Abstract
This phase 3, randomized, double-blind study assessed the efficacy and safety of lenalidomide in 205 red blood cell (RBC) transfusion-dependent patients with International Prognostic Scoring System Low-/Intermediate-1-risk del5q31 myelodysplastic syndromes. Patients received lenalidomide 10 mg/day on days 1-21 (n = 69) or 5 mg/day on days 1-28 (n = 69) of 28-day cycles; or placebo (n = 67). Crossover to lenalidomide or higher dose was allowed after 16 weeks. More patients in the lenalidomide 10- and 5-mg groups achieved RBC-transfusion independence (TI) for ≥ 26 weeks (primary endpoint) versus placebo (56.1% and 42.6% vs 5.9%; both P < .001). Median duration of RBC-TI was not reached (median follow-up, 1.55 years), with 60% to 67% of responses ongoing in patients without progression to acute myeloid leukemia (AML). Cytogenetic response rates were 50.0% (10 mg) versus 25.0% (5 mg; P = .066). For the lenalidomide groups combined, 3-year overall survival and AML risk were 56.5% and 25.1%, respectively. RBC-TI for ≥ 8 weeks was associated with 47% and 42% reductions in the relative risks of death and AML progression or death, respectively (P = .021 and .048). The safety profile was consistent with previous reports. Lenalidomide is beneficial and has an acceptable safety profile in transfusion-dependent patients with Low-/Intermediate-1-risk del5q myelodysplastic syndrome. This trial was registered at www.clinicaltrials.gov as #NCT00179621.
Introduction
International Prognostic Scoring System (IPSS)1 Low- or Intermediate-1-risk myelodysplastic syndromes (MDS) with a 5q deletion (del5q) cytogenetic abnormality is characterized by macrocytic anemia and short response duration when treated with erythropoiesis-stimulating agents.2-4 Anemia negatively affects quality of life (QoL) and disease course,5 with red blood cell (RBC) transfusion dependency and subsequent iron overload associated with poor outcomes in patients with MDS.6,7
Lenalidomide is an immunomodulatory agent with multiple mechanisms of action, which are thought to include the direct targeting of MDS clones, immunomodulation, erythropoiesis restoration, and angiogenesis inhibition.8-11
In a phase 2 study of lenalidomide 10 mg in 148 RBC transfusion-dependent patients with IPSS Low- or Intermediate-1-risk MDS with del5q31, with or without additional cytogenetic abnormalities, 67% of patients achieved RBC transfusion independence (TI) for ≥ 8 weeks, and 73% (62 of 85) experienced cytogenetic improvement, including 45% (38 of 85) complete cytogenetic remissions.12 However, because this was a single-arm study, comparisons with placebo could not be made.
The current phase 3 study is the first randomized, placebo-controlled study of lenalidomide in MDS. It compares the efficacy and safety of lenalidomide (10 mg and 5 mg) against placebo in RBC transfusion-dependent patients with IPSS Low- or Intermediate-1-risk MDS with del5q31.
Methods
This phase 3, multicenter, randomized, double-blind, placebo-controlled study enrolled patients from July 8, 2005 to June 26, 2007 at 37 study sites in the United Kingdom, France, Germany, Italy, Spain, Belgium, The Netherlands, Sweden, and Israel (registered at www.clinicaltrials.gov as #NCT00179621). The study conformed to the Declaration of Helsinki and was approved by individual institutional review boards of all participating institutions. All patients provided written informed consent.
Inclusion and exclusion criteria
Patients 18 years of age or older with investigator-documented IPSS Low- or Intermediate-1-risk MDS with del5q31, with or without additional cytogenetic abnormalities, and RBC transfusion-dependent anemia (no 8 consecutive weeks without RBC transfusions within the 16 weeks before randomization) were included. Confirmation of del5q31 status (karyotype analysis) and bone marrow morphology was performed by central hematologic review after randomization. Exclusion criteria included: proliferative (white blood cell count ≥ 12 000/μL) chronic myelomonocytic leukemia; grade ≥ 2 neuropathy; prior use of lenalidomide; use of recombinant erythropoietin (EPO), chemotherapy, or treatment with any other investigational agent within the last 28 days or long-acting erythropoiesis-stimulating agents within the last 8 weeks; and abnormal laboratory values (absolute neutrophil count < 500/μL, platelet count < 25 000/μL, serum creatinine > 2.0 mg/dL, serum transaminases > 3.0 × upper limit of normal [unless resulting from iron overload from blood transfusions], and serum total bilirubin > 1.5 mg/dL).
