Abstract
Currently, there are no reliable RBC invasion assays to guide the discovery of vaccines against Plasmodium vivax, the most prevalent malaria parasite in Asia and South America. Here we describe a protocol for an ex vivo P vivax invasion assay that can be easily deployed in laboratories located in endemic countries. The assay is based on mixing enriched cord blood reticulocytes with matured, trypsin-treated P vivax schizonts concentrated from clinical isolates. The reliability of this assay was demonstrated using a large panel of P vivax isolates freshly collected from patients in Thailand.
Introduction
Vivax malaria remains one of the most important tropical diseases in Asia and South America.1 Effective control of Plasmodium vivax is hampered by the presence of the dormant liver forms (hypnozoites) that cause repeated clinical and parasitologic episodes (relapses), and by the continuing spread of drug-resistant parasites.2 A renewed appreciation of the substantial contribution of P vivax to global malaria morbidity,1,3,4 as well as the adoption of elimination as a strategic goal, have provided impetus for the development of a vaccine against P vivax.5 Merozoite invasion of RBCs by P vivax is a particularly attractive vaccine target because it depends on a single host cell receptor, the Duffy Ag receptor for chemokines (DARC).6,7 A major impediment to guide vaccine development is the lack of a consistent assay for assessing the inhibition of invasion induced by various vaccine formulations. There are 2 principal obstacles to develop such an assay. First, P vivax merozoites have a strict preference for reticulocytes,8 which represent only a minor and short-lived population in the peripheral blood. Second, given that P vivax cannot as yet be practically maintained in culture, blood from infected patients is the only routinely available P vivax source; however, the parasite density in these samples is generally too low for making meaningful observations.
In the late 1980s, 2 research groups independently developed a promising strategy to overcome these obstacles.9-12 The aim was to create optimal conditions for invasion by mixing highly enriched fractions of mature schizonts and host reticulocytes. In both cases, enrichment was achieved by centrifugation of whole blood or of ex vivo–matured, P vivax–infected RBCs (IRBCs) on density gradients. Mons et al assessed their protocol on P vivax isolates obtained from 13 patients11 or from P vivax–infected Aotus monkeys10 (Palo Alto strain) using reticulocytes from several sources including human cord blood. The efficiency of the invasion of enriched human reticulocytes (postinvasion parasitemias 0.2%-2.5%) was relatively low.11 The protocol of Barnwell and colleagues led to significantly better invasion efficiencies.9,12 Using blood from squirrel monkeys infected with the P vivax Belem strain, they obtained adequate parasitemia (∼ 3%-14%) after invasion of the reticulocytes enriched from peripheral human or simian blood.12 However, neither protocol was further optimized, or applied widely to investigations on P vivax invasion, or used in studies published after the 1980s.
Here, we present a protocol that enables the conduct invasion assays on fresh P vivax isolates, practicably, rapidly, and reproducibly.
Methods
Reticulocyte enrichment
Twenty milliliters of cord blood were collected on lithium heparin from umbilical cords immediately after the delivery of the child (n = 28). The blood group was determined using a standard ABO antisera kit. After plasma removal, the packed red cells were washed in McCoy 5A medium. Host white blood cells and platelets were depleted from the cord RBCs using 2 rounds of CF11 (Whatman) column filtration.13 Cord blood red cells were then adjusted to a 50% hematocrit using McCoy 5A medium, and the mixture split into 5-mL aliquots that were each carefully layered on a 6 mL 70% Isotonic Percoll cushion. After centrifugation for 15 minutes at 1200g, the resulting fine band of concentrated reticulocytes formed on the Percoll interface was carefully removed and washed twice in McCoy 5A medium. The washed and concentrated reticulocyte preparations were kept at 4°C in McCoy 5A medium at a 20% hematocrit, and were used for the invasion assays within 1 month of preparation. Before use, the proportion of reticulocytes (erythrocytes containing reticular matter) was determined by supravital staining with new methylene blue (Sigma-Aldrich). For some assays, the normocytes present in the pellet after the Percoll step were used for the invasion after 2 washes in complete McCoy 5A medium.
P vivax mature schizont enrichment
Between November 2008 and January 2011, blood samples from 98 P vivax– and 3 Plasmodium falciparum–infected patients attending the clinics of the Shoklo Malaria Research Unit (SMRU), Mae Sot region northwest of Thailand, were collected after written informed consent. The clinical samples examined in this study were collected under the following ethical guidelines in the approved protocols: OXTREC 027-025 (Center for Clinical Vaccinology and Tropical Medicine, University of Oxford, Oxford, United Kingdom) and MUTM 2008-215 from the Ethics Committee of the Faculty of Tropical Medicine (Mahidol University, Bangkok, Thailand). Patients, who took antimalarial or antimicrobial drugs within a month before sample collection, or whose admission parasitemia was < 0.1%, were excluded. The isolates where < 75% of the parasites were at the mature trophozoite development stage (∼ 20 hours after invasion in vivo) were excluded. The 5-mL blood samples collected by venepuncture on lithium-heparin were kept at room temperature for a maximum of 4 hours before transport to the culture laboratory at SMRU. White blood cells and platelets were removed using a CF11 column.13 The filtrate was then washed twice in McCoy 5A medium and the parasites present in the packed cells (∼ 1.5 mL per isolate) were cultured to the schizont stage in 12 mL of McCoy 5A medium supplemented with 2.4 g/L D-glucose, 40 mg/mL gentamycin sulfate, and 20% heat-inactivated human AB serum, in an atmosphere of 5% O2 at 37.5°C for 22 hours.
