E). In contrast, forskolin, an activator of adenylate cyclase, induced VASP phosphorylation in both sGC βfl/fl/Cre and sGC β1fl/fl platelets. Together, these data demonstrate that the sGC activity is abolished in sGC β1fl/fl/Cre platelets.
The role of sGC in platelet agonist-induced cGMP production
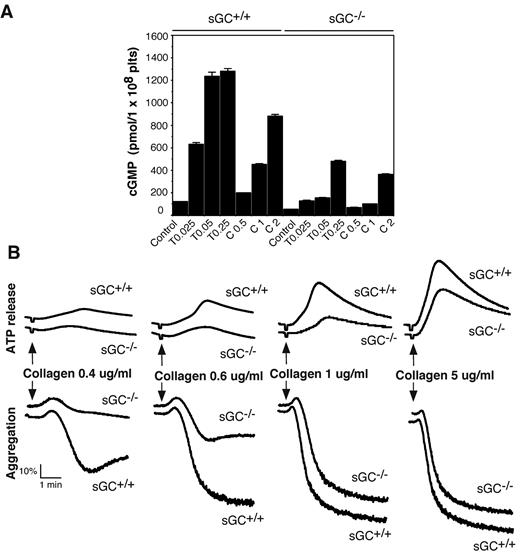
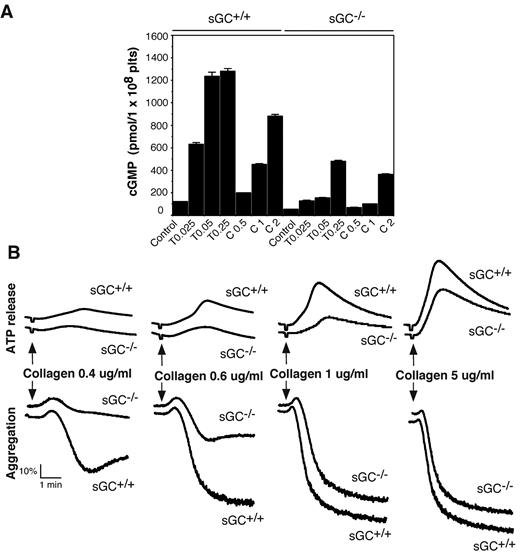
Intracellular cGMP concentrations are significantly increased in platelets on agonist stimulation.7,8,20,29,30 To determine whether sGC is responsible for agonist-induced cGMP production, washed platelets from sGC deficient mice or wild type littermates, were stimulated with thrombin or collagen, and intra-platelet cGMP concentrations were measured by an ELISA assay. As shown in Figure 2A, intracellular cGMP concentrations were increased significantly in platelets from sGC β1fl/fl mice in response to thrombin and collagen, respectively. In contrast, the agonist-induced cGMP elevation in sGC β1-deficient platelets was reduced markedly to < 15% of control sGC β1fl/fl platelets at a low concentration (0.025 U/mL) and < 40% of control at a high concentration of thrombin (0.25 U/mL). However, there was still residual agonist-induced cGMP synthesis in sGC β1-deficient platelets, particularly at higher concentrations of agonists. These results demonstrate an important role for sGC in agonist-induced cGMP synthesis in platelets. Our results also suggest a sGC-independent alternative pathway contributing to the agonist-induced elevation in intra-platelet cGMP concentrations.
Agonist-induced cGMP production is diminished and collagen-induced platelet secretion and aggregation are attenuated in sGC β1 deficient platelets. (A) Washed platelets from wild-type (sGC+/+) or sGC β1 deficient mice (sGC−/−) resuspended in Tyrode's solution were stimulated with selected concentrations of either thrombin or collagen at 37°C for 5 minutes and solubilized by adding ice-cold 12% (wt/vol) trichloroacetic acid. cGMP concentrations were determined using a cGMP enzyme immunoassay kit. Results are expressed as mean ± SD (n = 3). Means and SD of triplicates are shown from 1 representative experiment of 3. (B) Washed platelets from wild-type (sGC+/+) or sGC β1 deficient mice (sGC–/–) were stimulated with various concentrations of collagen in a lumi-aggregometer at 37°C. Real-time ATP secretion and platelet aggregation were recorded simultaneously. Data shown are representative of 4 independent experiments.
Agonist-induced cGMP production is diminished and collagen-induced platelet secretion and aggregation are attenuated in sGC β1 deficient platelets. (A) Washed platelets from wild-type (sGC+/+) or sGC β1 deficient mice (sGC−/−) resuspended in Tyrode's solution were stimulated with selected concentrations of either thrombin or collagen at 37°C for 5 minutes and solubilized by adding ice-cold 12% (wt/vol) trichloroacetic acid. cGMP concentrations were determined using a cGMP enzyme immunoassay kit. Results are expressed as mean ± SD (n = 3). Means and SD of triplicates are shown from 1 representative experiment of 3. (B) Washed platelets from wild-type (sGC+/+) or sGC β1 deficient mice (sGC–/–) were stimulated with various concentrations of collagen in a lumi-aggregometer at 37°C. Real-time ATP secretion and platelet aggregation were recorded simultaneously. Data shown are representative of 4 independent experiments.