Study design
The study included double-blind treatment and open-label extension phases. Patients were randomized 1:1:1 (centrally using a validated interactive voice response system) to lenalidomide 10 mg/day on days 1 to 21, lenalidomide 5 mg/day on days 1 to 28, or placebo on days 1 to 28 (all 28-day cycles). Patients were stratified according to IPSS karyotype score (0 vs > 0; ie, isolated del5q31 vs del5q31 plus ≥ 1 additional cytogenetic abnormality). Baseline complete blood count, ferritin, and EPO levels were measured.
Patients with at least a minor erythroid response (ie, 50% decrease in transfusion requirements) by week 16 could continue double-blind treatment for up to 52 weeks or until erythroid relapse, disease progression, or unacceptable toxicity. Those without minor erythroid response by week 16 were discontinued from the double-blind phase, unblinded, and eligible for open-label treatment. Those completing the double-blind phase without disease progression or erythroid relapse were unblinded and could start open-label treatment at their current lenalidomide dose. Patients in the placebo or lenalidomide 5 mg groups without minor erythroid response by week 16 or those who had an erythroid relapse could cross over to lenalidomide 5 mg or 10 mg, respectively, in the open-label extension phase. Open-label treatment was continued for up to 156 weeks of total study participation. Patients with disease progression at any time and patients randomized to lenalidomide 10 mg without minor erythroid response by week 16 were withdrawn from the study and were ineligible for open-label treatment. Concurrent use of erythropoiesis-stimulating agents, chemotherapy, or treatment with any other investigational agent was prohibited.
Efficacy and safety measurements were performed every 28 days. Complete blood counts were monitored weekly for the first 8 weeks, every 2 weeks for the next 8 weeks, and every 4 weeks thereafter. Bone marrow aspirate and cytogenetic studies were performed at baseline, weeks 12 and 24, every 24 weeks thereafter, and for disease progression. Bone marrow aspirates were submitted for central pathology and central cytogenetics review. All patients were followed for overall survival (OS) and acute myeloid leukemia (AML) progression.
Lenalidomide dosing was reduced as follows: lenalidomide 5 mg (starting dose), dose level −1 (5 mg every other day), dose level −2 (5 mg twice a week), and dose level −3 (5 mg weekly); lenalidomide 10 mg (starting dose), dose level −1 (5 mg daily), dose level −2 (5 mg every other day), and dose level −3 (5 mg twice a week); patients not tolerating dose level −3 discontinued treatment. For grade 4 neutropenia, lenalidomide was interrupted and resumed at the next dose level down when absolute neutrophil counts recovered to ≥ 500/μL. For grade 4 thrombocytopenia, lenalidomide was interrupted and resumed at a decreased dose level when the platelet count recovered to ≥ 25 000/μL and < 50 000/μL on ≥ 2 occasions for ≥ 7 days; or ≥ 50 000/μL at any time. Granulocyte colony-stimulating factors (G-CSFs) and granulocyte-macrophage colony-stimulating factors (GM-CSFs) were allowed for neutropenia.
Study endpoints
The primary endpoint was RBC-TI for ≥ 26 consecutive weeks. Secondary endpoints included erythroid response, duration of RBC-TI, cytogenetic response, OS, AML progression, safety, and health-related QoL (HRQoL). Changes in hemoglobin levels were determined from baseline.
Erythroid response was assessed using International Working Group (IWG) 200013 and 200614 criteria. Duration of RBC-TI (IWG 2000 criteria13 ) was defined as the number of days between the last transfusion before the start of the TI period or the first dose of lenalidomide, whichever occurred later, and the first transfusion after the TI period. Karyotyping (≥ 20 metaphases) and fluorescence in situ hybridization (≥ 100 cells evaluated) were performed. Cytogenetic response was assessed using IWG 2000 criteria and was determined based on karyotyping results.13
OS was defined as time from randomization to death from any cause. Time to AML progression was defined as the time from randomization to diagnosis of AML (French-American-British criteria).15 Disease progression to more advanced MDS subtypes is also presented. Adverse events (AEs) were classified using MedDRA and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0. Patient-reported HRQoL was assessed using the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire.16 FACT-An was administered at baseline and at weeks 12, 24, 36, and 48. Changes from baseline of ≥ 7 points for FACT-An are associated with clinically important improvements in HRQoL.17
Definitions of study populations
The modified intent-to-treat (mITT) population was used to assess efficacy and included patients with centrally confirmed Low- or Intermediate-1-risk MDS with del5q31 and documented RBC transfusion dependence, who received ≥ 1 dose of study drug. The intent-to-treat (ITT) population included randomized patients and was used to verify the mITT findings. The safety population included randomized patients who received ≥ 1 dose of study medication and was used to describe AEs, AML progression, OS, and HRQoL.