The resulting blood cells were recovered by centrifugation and resuspended in McCoy 5A medium to a 50% hematocrit before adding 3 mL of 0.125% w/v trypsin-versene in PBS and incubating for 15 minutes at 37.5°C. The trypsin digestion was halted by washing the cells once in McCoy 5A medium followed by addition of 1 mL of AB serum to the pellet and incubation for 5 minutes at room temperature. McCoy 5A medium was then added to the trypsinised IRBC pellet to a total volume of 5 mL, and the suspension was overlaid on a 45% Percoll (isotonic) cushion14 and centrifuged for 15 minutes at 1200g. The fine band of concentrated schizonts found on the Percoll interface was carefully removed and washed twice in McCoy 5A medium before use in the invasion assay. Enriched P falciparum schizonts were obtained from patient isolates following the protocol above except that a 70% Percoll cushion was used.
Abs
The mAb FY6, which recognizes the 2C3 epitope on the DARC N-terminal region located on the RBC surface membrane, was obtained as previously described.15 Polyclonal anti–P vivax Duffy-binding protein (PvDBP) IgG raised against a purified recombinant protein spanning regions II-IV of PvDBP from P vivax Sal 1 as previously described16 and prebleed control IgG, were purified by overnight incubation with protein G agarose (Millipore) followed by elution with a glycine solution and dialysed in PBS using Vivaspin (Sartorius Stedim).
Invasion assays
The concentrated mature schizont preparation was mixed with the enriched reticulocyte fraction at 1:6 ratio (thus, the starting schizont parasitemia of the invasion assays was ∼ 14%). The mixture was diluted to a 1.3% hematocrit in 300 μL of complete McCoy 5A medium and then cultured. Maturation was obtained after incubation in an atmosphere of 5% O2 at 37.5°C for an average of 24 hours, a period that was extended to 30 hours or curtailed to 20 hours depending on the extent of parasite maturation, which was assessed after 20 hours by microscopy. Abs were tested for inhibitory potential by adding the Fy6 mAb or the IgG purified from the anti-PvDBP polyclonal serum to the final invasion assay mixture to a final concentration of 25 μg/mL (FY6 mAb) or 100 μg/mL (anti-PvDBP IgG); purified prebleed rabbit IgG was used at a 100 μg/mL as a negative control. At the end of the incubation period, 4 thin smears (each made with 1 μL packed cells) were made, one of which was stained with Giemsa (Sigma-Aldrich) while the other 3 were air-dried and kept sealed with anhydrous silica gel for use in immunofluorescence assays. The number of rings/trophozoites in 4000 erythrocytes was determined by examining the Giemsa thin film smears by light microscopy. A thin smear was made before the incubation and the number of ring/trophozoite stage parasites was subtracted from that determined in the assay wells after incubation, and the resulting value was used to calculate the percentage of newly invaded RBCs, which we termed invasion efficiency.
To determine the “quantitation limit” for inhibition assays that have a 90% chance of detecting significant changes in parasitemia between 2 paired treatment groups (for example, Ab vs control), one needs to count at least 20 infected RBCs in the control. Therefore, for the standard thin film count of 4000 erythrocytes, the final parasitemia in the prebleed control should be ≥ 0.5%.
Immunofluorescence microscopy
Thin smear preparations of parasite samples were made on glass slides and then fixed with cold acetone for 15 minutes. After drying, the slides were blocked with 3% BSA in PBS for 30 minutes at 37.5°C in a humidified incubator. The primary anti-PvDBP Ab was then applied and incubated for 60 minutes at 37°C in a humidified incubator. Slides were then washed 3 times with PBS before addition of goat anti–rabbit IgG conjugated to Alexa Fluor 647 (Molecular Probes) at a concentration of 4 μg/mL together with DAPI (250 ng/mL) and incubation for 60 minutes. Binding was visualized using an Olympus FV1000 confocal microscope after 3 washes in PBS, rinsing in distilled water and mounting in FluorSave (Calbiochem).
The percentage of IRBCs that contained hemoglobin F (HbF) after the incubation period, was determined in dried thin smears fixed in an acetone: ethanol: methanol (6:2:2 v/v) using a simple single-step immunofluorescence assay17 using a FITC linked anti-HbF Ab (BD Biosciences).
Amplification and sequencing of the PvDBP region II
Genomic DNA was extracted from an aliquot (200 μL) of the admission blood sample using the DNeasy Blood and Tissue kit (QIAGEN). PCR amplification was performed on 1 μL of the purified genomic DNA in a total volume of 50 μL, containing 1× PCR buffer, 2.5mM MgCl2, 0.2mM of each dNTP, 2μM of each primer (forward 5′-gtgactgggcatgagggaaattctcg and reverse 5′-gcgtagaatctcctggaaccttctcc), 1.25 U of AmpliTaq Gold DNA polymerase (Applied Biosystems). The cycling conditions were: 95°C for 5 minutes followed by 30 cycles at 94°C for 1 minute, 63°C for 2 minutes, and 72°C for 2 minutes. The product was purified using the QIAquick PCR purification kit (QIAGEN) and then sequenced commercially.
Statistical analysis
Comparison of 2 sets of nonparametric data (see Figure 2) was analyzed by a Mann-Whitney test. Nonparametric comparisons between more than 2 unmatched and matched observations used Kruskal-Wallis and Friedman tests, respectively. Associations between ratio data presented in Figure 4A used a Fisher exact test. A 2-way repeated measures ANOVA was used to compare the data presented in Figure 4B. Posthoc analysis used a Dunn test (see Figures 5, 6). Parameters for the power calculation were as follows: power = 0.9, significance level (adjusted for sidedness) = 0.025, SD = 2%, location of mean in one group as a percentile of the other group = 0.93. All statistical analysis used Prism 5 for Windows (Version 5.01), Mackiev software.