A stimulatory role of sGC in platelet activation
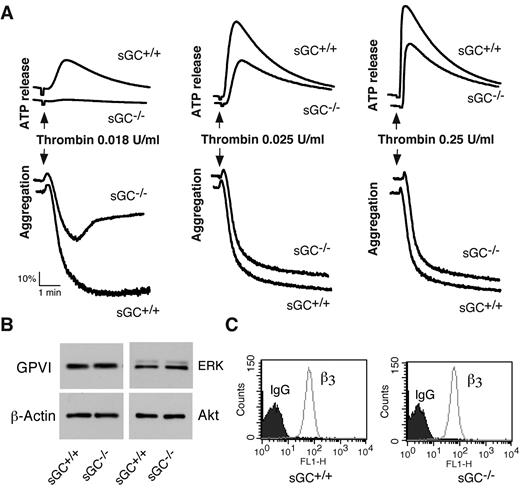
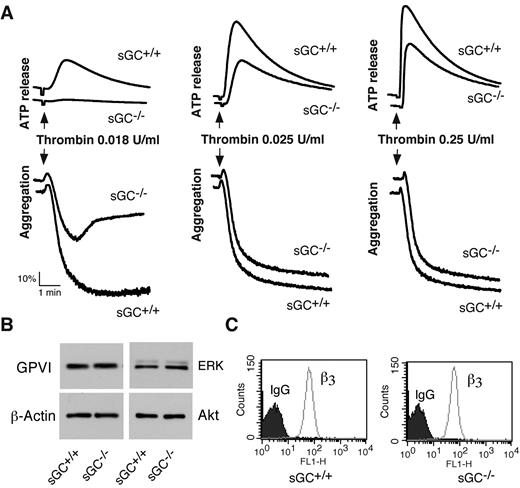
To determine whether sGC-mediated cGMP synthesis exerted a stimulatory role in platelet activation, we examined the effect of sGC deficiency on agonist-induced platelet ATP release and platelet aggregation. Platelet aggregation and ATP release (as a marker for dense granule secretion) were monitored in real time, using a Chrono-Log Lumi-aggregometer. Platelets lacking sGC β1 showed a marked defect in granule secretion and aggregation in response to low concentrations of either collagen (Figure 2B) or thrombin (Figure 3A), compared with control sGC β1fl/fl platelets. However, platelet aggregation and secretion were identical in sGC β1 deficient and control wild type platelets when stimulated with high concentrations of agonists (Figures 2B and 3A).
Thrombin-induced platelet activation is attenuated in sGC β1 deficient platelets, and expression of receptors and signaling molecules is not affected by sGC deficiency. (A) Washed platelets from wild-type (sGC+/+) or sGC β1 deficient mice (sGC–/–) were stimulated with increasing concentrations of thrombin in a lumi-aggregometer at 37°C. Real-time ATP secretion and platelet aggregation were recorded simultaneously. Data shown are representative of 4 independent experiments. (B) Washed platelets from wild type (sGC+/+) or sGC β1 deficient mice (sGC–/–) were solubilized in 2 × SDS sample buffer. GPVI, Akt, ERK, and β-actin were detected by Western blot. (C) Expression integrin β3 on platelets from wild-type (sGC+/+) or sGC β1 deficient mice (sGC–/–) was analyzed by flow cytometry with a FITC-labeled anti–mouse β3 monoclonal antibody. Data shown in panels B and C are representative of 2 independent experiments
Thrombin-induced platelet activation is attenuated in sGC β1 deficient platelets, and expression of receptors and signaling molecules is not affected by sGC deficiency. (A) Washed platelets from wild-type (sGC+/+) or sGC β1 deficient mice (sGC–/–) were stimulated with increasing concentrations of thrombin in a lumi-aggregometer at 37°C. Real-time ATP secretion and platelet aggregation were recorded simultaneously. Data shown are representative of 4 independent experiments. (B) Washed platelets from wild type (sGC+/+) or sGC β1 deficient mice (sGC–/–) were solubilized in 2 × SDS sample buffer. GPVI, Akt, ERK, and β-actin were detected by Western blot. (C) Expression integrin β3 on platelets from wild-type (sGC+/+) or sGC β1 deficient mice (sGC–/–) was analyzed by flow cytometry with a FITC-labeled anti–mouse β3 monoclonal antibody. Data shown in panels B and C are representative of 2 independent experiments
We next examined whether loss of sGC affected expression of receptors and signaling molecules in platelets, to exclude the possibility that defect in activation of sGC-deficient platelets results from an effect of sGC on protein expression. Expression of the collagen receptor GPVI in sGC-deficient platelets, as detected by Western blot with a rat anti–mouse GPVI monoclonal antibody, was comparable with wild type platelets (Figure 3B). Integrin αIIbβ3 was detected by flow cytometry with a FITC-labeled rat anti-mouse β3 monoclonal antibody. The abundance of integrin αIIbβ3 in sGC-deficient platelets was also similar to wild type platelets (Figure 3C). Lack of sGC did not affect expression of the signaling molecules that are important for platelet activation, including Akt, ERK (Figure 3B), and Src family kinases (data not shown), because abundance of these proteins in sGC-deficient platelets was identical to wild type platelets. These results suggest that expression of these receptors and signaling molecules in platelets is not affected by deficiency of sGC.