Statistical methods
Assuming response rates (RBC-TI for ≥ 26 weeks) of 0.400 and 0.100 in the active and placebo groups, respectively, a sample size of 45 patients per group (mITT population) and a 2-group continuity corrected χ2 test with a .025 2-sided significance level (α split to adjust multiple comparisons) has an 80% power to detect differences between each active treatment group and placebo. Descriptive statistics were used to compare lenalidomide 10 mg and 5 mg; the study was not powered to detect differences between the lenalidomide groups. Initial enrollment for the study was 162 patients. On June 8, 2006, the target enrollment was expanded to 205 to ensure the prespecified number of evaluable patients (N = 135).
The Mantel-Haenszel procedure stratifying on IPSS karyotype score (0 vs > 0) compared response rates for lenalidomide 10 mg and 5 mg versus placebo. For the primary endpoint, a step-wise modified Bonferroni procedure controlled the experiment-wise error rate. Patients who discontinued double-blind treatment were considered treatment failures. For erythroid and cytogenetic responses, results are summarized by treatment group. Cytogenetic responses are best postbaseline responses. Duration of response, AML progression, and OS were characterized using Kaplan-Meier curves. Duration for time-to-event analyses was calculated from randomization to the date of death or censoring (date of last contact), whichever was earliest. For duration of RBC-TI, data are included until the last date with available information on transfusions. This date is indicated as censored for patients who died or who remained RBC-TI at data cut-off.
Analysis of variance was used to analyze changes from baseline in hemoglobin concentration. Response rates were compared within prespecified subgroups of baseline serum or plasma EPO levels (≤ 500 vs > 500 mIU/mL), and isolated del5q31 versus del5q31 plus ≥ 1 additional abnormality using a χ2 test. Analysis of variance was performed to compare FACT-An change from baseline scores at week 12 in each lenalidomide group versus placebo. Longitudinal assessment of FACT-An scores to week 48 for patients who achieved RBC-TI for ≥ 26 weeks with lenalidomide is presented.
A multivariate logistic regression model identified external factors that predict RBC-TI. A backward procedure eliminated the less significant (P < .05) factors to build the final logistic regression model. The following variables were used: treatment group (lenalidomide 5 mg vs placebo, lenalidomide 10 mg vs placebo); age (> 65 vs ≤ 65 years); MDS duration (> 2 vs ≤ 2 years); centrally confirmed IPSS score (Intermediate-1- vs Low-risk); transfusion burden (> 4 vs ≤ 4 units/8 weeks); bone marrow blasts (≥ 5% vs < 5%); baseline EPO level (> 500 vs ≤ 500 mIU/mL); baseline platelet count (≥ 150 vs < 150 × 109/L); and G-CSF or GM-CSF use (yes vs no). Age, MDS duration, and bone marrow blasts were also investigated as continuous variables.
A Cox proportional hazard model was used to evaluate the effect of potential baseline risk factors and RBC-TI ≥ 8 weeks (IWG 2000 criteria13 ) on AML-free survival and OS, with RBC-TI (≥ 8 weeks) as a time-dependent covariate. A landmark analysis (6 months) was performed to reduce potential bias regarding the fact that responding patients must have survived long enough to attain response. Univariate Cox proportional hazard models first assessed each individual risk factor. Once potentially significant (P < .15) risk factors were identified, a multivariate model simultaneously determined the most important prognostic variables using a backward elimination variable-selection approach (variables were eliminated until all remaining variables had P < .15). These analyses included data through completion of the open-label phase for patients randomized to lenalidomide (dose groups combined); patients randomized to placebo were excluded because all, except 11 patients, crossed over to lenalidomide 5 mg. Identification of prognostic factors for RBC-TI and long-term outcomes was a predefined objective.
Final data for the double-blind phase are presented for all endpoints except for duration of response, AML progression, and OS, which include open-label data up to data cut-off (July 9, 2010; 156 weeks after last patient accrual).