Results
Concentration of human reticulocytes
Relatively modest reticulocyte enrichment was considered to be an important impediment to efficient invasion rates.11 Therefore, we opted for cord blood as a source of reticulocytes because it contains a significantly higher percentage of immature RBCs than peripheral blood. We considered it important to deplete leukocytes to minimize phagocytosis of merozoite or IRBCs during the assay.18 This depletion, achieved using CF11 filtration,13 was best carried out before Percoll enrichment. It was necessary to perform 2 rounds of CF11 filtration to bring lymphocyte contamination to undetectable levels. We then optimized Percoll gradients for the enrichment of reticulocytes from the leukocyte-depleted cord blood. Using the new methylene blue stain (Sigma-Aldrich) to identify reticulocytes, we established that optimal reticulocyte enrichment is obtained on a 70% Percoll cushion. A mean of 57.8% reticulocytes (95% confidence interval [CI]: 51.1%-64.5%) was routinely obtained from cord blood that initially contained an average of 4.6% (95% CI: 3.9%-5.2%) reticulocytes (Figure 1). Generally, 20 mL of whole cord blood yielded a mean 191 μL (95% CI: 152 μL-230 μL; n = 28) of packed reticulocyte-enriched blood.
An overview of the assay methodology. Thin smears stained with new methylene blue (A-B) or Giemsa (D-G) illustrating the key methodologic steps of the ex vivo P vivax invasion assay. (A) Cord blood at collection contains a mixture of normocytes, reticulocytes, and leukocytes. (B) After 2 rounds of leukocyte depletion on a CF-11 cellulose column, reticulocytes form the majority of the cells present in the band (seen at the 5.5-mL mark on the tube pictured in panel C) obtained by enrichment on a 70% Percoll cushion. (D) Thin smear showing mature trophozoites (black arrows) and leukocytes typically observed from P vivax isolates collected from patients. (E) Typical yield of concentrated mature P vivax parasites obtained on a 45% Percoll cushion (from a band similar to the one depicted in panel C after a single round of leukocyte depletion on CF-11 and 20 hours ex vivo maturation). (F) The concentrated reticulocyte target cells (B) and mature P vivax schizonts (E) are mixed at a ratio of 6:1 for the invasion assay. (G) After culturing for ∼ 24 hours, the invasion assay mixture shows remnants of ruptured schizonts (*), and RBCs newly invaded by one (black arrow) or multiple merozoites (orange arrowhead). Black scale bar on the micrographs corresponds to 10 μm.
An overview of the assay methodology. Thin smears stained with new methylene blue (A-B) or Giemsa (D-G) illustrating the key methodologic steps of the ex vivo P vivax invasion assay. (A) Cord blood at collection contains a mixture of normocytes, reticulocytes, and leukocytes. (B) After 2 rounds of leukocyte depletion on a CF-11 cellulose column, reticulocytes form the majority of the cells present in the band (seen at the 5.5-mL mark on the tube pictured in panel C) obtained by enrichment on a 70% Percoll cushion. (D) Thin smear showing mature trophozoites (black arrows) and leukocytes typically observed from P vivax isolates collected from patients. (E) Typical yield of concentrated mature P vivax parasites obtained on a 45% Percoll cushion (from a band similar to the one depicted in panel C after a single round of leukocyte depletion on CF-11 and 20 hours ex vivo maturation). (F) The concentrated reticulocyte target cells (B) and mature P vivax schizonts (E) are mixed at a ratio of 6:1 for the invasion assay. (G) After culturing for ∼ 24 hours, the invasion assay mixture shows remnants of ruptured schizonts (*), and RBCs newly invaded by one (black arrow) or multiple merozoites (orange arrowhead). Black scale bar on the micrographs corresponds to 10 μm.
Concentration of mature P vivax IRBCs
The IRBC levels in the peripheral blood samples collected from P vivax–infected patients rarely exceed 50 000/μL. Moreover, the circulating parasites are often at different developmental stages of their 48-hour erythrocytic cycle. Thus, an ex vivo maturation step before enrichment is needed to increase the proportion of mature schizonts. A total of 98 isolates were collected from malaria patients with microscopically confirmed P vivax at several SMRU clinics on the Thai-Myanmar border. The mean parasitemia observed in these samples was 0.41% (95% CI: 0.36%-0.44%). The samples were processed for the invasion assay within 6 hours after collection. A single round of leukocyte depletion on CF11 columns was carried out before initiating the ex vivo maturation step. Eighty-five isolates of the 98 collected were successfully cultured to late schizogony, the remaining failed to mature or became contaminated. We then established that a 45% Percoll cushion best concentrated the mature parasites.14
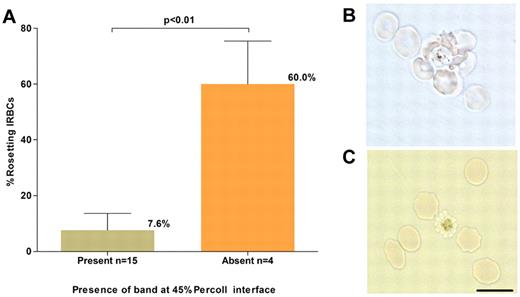
In preliminary experiments, it was noted that no band was obtained in the 45% Percoll cushion for some P vivax isolates despite successful parasite maturation (R. Suwanarusk, unpublished observations, December 20, 2008). Phase contrast microscopic examination of unstained and unfixed wet preparations of the IRBCs collected from the Percoll gradient pellet of these isolates revealed numerous rosetting forms, a phenomenon in which uninfected RBCs cluster to a single mature IRBC.19,20 This explained the failure to concentrate these matured parasites on Percoll because trypsin treatment of the pelleted parasites abrogated rosette formation, and the schizonts present were then successfully concentrated on the 45% Percoll cushion. The occurrence of rosetting was tested in a subset of 19 isolates collected for this study; 4 (21%) failed to yield the expected band on the Percoll cushion. For these isolates, the mean percentage of rosetting forms observed before Percoll enrichment was high 60% (SD: 15.5%), while for the isolates that yielded the expected band on the Percoll cushion it was much lower, 7.6% (SD: 6.1%; Figure 2) Therefore, trypsin treatment before Percoll cushion enrichment was performed for all ex vivo–matured P vivax isolates.