sGC deficiency did not affect calcium mobilization, but inhibited phosphorylation of ERK and Akt in response to agonists
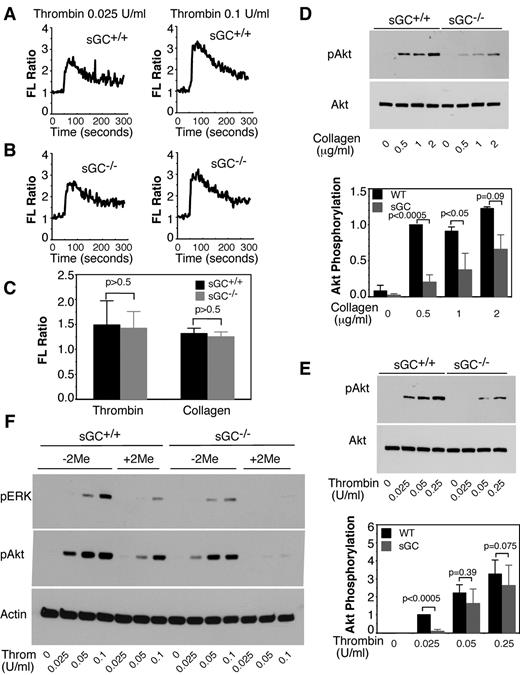
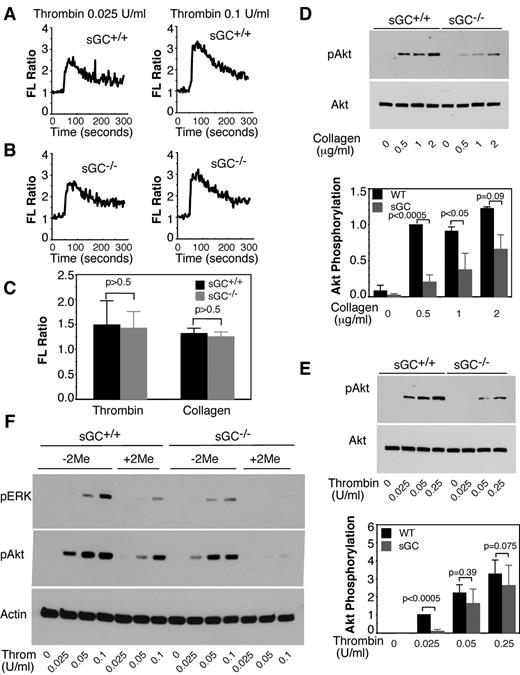
Agonist-induced increase in intra-platelet calcium is a key signaling event to platelet secretion and aggregation. We then examined calcium concentrations in sGC-deficient platelets in response to agonists to determine whether sGC plays a role in calcium mobilization. Washed platelets from sGC-deficient mice or wild type littermates were labeled with a calcium probe, Fura-2/AM. Intra-platelet calcium concentrations elicited by thrombin or collagen were comparable between wild type (Figure 4A,C) and sGC-deficient platelets (Figure 4B-C), suggesting that calcium mobilization in sGC-deficient platelets was not defective.
sGC deficiency does not affect agonist-induced calcium mobilization, but inhibits Akt and ERK phosphorylation in response to either thrombin or collagen. (A-C) Washed platelets from wild-type (sGC+/+; A) or sGC β1 deficient mice (sGC–/–; B) were labeled with 12.5μM Fura-2/AM/0.2% Pluronic F-127 and resuspended in Tyrode's solution. Platelets were then stimulated with selected concentrations of thrombin. Changes in intracellular free calcium concentrations were measured every 2 seconds and expressed as a ratio of fluorescence (FL) detected at 509 nm emission with an excitation wavelength of 340 nm and 380 nm. Statistical data from 3 experiments were shown in panel C. (D-E) Washed platelets from wild type (sGC+/+) or sGC β1 deficient mice (sGC–/–) mice were stimulated with either collagen (D) or thrombin (E) at 37°C for 5 minutes and solubilized in 2× SDS sample buffer. Phosphorylation of Akt was detected by Western blotting with a rabbit monoclonal antibody specifically recognizing the phosphorylated Akt residue Ser473. A rabbit polyclonal antibody against nonphosphorylated Akt was used to verify equal loading. Data shown are representative of 3 independent experiments. (F) Washed platelets from wild type (sGC+/+) or sGC β1 deficient mice (sGC–/–) mice were pre-incubated with 2MeSAMP (10μM) or buffer for 5 minutes, and then stimulated with thrombin at 37°C for 5 minutes and solubilized in 2× SDS sample buffer. Phosphorylation of Akt and ERK was detected by Western blotting using phospho-specific antibodies against phosphorylated Ser473 (Akt) and Thr-202/Tyr204 (ERK; Cell Signaling Technologies). A mouse monoclonal antibody against β-actin (Sigma-Aldrich) was used to verify equal loading.
sGC deficiency does not affect agonist-induced calcium mobilization, but inhibits Akt and ERK phosphorylation in response to either thrombin or collagen. (A-C) Washed platelets from wild-type (sGC+/+; A) or sGC β1 deficient mice (sGC–/–; B) were labeled with 12.5μM Fura-2/AM/0.2% Pluronic F-127 and resuspended in Tyrode's solution. Platelets were then stimulated with selected concentrations of thrombin. Changes in intracellular free calcium concentrations were measured every 2 seconds and expressed as a ratio of fluorescence (FL) detected at 509 nm emission with an excitation wavelength of 340 nm and 380 nm. Statistical data from 3 experiments were shown in panel C. (D-E) Washed platelets from wild type (sGC+/+) or sGC β1 deficient mice (sGC–/–) mice were stimulated with either collagen (D) or thrombin (E) at 37°C for 5 minutes and solubilized in 2× SDS sample buffer. Phosphorylation of Akt was detected by Western blotting with a rabbit monoclonal antibody specifically recognizing the phosphorylated Akt residue Ser473. A rabbit polyclonal antibody against nonphosphorylated Akt was used to verify equal loading. Data shown are representative of 3 independent experiments. (F) Washed platelets from wild type (sGC+/+) or sGC β1 deficient mice (sGC–/–) mice were pre-incubated with 2MeSAMP (10μM) or buffer for 5 minutes, and then stimulated with thrombin at 37°C for 5 minutes and solubilized in 2× SDS sample buffer. Phosphorylation of Akt and ERK was detected by Western blotting using phospho-specific antibodies against phosphorylated Ser473 (Akt) and Thr-202/Tyr204 (ERK; Cell Signaling Technologies). A mouse monoclonal antibody against β-actin (Sigma-Aldrich) was used to verify equal loading.