P vivax rosetting and its effect on schizont concentration. (A). The relationship between the percentage of rosetting P vivax IRBCs (in the 20-hour matured isolates) before concentration and presence or absence of a concentrated schizont band at the 45% Percoll interface. (B) A micrograph of an unstained wet preparation showing a P vivax rosette (more than one RBC attached to a central IRBC) collected from the pellet of a isolate that did not yield a band on the Percoll cushion. (C) Rosetting forms were not observed following treatment of the pellet with trypsin, and a band was subsequently obtained on the Percoll cushion. Black scale bar corresponds to 10 μm for both micrographs.
P vivax rosetting and its effect on schizont concentration. (A). The relationship between the percentage of rosetting P vivax IRBCs (in the 20-hour matured isolates) before concentration and presence or absence of a concentrated schizont band at the 45% Percoll interface. (B) A micrograph of an unstained wet preparation showing a P vivax rosette (more than one RBC attached to a central IRBC) collected from the pellet of a isolate that did not yield a band on the Percoll cushion. (C) Rosetting forms were not observed following treatment of the pellet with trypsin, and a band was subsequently obtained on the Percoll cushion. Black scale bar corresponds to 10 μm for both micrographs.
The matured parasite fractions obtained from the 85 isolates successfully enriched on the Percoll gradient were composed of 93.8% P vivax IRBCs (95% CI: 93.7%-97.0%), and contained predominantly fully mature schizonts (95.9%, 95% CI: 93.7%-97.0%), with a minor component of gametocytes (3.62%, 95% CI: 1.8%-5.4%) and trophozoites (0.4%, 95% CI: 0.1%-0.7%; Figure 1). The mean volume of the P vivax schizont concentrates obtained from a 5-mL isolate was 4.5 μL (95% CI: 3.2 μL-5.8 μL).
Ex vivo invasion assay
For the invasion assay, enriched reticulocytes and mature P vivax schizonts were incubated together for 24 hours, and newly infected RBCs were determined by microscopy. Only ring and early trophozoites were enumerated because these forms alone were considered to result from invasion events that occurred during the incubation. Any mature schizonts, gametocytes, degenerate parasites, or forms with evidence of nuclear division that were occasionally observed were excluded when calculating invasion efficiency.
At present a standard volume of 300 μL of medium with 1.4% hematocrit was used for Plasmodium invasion assays, principally because it would have been difficult to obtain thin smears of sufficient quality to enumerate parasites at a lower volume or hematocrit. In preliminary experiments, the invasion efficiency decreased when the number of host cells per parasite increased above 6 (data not shown). Therefore, a maximum of 6 invasion assays could be undertaken from the enriched parasite fraction (4.5 μL) originating from the 5 mL of blood sample collected from each patient. On average, a total of 55 assays are possible from the enriched reticulocyte fraction (191 μL) originating from each 20-mL cord blood sample.
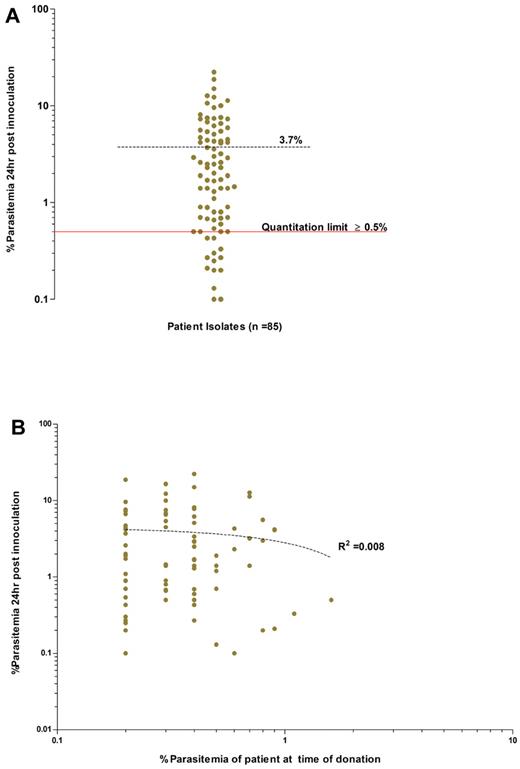
Successful invasion assays were conducted for the parasites enriched from 85 patients. Invasion efficiency was expressed as the percentage of RBCs with rings/trophozoites (ie, newly invaded cells) per 4000 target host cells following the 24-hour incubation. The invasion efficiencies observed were highly variable, from 0.1% to 22.3%, with a mean of 3.7% (95% CI: 2.8%-4.6%; Figure 3). Such variability may be because of one or several factors related to either reticulocyte-enriched fraction (target cell), or the schizont-enriched P vivax inoculum, or to both. Despite this variability, 83% (71 of 85) of the isolates were equal to or above the “quantitation limit” threshold of 0.5% (Figure 3A) and would have proven to be useful in invasion assays.
Overall invasion efficiencies of the P vivax field isolates used in the invasion assays. (A) Average invasion efficiency of enriched ex vivo–matured P vivax schizonts isolated from 85 isolates in concentrated reticulocytes collected from 28 cord blood isolates (experiments conducted in triplicate). The mean percentage of newly invaded RBCs (dashed black line) after ∼ 24 hours of incubation was 3.7% (95% CI: 2.8-4.7%, range 0.1-22.3%). Isolates with an invasion efficiency above the quantitation limit of ≥ 0.5% parasitemia (solid red line), provide those conducting assay with a 90% (power = 0.9) chance of detecting significance changes in parasitemia between 2 paired treatment groups (for example, Ab vs control). (B) Absence of any relationship between the ex vivo invasion efficiency and the in vivo admission parasitemia for each isolate (dotted line, goodness of fit: R2 = 0.008, P = .397).