The PI3K/Akt pathway has been implicated in platelet secretion and aggregation. Akt phosphorylation in response to collagen (Figure 4D) and thrombin (Figure 4E), respectively, was significantly reduced in sGC-deficient platelets compared with wild type platelets. We have previously reported that the agonist-induced activation of Akt is upstream of the NOS/cGMP pathway.31,32 Therefore, Akt phosphorylation induced by thrombin should not be affected by sGC deficiency. We hypothesize that reduced Akt phosphorylation in sGC deficient platelets might be because of reduced secretion of ADP, which can activate the Akt pathway through its P2Y12/Gi signaling. Indeed, thrombin-induced Akt phosphorylation was significantly reduced by incubation with an antagonist of P2Y12, 2MeSAMP. Thus, the secreted ADP greatly amplified thrombin-stimulated Akt phosphorylation (Figure 4F), which was diminished in sGC-deficient platelets. These results suggest a positive feedback mechanism in which sGC stimulates granule secretion of ADP, which greatly amplify Akt activation that activates the NO-sGC pathway. We have shown that the agonist-induced activation of ERK MAPK pathway is downstream from cGMP/PKG pathway.18 As expected, ERK phosphorylation was lower in sGC deficient platelets, compared with wild type platelets (Figure 4F).
An important role of sGC in hemostasis and thrombus formation in vivo
The above in vitro data suggest that sGC promotes platelet activation. To investigate whether platelet sGC exerts a role in hemostasis in vivo, tail-bleeding time tests were performed in 7- 8-week-old isoflourance-anesthetized littermates generated from mating of sGC β1fl/fl/Cre male mice with sGC β1fl/fl female mice as breeders. Genotyping of the offspring was subsequently determined by PCR analysis, using DNA extracted from tail tissue after bleeding time tests. Among the 66 mice tested, 31 were wild type mice (sGC β1fl/fl) with a median bleeding time of 198 seconds. In contrast, the median bleeding time of 35 megakaryocyte and platelet specific sGC-deficient mice (sGC β1fl/fl/Cre; 316 seconds) was significantly prolonged (P = .025; Figure 5A). Thus, our data indicate that sGC is required for normal hemostasis. sGC deficiency results in a longer bleeding time.
sGC deficient mice have prolonged tail-bleeding times, impaired thrombus formation, but normal systolic blood pressure. (A) Bleeding time tests were performed in littermate mice (7-8 weeks old) generated from mating with sGC β1fl/fl/Cre+ male and sGC β1fl/fl female mice as breeders, using methods described under “Bleeding time.” Genotyping of the tested mice was subsequently determined by PCR analysis. The solid triangles represent bleeding times of a single mouse. The bar represents median bleeding times of the group. Note that although the variance of bleeding times were broad in both wild type (n = 31) and sGC β1 deficient mice (n = 35), the difference between groups is statistically significant (P = .025). (B) FeCl3-induced carotid artery injury was performed and time to occlusive thrombus formation recorded as described under “In vivo thrombosis.” The occlusion time of each mouse is shown as a solid circle. The bars represent the median occlusion time (442 seconds for sGC β1fl/fl/Cre mice [n = 15], and 252 seconds for β1fl/fl mice [n = 15], P = .012). (C) Systolic blood pressure was measured by volume pressure recording of the tail using the CODA noninvasive blood pressure system on 5 consecutive days. Systolic blood pressures were similar in platelet-specific sGC deficient mice compared with wild-type littermates. Data are means ± SE from n = 6 mice/group.
sGC deficient mice have prolonged tail-bleeding times, impaired thrombus formation, but normal systolic blood pressure. (A) Bleeding time tests were performed in littermate mice (7-8 weeks old) generated from mating with sGC β1fl/fl/Cre+ male and sGC β1fl/fl female mice as breeders, using methods described under “Bleeding time.” Genotyping of the tested mice was subsequently determined by PCR analysis. The solid triangles represent bleeding times of a single mouse. The bar represents median bleeding times of the group. Note that although the variance of bleeding times were broad in both wild type (n = 31) and sGC β1 deficient mice (n = 35), the difference between groups is statistically significant (P = .025). (B) FeCl3-induced carotid artery injury was performed and time to occlusive thrombus formation recorded as described under “In vivo thrombosis.” The occlusion time of each mouse is shown as a solid circle. The bars represent the median occlusion time (442 seconds for sGC β1fl/fl/Cre mice [n = 15], and 252 seconds for β1fl/fl mice [n = 15], P = .012). (C) Systolic blood pressure was measured by volume pressure recording of the tail using the CODA noninvasive blood pressure system on 5 consecutive days. Systolic blood pressures were similar in platelet-specific sGC deficient mice compared with wild-type littermates. Data are means ± SE from n = 6 mice/group.
To further evaluate the role of platelet sGC in thrombus formation in vivo, we compared thrombus formation between wild type and megakaryocyte-specific sGC-deficient mice using the FeCl3-injured carotid artery thrombosis model. The time to formation of stable thrombi in the megakaryocyte-specific sGC-deficient mice was significantly prolonged compared with wild-type littermates (442 seconds for megakaryocyte-specific sGC-deficient mice, 252 seconds for wild type littermates, P = .012; Figure 5B). These data demonstrate that platelet sGC participates in thrombus formation and stability in vivo, in agreement with the observed stimulatory role of sGC in platelet activation.