Overall invasion efficiencies of the P vivax field isolates used in the invasion assays. (A) Average invasion efficiency of enriched ex vivo–matured P vivax schizonts isolated from 85 isolates in concentrated reticulocytes collected from 28 cord blood isolates (experiments conducted in triplicate). The mean percentage of newly invaded RBCs (dashed black line) after ∼ 24 hours of incubation was 3.7% (95% CI: 2.8-4.7%, range 0.1-22.3%). Isolates with an invasion efficiency above the quantitation limit of ≥ 0.5% parasitemia (solid red line), provide those conducting assay with a 90% (power = 0.9) chance of detecting significance changes in parasitemia between 2 paired treatment groups (for example, Ab vs control). (B) Absence of any relationship between the ex vivo invasion efficiency and the in vivo admission parasitemia for each isolate (dotted line, goodness of fit: R2 = 0.008, P = .397).
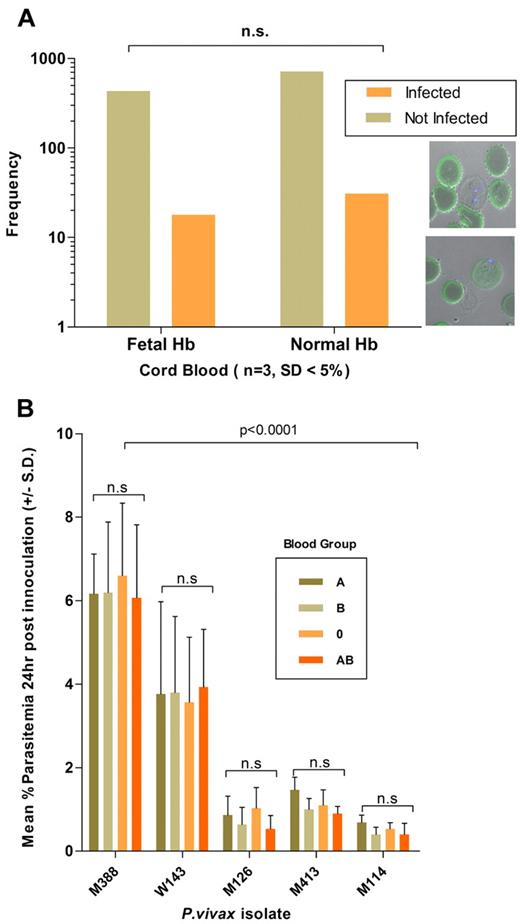
Invasion efficiency was not influenced by the proportion (generally above 50%) of reticulocytes in the inoculum added to the assay (data not shown), nor by the storage period of the host target cell fraction used, which could extend to a month at 4°C without significant reticulocyte loss (data not shown). Hemoglobin F, present in a variable proportion of cord blood, did not affect the invasion efficiency (n = 3 isolates, Fisher exact test, P = 1.00) in the target cells (Figure 4A). Finally, invasion efficiency remained constant when a specific isolate (n = 5) was tested side-by-side in reticulocytes originating from donors with different ABO blood groups (Figure 4B). This latter experiment showed that 85.79% of the total variance is dependent on the isolate type (2-way repeated measures ANOVA; P < .0001, F distribution [F] = 60.1, degrees of freedom [DF] 4, 10) while the host cell blood group only accounted for only 0.17% of the total variability in invasion efficiency (P = .9, F = 0.16, DF 3, 30). Finally, the invasion efficiency did not correlate with the patient's admission parasitemia (Figure 3B; R2 = 0.008, P = .39).
Effect of host cell attributes on invasion efficiency. (A) P vivax invasion efficiency, determined from 1200 target cells (n = 3 isolates), was not significantly associated with the presence of fetal Hb (stained in green). The bottom right inset shows a P vivax (nucleus stained with Dapi blue) in a newly invaded RBCs devoid (top micrograph) or containing fetal Hb. (B) The P vivax invasion efficiency (n = 5 isolates) was not affected by the ABO blood group of the reticulocytes used in the invasion assay. Invasion efficiencies were significantly associated with the particular isolate used (isolates M388 and W143 consistently invaded the distinct target cell preparation at a higher efficiency than that observed for isolates M126, M413, and M114).
Effect of host cell attributes on invasion efficiency. (A) P vivax invasion efficiency, determined from 1200 target cells (n = 3 isolates), was not significantly associated with the presence of fetal Hb (stained in green). The bottom right inset shows a P vivax (nucleus stained with Dapi blue) in a newly invaded RBCs devoid (top micrograph) or containing fetal Hb. (B) The P vivax invasion efficiency (n = 5 isolates) was not affected by the ABO blood group of the reticulocytes used in the invasion assay. Invasion efficiencies were significantly associated with the particular isolate used (isolates M388 and W143 consistently invaded the distinct target cell preparation at a higher efficiency than that observed for isolates M126, M413, and M114).
RBCs that contained more than 1 P vivax ring or trophozoites were consistently observed in about a third of all newly infected cells (Figures 1, 5). Invasion efficiency was expressed in terms of total numbers of infected target cells, rather than in terms of the number of merozoites having successfully invaded a target cell. The occurrence and the proportion of such multiple invasions did not correlate with invasion efficiency because their frequency varied little between the 85 isolates tested.
Inhibition of P vivax invasion by a mAb (25 μg/mL) specific to the 2C3 epitope of DARC, the obligatory receptor for P vivax merozoites. (A) Inhibition of P vivax (n = 10 isolates) invasion was near-total (mean 7.5% SD ± 2.9 vs 0.15% SD ± 0.28, P < .01, Wilcoxon sum of signed ranks [W] = 55.), whereas little inhibition was observed (P = .25, W = 6.0) when P falciparum (n = 3 isolates) were used in the invasion assay. The assays were conducted in triplicate for each isolate. (B) Representative micrographs are shown for the (i-ii) P vivax or (iii-iv) P falciparum invasion assays (annotations as in Figure 1). Remnants of ruptured schizonts (*), and RBCs newly invaded by 1 (black arrow) or multiple merozoites (orange arrowhead). Black scale bars on micrograph represent 10 μm.
Inhibition of P vivax invasion by a mAb (25 μg/mL) specific to the 2C3 epitope of DARC, the obligatory receptor for P vivax merozoites. (A) Inhibition of P vivax (n = 10 isolates) invasion was near-total (mean 7.5% SD ± 2.9 vs 0.15% SD ± 0.28, P < .01, Wilcoxon sum of signed ranks [W] = 55.), whereas little inhibition was observed (P = .25, W = 6.0) when P falciparum (n = 3 isolates) were used in the invasion assay. The assays were conducted in triplicate for each isolate. (B) Representative micrographs are shown for the (i-ii) P vivax or (iii-iv) P falciparum invasion assays (annotations as in Figure 1). Remnants of ruptured schizonts (*), and RBCs newly invaded by 1 (black arrow) or multiple merozoites (orange arrowhead). Black scale bars on micrograph represent 10 μm.
Ab inhibition assays
To validate whether the invasion assay can measure Ab-mediated inhibition of invasion, a mAb (mAb FY6) raised against a specific surface-exposed DARC epitope, the obligatory host cell receptor for P vivax merozoite invasion, was added to the assay. Almost total inhibition (99%) of invasion was obtained by adding 25 μg/mL AB FY6 to assays conducted with parasites enriched from 10 different P vivax and reticulocytes enriched from 6 different donors (Figure 5). The inhibition was specific to P vivax invasion because adding 25 μg/mL mAb FY6 to an assay where P falciparum–enriched schizonts were used did not significantly (8% inhibition) affect the invasion of the target cells (Figure 5).
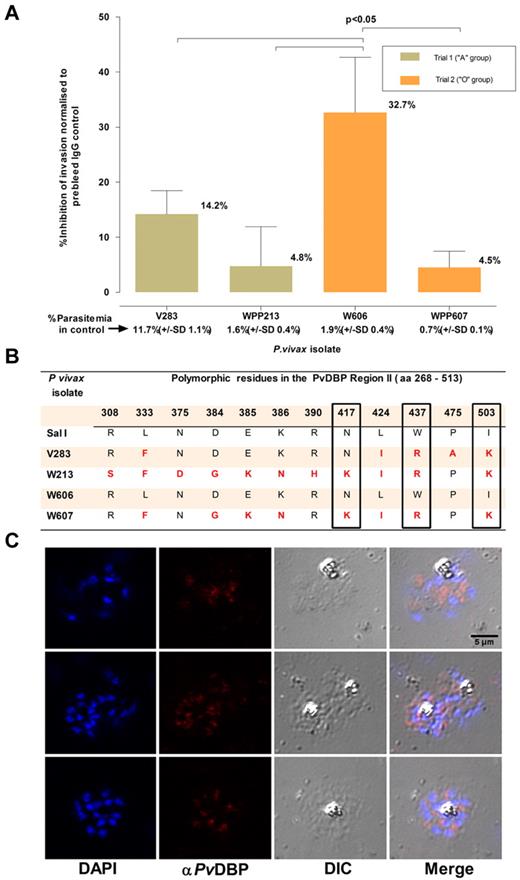
The ability of the assay to measure invasion inhibitory activity was ascertained by using a polyclonal Ab (purified IgG fraction) against a recombinant protein that encompasses regions II-IV of the PvDBP. This protein, a leading vaccine candidate, is located in the apical region of the P vivax merozoite (Figure 6C) and is the ligand for DARC. Region II of PvDBP, which is considered to contain the domain that binds DARC,16 is polymorphic. Four isolates were tested for invasion inhibition by this polyclonal Ab at a final concentration of 100 μg/mL. Significant inhibition (32.7%, SD: 10.0%, P < .01) was only observed for one of these isolates (W606, Figure 6). It was interesting to note that although the PvDBP region II predicted amino acid sequence differed among the 4 isolates, isolate W606 alone had a sequence identical (Figure 6) to that of the P vivax Salvador 1 (Sal1) line genome, against which the polyclonal serum was raised.16
Inhibition of P vivax invasion by anti-PvDBP II IgG Abs. (A) The mean inhibition (percentage ± % SD) of anti–PvDBP II IgG Abs (100 μg/mL) on the P vivax invasion efficiency. The inhibition was normalized to invasion efficiencies obtained in the presence of IgG Abs (100 μg/mL) purified from a rabbit prebleed serum. The actual mean invasion efficiencies (percentage ± % SD) in the prebleed controls are included below the corresponding isolate identifier. The assays were conducted in triplicate on 4 isolates in 2 separate experiments involving reticulocyte concentrates from 2 cord blood samples of different ABO type. (B) Polymorphisms in the predicted amino acid sequence of the PvDBP region II region in the parasites present in the 4 isolates used in the assay. The sequences were compared with that present in the P vivax reference strain (Sal 1). Nonsynonymous mutations at 3 residues (417, 437, and 503) that were previously suggested to interfere with the inhibition of PvDBP region II binding to DARC are enclosed in black frames. (C) Immunofluorescence microscopy with anti–P vivax DBPII IgG reacted with merozoites from a P vivax isolate (3 examples given) used in this assay.
Inhibition of P vivax invasion by anti-PvDBP II IgG Abs. (A) The mean inhibition (percentage ± % SD) of anti–PvDBP II IgG Abs (100 μg/mL) on the P vivax invasion efficiency. The inhibition was normalized to invasion efficiencies obtained in the presence of IgG Abs (100 μg/mL) purified from a rabbit prebleed serum. The actual mean invasion efficiencies (percentage ± % SD) in the prebleed controls are included below the corresponding isolate identifier. The assays were conducted in triplicate on 4 isolates in 2 separate experiments involving reticulocyte concentrates from 2 cord blood samples of different ABO type. (B) Polymorphisms in the predicted amino acid sequence of the PvDBP region II region in the parasites present in the 4 isolates used in the assay. The sequences were compared with that present in the P vivax reference strain (Sal 1). Nonsynonymous mutations at 3 residues (417, 437, and 503) that were previously suggested to interfere with the inhibition of PvDBP region II binding to DARC are enclosed in black frames. (C) Immunofluorescence microscopy with anti–P vivax DBPII IgG reacted with merozoites from a P vivax isolate (3 examples given) used in this assay.