Deficiency of sGC in platelets did not affect mouse blood pressure
The NO/sGC/PKG I pathway plays a critical role in regulation of blood pressure. It has been shown that eNOS, sGC, or PKG I deficient mice have an increased blood pressure compared with wild type mice.12,33,34 Thus, we measured mouse blood pressure to determine whether platelet sGC contributed to blood pressure regulation. Mouse blood pressure was measured by a CODA tail-cuff noninvasive blood pressure system.24 Figure 5C shows that systolic blood pressures in megakaryocyte-specific sGC-deficient mice were similar to that of wild-type littermates (129 ± 4 in megakaryocyte-specific sGC-deficient mice vs 130 ± 7 mmHg in wild type mice, respectively; Figure 5C). These results demonstrate that unlike whole body sGC-deficient mice, megakaryocyte-specific sGC deficiency did not affect mouse blood pressure.
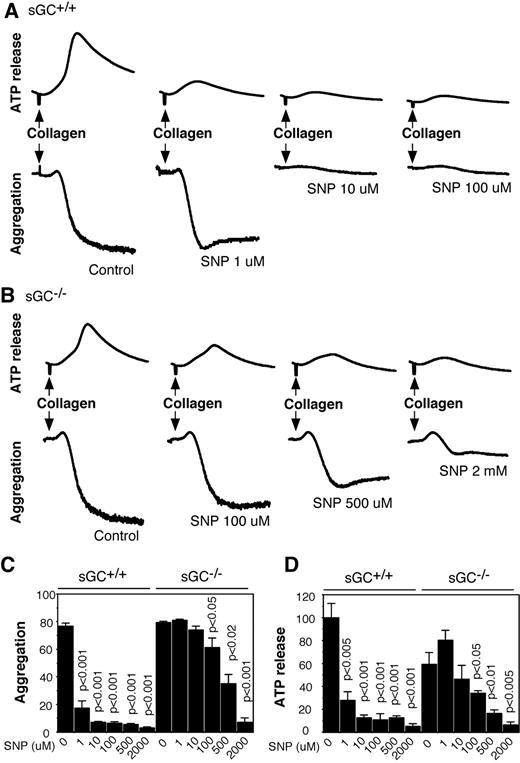
SNP inhibited agonist-induced platelet activation via sGC/cGMP-dependent and -independent pathways
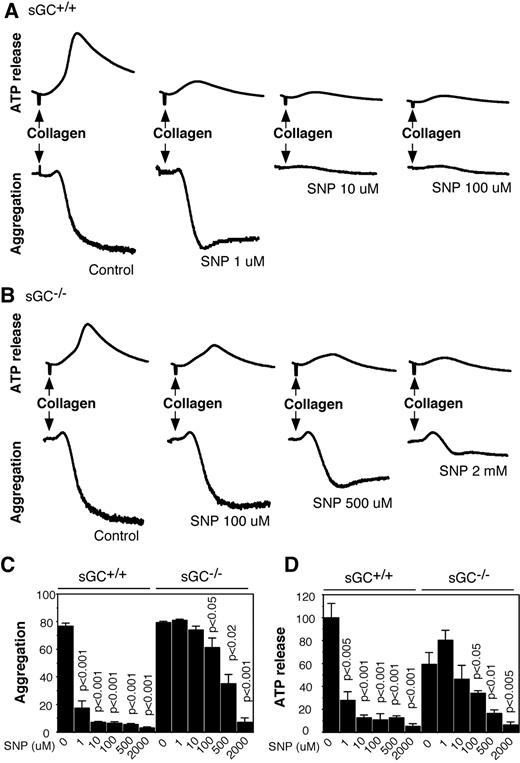
To elucidate the role of sGC in NO donor-induced inhibition of platelet activation, washed platelets from sGC deficient mice or wild type littermates were preincubated with increasing concentrations of SNP for 10 minutes, and then added with collagen to induce aggregation and secretion. In agreement with previous studies, SNP dose-dependently inhibited aggregation and ATP release of wild type platelets in response to collagen, and the maximal inhibition occurred at ∼ 10 μM of SNP (Figures 6A,C,D). In contrast, SNP failed to inhibit aggregation and secretion of sGC-deficient platelets at concentrations < 100μM, suggesting that inhibition of platelet activation by SNP was predominantly mediated by the sGC/cGMP-dependent pathway. However, at high concentrations (≥ 100μM), SNP inhibited aggregation and secretion of sGC-deficient platelets (Figure 6B-D), demonstrating that both sGC-dependent and -independent pathways are involved in the inhibitory effect of NO on platelet activation in response to collagen. Similarly, SNP at low concentrations inhibited thrombin-induced aggregation and secretion of wild type, but not sGC-deficient platelets, but at high concentrations, it also inhibited aggregation and secretion of sGC β1 deficient platelets (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These results demonstrated that platelet inhibition by NO donors is mediated through sGC-dependent mechanisms at commonly used pharmacologic concentrations, but is independent of sGC at high concentrations.