Discussion
We present a standardized protocol for routine ex vivo P vivax invasion assays that was validated on P vivax isolates obtained directly from patients. The assay is highly reproducible, yielding informative invasion data from 85 of 98 P vivax isolates, and can be established as a routine procedure in field-based laboratories with limited resources. The equipment required for all steps is limited to a microscope, a bench-top centrifuge, and a simple 37°C incubator (P vivax maturation can be performed in a candle jar21 ).
The methodology we present has relatively few limitations: (1) the assay must be conducted on the day the infected blood is collected; (2) for some isolates (14 of 85) the efficiency of the invasion was relatively low (< 0.5%), necessitating more time for the enumeration of the newly invaded RBCs; and (3) the number of assays that can be conducted from a single isolate is small (about 6 assays from a 5-mL blood sample) because of the need to obtain good quality postincubation thin blood smears for counting newly invaded RBCs accurately. We are currently testing various strategies to minimize these limitations. A protocol for the cryopreservation of infected blood samples that would allow the maturation step to be carried out at a later date of choice is under development. This would complement the flexibility afforded by the ability to store enriched reticulocyte fractions for a month without loss in invasion efficiency, increasing the flexibility of the assay. Larger volumes (> 5 mL) of infected blood collected would increase the potential number of assays from a single isolate, though this would restrict the sampling to adults. We are exploring automated cell counting in field settings as a means to decrease the quantity of enriched P vivax schizonts needed for each assay. This would increase up to 60 the number of assays possible from a 5-mL blood sample. Automated counting of newly invaded RBCs would also obviate the need for time-consuming parasite enumeration by microscopy, especially for those P vivax isolates with low invasion efficiencies.
The availability of a reliable P vivax invasion assay will be highly beneficial for the development of vaccines against P vivax. Currently, the evaluation of induced immune responses targeting P vivax merozoite invasion is limited by the fact that it can only be conducted in a restricted number of costly primate models. The ex vivo invasion assay reported here provides a cheap and practicable alternative to the primate models. The leading P vivax anti-erythrocytic stage vaccine candidate5,6 is PvDBP, which is the ligand for DARC, the obligate receptor for invasion. One concern is that the majority of the recorded polymorphisms in PvDBP map to the region II domain that mediates binding to DARC.22-26 In the absence of an informative invasion assay, variations in the binding of PvDBP to DARC are used as a surrogate for invasion efficiency.6 Although the binding strengths in these experiments were correlated with a subset of polymorphic sites,23 it is not known whether, or to what degree, they correlate with the actual P vivax invasion efficiency.
In an attempt to address this issue, an assay measuring invasion in 2 ex vivo–matured clinical P vivax isolates, simply incubated for 24 hours with or without anti-PvDBP region II polyclonal Abs, was recently described.27 The RBC invasion inhibition observed was partial (64%, n = 2 isolates), but the statistical reliability of the assay was doubtful because of the very low invasion efficiencies (< 0.05%), which was confounded by the ambiguity in distinguishing truly newly invaded host cells. Moreover, the sequence encoding for region II in the P vivax from these 2 isolates was not described.27
The usefulness of our ex vivo invasion assay to vaccine development was provided, as a proof-of-principle, by a preliminary functional characterized of polyclonal Abs against a PvDBP fragment bearing region II on parasites from 4 P vivax isolates (Figure 6). We could not perform a dose-response analysis for inhibition because of the limited amount of material available. The invasion inhibitions observed in the presence of high levels of specific IgG (100 μg/mL) were modest similar to anti-EBA175 serum IgG inhibition of P falciparum laboratory strains.28 Merozoites from the different P vivax isolates were clearly differentially susceptible to invasion inhibition. When the predicted amino acid sequence of the PvDBP region II in the parasites from the 4 isolates tested was obtained, the parasites from the most inhibited isolate (W606) had a sequence identical to that of the Sal1 PvDBP construct used to raise the polyclonal Abs. It was of particular interest that there was a negative correlation between levels of inhibition and mutations in DBP region II of the isolates tested. Three residues (417, 437, and 503 shown in Figure 6B) that were previously suggested to interfere with the inhibition of PvDBP region II binding to DARC23 by the polyclonal Abs were found in the parasites from the 3 isolates least inhibited by these Abs. Furthermore, there was a clear dichotomy in sensitivity to Ab inhibition and sequence identity in the most polymorphic human B cell epitope of PvDBP, residues 384-386 (“DEK”).29 These observations support the notion that PvDBP region II polymorphisms are biologically and immunologically functional. It will now be possible to formulate a strategy based on our ex vivo assay to test sera induced by experimental PvRBP-based vaccine formulations on a broad range of P vivax isolates. This type of approach can also be equally applied to other potential vaccine candidates targeting invasion. Moreover, the assay can also serve to identify compounds that inhibit P vivax invasion of red cells.
In P vivax, observations of rosetting have been limited to 2 studies, in which rosettes were observed in most isolates collected from patients admitted to the Tropical Disease Hospital (Mahidol University, Bangkok, Thailand).30,31 We also observed rosettes in all 19 isolates we examined, and these fell into 2 distinct groups: the majority were “low rosetters” (7.6% of infected RBCs, SD: 6.1%), while only 4 were “high rosetters” (60%, SD: 15.5%). There was no correlation between rosetting, admission parasitemia, and invasion efficiency, though the number of isolates tested was low. Importantly, the presence of this significant proportion of “high-rosetting” isolates in the Thai P vivax population necessitates the novel trypsinization step outlined in this protocol. Further studies will be needed to establish whether similar patterns occur in P vivax populations in other endemic areas, or whether a high-rosetting rate is of any biologic or clinical significance.