SNP inhibits collagen-induced platelet aggregation and secretion in wild-type and sGC deficient platelets. (A-B) Washed platelets from wild-type (sGC+/+; A) or sGC β1 deficient mice (sGC–/–; B) were incubated with selected concentrations of SNP for 10 minutes, and collagen (1 μg/mL) was added to induce aggregation and secretion. (C-D) Statistical data of aggregation (C) and secretion (D) shown are from 4 independent experiments. Values were normalized with respect to wild type platelets stimulated with collagen in the absence of SNP. Statistical significance between platelets in the absence of SNP and platelets preincubated with a certain concentration of SNP was determined using a Student t test.
SNP inhibits collagen-induced platelet aggregation and secretion in wild-type and sGC deficient platelets. (A-B) Washed platelets from wild-type (sGC+/+; A) or sGC β1 deficient mice (sGC–/–; B) were incubated with selected concentrations of SNP for 10 minutes, and collagen (1 μg/mL) was added to induce aggregation and secretion. (C-D) Statistical data of aggregation (C) and secretion (D) shown are from 4 independent experiments. Values were normalized with respect to wild type platelets stimulated with collagen in the absence of SNP. Statistical significance between platelets in the absence of SNP and platelets preincubated with a certain concentration of SNP was determined using a Student t test.
Discussion
Overall, we have made 2 important findings in this study. First, we have discovered that, in contrast to the prevailing belief that sGC plays a physiologic inhibitory role in platelet activation, we provide in vitro and in vivo evidence, indicating that sGC plays a stimulatory role in platelet activation and thrombus formation. Secondly, we have shown that while the inhibitory role of NO donors in platelet activation is mainly mediated by a sGC-dependent mechanism in agreement with previous studies by Dangel et al,13 the NO donor SNP at millimolar concentrations can mediate sGC-independent platelet inhibition. This latter observation is consistent with the roles of NO-mediated protein nitrosylation and nitration in inhibiting platelet function as suggested by previous studies. Thus our studies reveal different mechanisms of NO-mediated platelet inhibition at different concentrations of NO donors, which may help clarify previous controversies on whether NO-mediated platelet inhibition is cGMP-dependent or not.
Previously, Akt has been shown to stimulate activation of the eNOS/cGMP pathway in response to agonists including thrombin.31,32 Here we show that Akt phosphorylation in response to agonists is diminished in sGC deficient platelets. Inhibition of Akt activation in sGC-deficient platelets is likely because of the role of sGC in stimulating ADP secretion from dense granules and ADP/P2Y12-mediated amplification of Akt activation. These results suggest existence of positive feedback mechanism in which Akt activates NO-dependent sGC activation and cGMP synthesis, leading to granule secretion. ADP secreted from dense granules conversely greatly amplifies Akt activation. Our data also indicate that cGMP production in response to agonists at high concentrations was not totally abolished, suggesting that a sGC-independent mechanism contributes to agonist-induced cGMP production.
The data supporting a stimulatory role for sGC in platelet activation and thrombus formation in vivo was obtained using megakaryocyte and platelet specific sGC-deficient mice, which exclude the compound effects of multiple tissue sGC knockout shown in previous studies12,13,35 and clearly reveal the platelet specific role of sGC. Our data that megakaryocyte and platelet specific sGC-deficient mice had a significantly prolonged bleeding time is in contrast to a recent study showing that mouse tail-bleeding times were shorter in whole body sGC β1 deficient mice.13 However, cGMP plays important roles not only in platelets, but also in the vasculature.11,12,35,36 Whole body sGC β1-deficient mice have a pronounced elevation in systolic blood pressure and significantly shorter life span. Approximately 60% of sGC β1 global knockout mice die within the first 2 days, and ∼ 90% of the remaining mice die between day 18 and 31.12 In addition, body weights of age and gender matched sGC-deficient mice were ∼ 40% less than wild-type mice.12 Therefore, it is unclear whether the reduction of bleeding time in whole body sGC -deficient mice results from effects on vascular cells or platelets or both. Bleeding times in the whole body sGC-deficient mice were < 15 seconds, with a median of 10 seconds, and complete lack of bleeding was observed in some of the whole body sGC deficient mice after the tail cut.16 These results strongly point to a role for vasoconstriction and remodeling in the markedly shortened bleeding times in the whole body sGC-deficient mice. In contrast, platelet-specific sGC-deficient mice develop normally and have a normal life span. The systolic blood pressure of megakaryocyte and platelet specific sGC deficient mice was similar to littermate wild-type controls (Figure 5). Thus, data from megakaryocyte and platelet specific sGC deficient mice unequivocally demonstrate a stimulatory role of platelet sGC in in vivo hemostasis and thrombosis. This conclusion is consistent with the findings by us and others that mildly defective thrombosis formation and/or prolonged bleeding time can be observed in mice that lack some key enzymes in the cGMP pathway5,6,37 and in PKG I deficient mice.20 Interestingly, in the study by Dangel et al,16 whose conclusion is opposite from ours, P-selectin expression in the absence of NO donors was reduced in sGC-deficient platelets, compared with wild-type platelets, consistent with a role of sGC in agonist-induced platelet secretion.
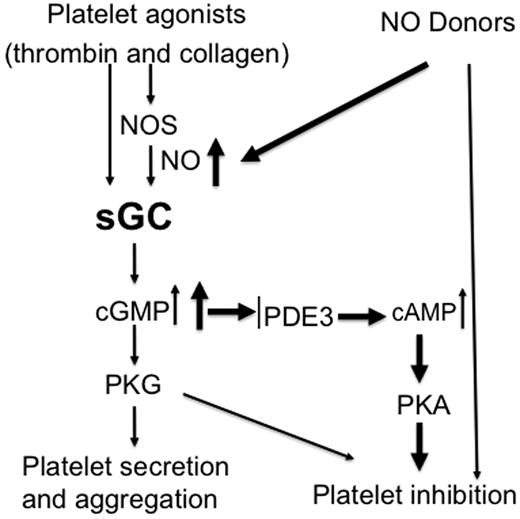
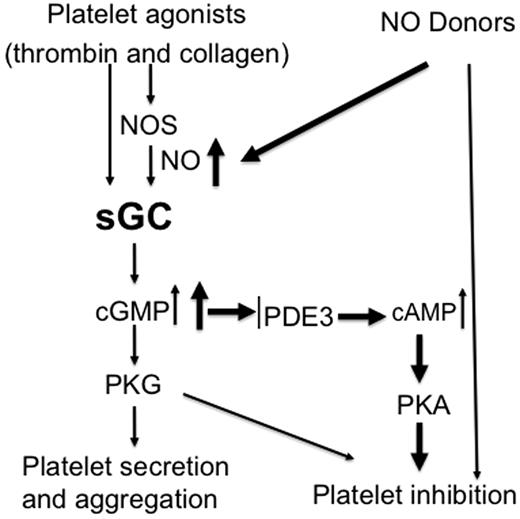
Together with previous findings showing inhibitory effects of relatively high concentrations of exogenous NO donors on platelet activation, our data support the concept that the NO/cGMP pathway plays biphasic roles in platelet activation and thrombus formation. This biphasic response may serve as a mechanism to fine-tune platelet activation, whereby NO plays a stimulatory role at physiologic concentration synthesized by platelets and an inhibitory role at the higher concentrations supplied exogenously by other vascular cells (Figure 7). This biphasic response facilitates hemostasis initially then may limit platelet over-activation to ensure that the response to injury halts bleeding without causing vascular occlusion. Pharmacologic manipulations of the system may often mimic the inhibitory events triggered by higher concentrations of exogenously supplies NO. Although NO activates sGC and thus the cGMP pathway, it can also covalently modify other proteins or their metal cofactors, for example, through S-nitrosylation (which converts thiol groups in proteins, into S-nitrosothiols [SNOs]),38-40 and nitration.41-44 When incubated with NO donors, many proteins in platelets can be modified by S-nitrosylation.40 However, whether the inhibitory effect of NO on platelet activation involves these sGC/cGMP-independent mechanisms is not known. While some studies suggested that sGC-independent mechanisms contribute to the inhibition of platelet activation by NO donors,38,39,43,45 other investigators reported that NO donors fail to inhibit aggregation of the platelets isolated from whole body sGC deficiency mice lacking either α1 or β1 subunit of sGC11,12,16 . Based on these later findings, NO-mediated inhibition of platelet activation was thought to be totally dependent on the sGC/cGMP pathway.35 In experiments with megakaryocyte-specific sGC-deficient mice, we show that SNP at low doses (< 100μM) fails to inhibit activation of sGC-deficient platelets. However, at ≥ 100μM concentrations, SNP inhibits activation of the sGC-deficient platelets (Figure 6 and supplemental Figure 1). The lack of cGMP production in sGC-deficient platelets, even at the highest concentrations of SNP, indicates that sGC activity is abolished and implicates cGMP-independent effects in platelet inhibition by NO donors. Given our results, it is unlikely that the ability of NO donors to inhibit activation of sGC-deficient platelets is because of incomplete deletion of the sGC β1 gene. It is more likely that the difference between our findings and those of Frebe, et al12,13 stem from fundamental differences in mice with megakaryocyte-specific sGC deletion and global, inherited deficiency of sGC. The use of higher concentrations of platelet agonists may also explain the differences, because in our hands, NO donors have minimal inhibitory effect on platelet aggregation induced by high concentrations of agonists, even in wild-type platelets.
Biphasic roles of sGC in regulating platelet activation. Physiologic platelet agonists such as thrombin and collagen activate sGC through NOS-dependent or -independent pathways, leading to platelet activation and formation of a hemostatic thrombus. Continued cGMP elevation in aggregated platelets or NO donors induces a second phase of inhibitory cGMP signaling, resulting in inhibition of further recruitment of platelets. This prevents the overgrowth of hemostatic thrombus.
Biphasic roles of sGC in regulating platelet activation. Physiologic platelet agonists such as thrombin and collagen activate sGC through NOS-dependent or -independent pathways, leading to platelet activation and formation of a hemostatic thrombus. Continued cGMP elevation in aggregated platelets or NO donors induces a second phase of inhibitory cGMP signaling, resulting in inhibition of further recruitment of platelets. This prevents the overgrowth of hemostatic thrombus.
Taken together, our data indicate that sGC plays important roles in both agonist-induced platelet activation and NO-dependent inhibition of platelet activation. Our data also suggest that high concentrations of NO donors may inhibit platelet activation via both sGC-dependent and independent mechanisms. We believe that our finding that sGC plays an important stimulatory role in platelet activation not only represents a major advance in our understanding NO/sGC signaling in platelets, but is also important for guiding the pharmacology and therapeutic applications of drugs targeting cGMP-dependent pathways such as the sGC activators.14,15
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sidney W. Whiteheart for helpful discussions and critically reading the manuscript. They also thank Steve P. Watson for providing helpful suggestions regarding the manuscript; Zhenhui Liu, Jiaping Xie, and Lijun Xia for their insightful suggestions and advice on experimental protocols for generating the platelet-specific sGC deficient mice; Weihua Gao (University of Illinois at Chicago) for her assistance with statistical analysis; and Anju Balakrishnan for her assistance with mouse blood pressure measurement.