In a previous study, observations of a higher than expected proportion of RBCs infected with more than one parasite in smears collected from 19 of 20 P vivax-infected patients suggested that about 13% of the RBCs are susceptible to invasion by P vivax, thereby extending the RBC preference of this parasite to young normocytes.32 This was not the case for the cord blood used for our invasion assay. Indeed, when 32 P vivax isolates were tested in parallel using the enriched reticulocyte fraction, which account for 2% of the RBCs present in the cord blood, and the matched normocyte pellets from the Percoll cushion (the remaining 98%), the mean invasions efficiency for the normocytes (0.09%, 95% CI: 0.02%-0.15%) was 54-fold lower than that for the enriched reticulocytes (4.9%, 95% CI: 3.1%-6.7%). A selectivity index32 was not calculated because the invasion events in the assay occur under static conditions precluding comparison with those occurring in vivo.
The ex vivo invasion assays were carried out for 85 P vivax isolates in the absence of the patient serum or host cells, yet the invasion efficiencies observed (Figure 3A) varied by 2 orders of magnitude (range 0.1%- 22.3%). The variations did not correlate with the nature or origin of the enriched reticulocytes invaded (Figure 4). Interestingly, the ex vivo invasion efficiencies did not correlate with the patient's parasite density either (Figure 3B). Therefore, the ex vivo invasion phenotype cannot be used as surrogate indicator for clinical virulence. It would thus appear that the P vivax populations circulating during a clinical episode display a remarkably wide range of invasive potential. It would be of fundamental biologic interest to confirm this observation, and then to investigate whether invasion efficiency varies during the course of infection by a single parasite line, or whether it is genetically determined, or a combination of these.
In conclusion, the ex vivo P vivax invasion assay presented here will significantly contribute to a broad range of investigations that are ultimately aimed at developing effective measures to control and then eliminate a major scourge of mankind.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the patients and staff of the SMRU for their contribution to this study. SMRU is sponsored by the Wellcome Trust of Great Britain, as part of the Oxford Tropical Medicine Research Program of Wellcome Trust–Mahidol University.
This study received funding from the Agency for Science Technology and Research (A*STAR; Singapore) and the Wellcome Trust (United Kingdom). L.R. and G.S. are currently part of an official collaboration between SIgN/A*-STAR and Inserm (Laboratoire International Associé, Inserm). C.B. and U.D. were supported by ITM Secondary Research Funding (SOFI-B).
Wellcome Trust
Authorship
Contribution: B.R. and R.S. conceptualized the study, designed and performed research, analyzed data, and wrote the manuscript; C.B. designed and performed research, analyzed data, and contributed to manuscript writing; F.T.M.C. contributed vital new methods/ideas and contributed to manuscript writing; C.S.C. and M.J.R. were involved in patient care and ethical sample collection processing, and contributed to manuscript writing; K.S. performed research and analyzed data; L.W., E.G.L.K., and B.M. were involved in research planning, reagent processing, performing the research, and contributing to manuscript writing; Y.C., O.B., and J.H.A. contributed vital new hematologic reagents (custom Abs and sera) and analytical tools, analyzed data, and contributed to manuscript writing; U.D. co-directed research, analyzed data, and contributed to manuscript writing; G.S. co-directed research, analyzed data, and wrote the manuscript; F.N. directed all aspects of the clinical work, obtained ethics clearance, and co-directed research; and L.R. co-directed scientific work, designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce Russell, Laboratory of Malaria Immunobiology, Singapore Immunology Network (SIgN), 8A Biomedical Grove, Immunos, Singapore 138648; e-mail: bruce_russell@immunol.a-star.edu.sg.
References
Author notes
B.R. and R.S. contributed equally to this study.


![Figure 5. Inhibition of P vivax invasion by a mAb (25 μg/mL) specific to the 2C3 epitope of DARC, the obligatory receptor for P vivax merozoites. (A) Inhibition of P vivax (n = 10 isolates) invasion was near-total (mean 7.5% SD ± 2.9 vs 0.15% SD ± 0.28, P < .01, Wilcoxon sum of signed ranks [W] = 55.), whereas little inhibition was observed (P = .25, W = 6.0) when P falciparum (n = 3 isolates) were used in the invasion assay. The assays were conducted in triplicate for each isolate. (B) Representative micrographs are shown for the (i-ii) P vivax or (iii-iv) P falciparum invasion assays (annotations as in Figure 1). Remnants of ruptured schizonts (*), and RBCs newly invaded by 1 (black arrow) or multiple merozoites (orange arrowhead). Black scale bars on micrograph represent 10 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/13/10.1182_blood-2011-04-348748/4/m_zh89991177610005.jpeg?Expires=1769443185&Signature=3S16fi~Qdejp-tkoSS4CR22~hs7QfG1xiy~IzLRsrEJzObpmikOyie9o1YqwTrd0WwFUxgUzVwr43VI4MAzlN3cP1AXyr7O46ZKWyTjoGWnUY~nWm8kdDJ0Vjgm4l4VvjDt8y6Im9UYf2Vjcbh4XWbJ1uByYYBdExPnbsbCi-~9M2MYRIRdYTOv0M0mkhpXvtw3IZ~kPD-zNlJvO0h4UajO4hfe60m~kgrT6EvDwq7rIAZIzQUI1FaamxCdwnVUXlYIrqn1N4zoGY87Lg8uxQTPi5lxltyjTO9uYTNUDcIm6xbKPXCrdmSIwj0z8p7NgJcIXq1Ub7lIvDAQSng9wkw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)