This work is supported by American Heart Association National Scientist Development Grant 0430095N and American Heart Association Midwest affiliate Grant-in-Aid 0855698G (to Z.L.), in part by the National Institutes of Health/National Center for Research Resources Centers of Biomedical Research Excellence in Obesity and Cardiovascular Disease Grant P20 RR021954, and by grants from the NHLBI HL068819 and HL062350 (to X.D.).
National Institutes of Health
Authorship
Contribution: G.Z. performed a major part of experiments, was involved in analyzed data and wrote the paper; B.X. performed important experiments; A. Dong contributed to experiments of thrombosis model; R.S. provided PF4-Cre transgenic mice; A. Daugherty contributed to experiments of blood pressure measurement; S.S. contributed to experiments of thrombosis model, and analyzed data, and wrote the paper; X.D. was involved in research design, analyzed data and wrote the paper; and Z.L. designed research, performed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zhenyu Li, Division of Cardiovascular Medicine, Saha Cardiovascular Center, 741 S Limestone St, BBSRB, Rm B251, University of Kentucky, Lexington, Kentucky 40536-0200; e-mail: zhenyuli08@uky.edu.

![Figure 5. sGC deficient mice have prolonged tail-bleeding times, impaired thrombus formation, but normal systolic blood pressure. (A) Bleeding time tests were performed in littermate mice (7-8 weeks old) generated from mating with sGC β1fl/fl/Cre+ male and sGC β1fl/fl female mice as breeders, using methods described under “Bleeding time.” Genotyping of the tested mice was subsequently determined by PCR analysis. The solid triangles represent bleeding times of a single mouse. The bar represents median bleeding times of the group. Note that although the variance of bleeding times were broad in both wild type (n = 31) and sGC β1 deficient mice (n = 35), the difference between groups is statistically significant (P = .025). (B) FeCl3-induced carotid artery injury was performed and time to occlusive thrombus formation recorded as described under “In vivo thrombosis.” The occlusion time of each mouse is shown as a solid circle. The bars represent the median occlusion time (442 seconds for sGC β1fl/fl/Cre mice [n = 15], and 252 seconds for β1fl/fl mice [n = 15], P = .012). (C) Systolic blood pressure was measured by volume pressure recording of the tail using the CODA noninvasive blood pressure system on 5 consecutive days. Systolic blood pressures were similar in platelet-specific sGC deficient mice compared with wild-type littermates. Data are means ± SE from n = 6 mice/group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/13/10.1182_blood-2011-03-341107/4/m_zh89991178530005.jpeg?Expires=1769291248&Signature=sJ5PBXAewfylZUZJfMNWCyGA8XUy3CuyEG4PBe07mfPXaAB9bSUZtQCT4cOBRHoXG2MBdxEBrQR6YpU6hP~tJ0C7pLMsNKV0TmK8uZcg-MubAP1w5EL6hoAOovqaBr-LbtdksMflrmXgMDSBzWo13cnDdIF7x0YQYYon1CguDRegdxOtXELrY1ZCSX58YbosKPZQrVLIeTmsERL89GANxL-TcGXWO0slljh7E~noq-jegQnYgaNfjKtqAouNzfIqPPFi8xFcDopqwR-utRrHTDa4RmhYGP0oSpkA4KOXSmY7YQTsPFmqQt8G7Vk6eadkyAka2Sb~hbOsiW8LDjNYJQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. sGC deficient mice have prolonged tail-bleeding times, impaired thrombus formation, but normal systolic blood pressure. (A) Bleeding time tests were performed in littermate mice (7-8 weeks old) generated from mating with sGC β1fl/fl/Cre+ male and sGC β1fl/fl female mice as breeders, using methods described under “Bleeding time.” Genotyping of the tested mice was subsequently determined by PCR analysis. The solid triangles represent bleeding times of a single mouse. The bar represents median bleeding times of the group. Note that although the variance of bleeding times were broad in both wild type (n = 31) and sGC β1 deficient mice (n = 35), the difference between groups is statistically significant (P = .025). (B) FeCl3-induced carotid artery injury was performed and time to occlusive thrombus formation recorded as described under “In vivo thrombosis.” The occlusion time of each mouse is shown as a solid circle. The bars represent the median occlusion time (442 seconds for sGC β1fl/fl/Cre mice [n = 15], and 252 seconds for β1fl/fl mice [n = 15], P = .012). (C) Systolic blood pressure was measured by volume pressure recording of the tail using the CODA noninvasive blood pressure system on 5 consecutive days. Systolic blood pressures were similar in platelet-specific sGC deficient mice compared with wild-type littermates. Data are means ± SE from n = 6 mice/group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/13/10.1182_blood-2011-03-341107/4/m_zh89991178530005.jpeg?Expires=1769374801&Signature=zyuiVmVzMSLrQOqYwpYBKivkIpawZkUSG0hSmD-o2IJNUB5JWjb0k2HrUaGMfpTt9u0zZx0DYv9WwE8jdAEDf5uBglfQZBTUt4L~dF2SiAFfRZVTelLyaE1pmcS4XMEzu49NMRyRw3GEjmCi13CSGkRShQ5otoRS1L7BC-MS~UUUEK0hNAEjGluMSZ4sBbLwxz5JEgnRA~PoyJxyrmCvnCXLOK-DFp~QitaP3bS~5YjHJVHgRaIPhTW52JrCvCj51T9LP~h-VF6lIxklymsRZR4Za3OKcVhKPYAP6VY~V6ibcJzZmMSGLiWQDVTYQVUj7T--I01Y1zkpUS8r2ufnyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)