Results
On June 26, 2008, 205 patients had either completed or discontinued from the double-blind phase and entered the open-label phase. Patient disposition details are presented in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Patients
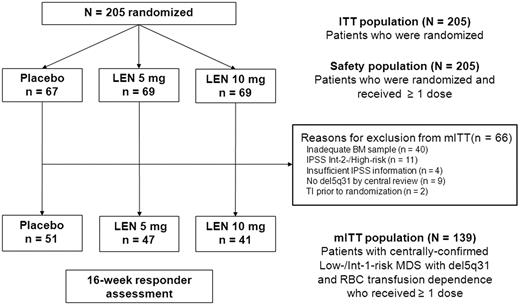
Patients (N = 205) were randomized to receive lenalidomide 10 mg (n = 69), lenalidomide 5 mg (n = 69), or placebo (n = 67) during the double-blind phase and were included in the ITT and safety populations. A total of 139 patients were included in the mITT population (lenalidomide 10 mg, n = 41; lenalidomide 5 mg, n = 47; and placebo, n = 51). Figure 1 outlines the reasons for exclusion from the mITT population. Baseline patient characteristics were similar to those of the ITT population and across treatment groups (Table 1). Duration of exposure data are presented in Table 2.
Study populations by randomized treatment group in the double-blind phase. BM indicates bone marrow; LEN, lenalidomide; and Int, Intermediate.
Study populations by randomized treatment group in the double-blind phase. BM indicates bone marrow; LEN, lenalidomide; and Int, Intermediate.
Erythroid response
In the double-blind phase, significantly more patients in the mITT population achieved the primary endpoint (RBC-TI for ≥ 26 weeks) with lenalidomide 10 mg (56.1%) and 5 mg (42.6%) versus placebo (5.9%; P < .001 vs both lenalidomide groups; Table 3). RBC-TI rates using IWG 2000 criteria13 (≥ 8 weeks) were 61.0% with lenalidomide 10 mg, 51.1% with lenalidomide 5 mg, and 7.8% with placebo (P < .001 for each comparison vs placebo). Similar results were found using the ITT population and IWG 2006 criteria14 (Table 3).
Time to erythroid response
Among patients who achieved RBC-TI for ≥ 26 weeks with lenalidomide (dose groups combined), onset of response occurred in 48.8% of patients during cycle 1, 37.2% during cycle 2, 9.3% in cycle 3, and 4.7% in cycle 4.
Duration of erythroid response
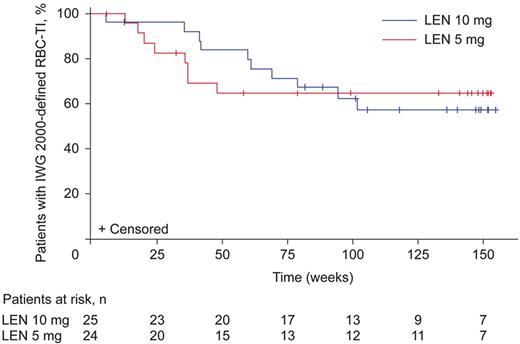
Median duration of IWG 2000-defined13 erythroid response (RBC-TI for ≥ 8 weeks) was not reached in either lenalidomide group (Figure 2), but the lower bound of the 95% confidence interval (CI) was 82.9 weeks with lenalidomide 10 mg and 41.3 weeks with lenalidomide 5 mg. Of these patients, 15 (60.0%) in the lenalidomide 10 mg group and 16 (66.7%) in the 5 mg group had ongoing responses and had been censored. Median duration of protocol-defined RBC-TI (≥ 26 weeks) was not reached.
Duration of IWG 2000-defined13 RBC-TI in patients randomized to lenalidomide (LEN) 10 mg or 5 mg (mITT population). Figure represents patients who achieved RBC-TI during the double-blind phase of the study. For duration of RBC-TI, data are included until the last date with available information on transfusions. This date is indicated as censored for patients who died or who remain RBC-TI at data cut-off. Median duration of RBC transfusion follow-up for all treatment groups combined was 1.55 years (RBC transfusion follow-up for ≥ 1, ≥ 2, and ≥ 3 years was available for 85, 54, and 9 patients, respectively).
Duration of IWG 2000-defined13 RBC-TI in patients randomized to lenalidomide (LEN) 10 mg or 5 mg (mITT population). Figure represents patients who achieved RBC-TI during the double-blind phase of the study. For duration of RBC-TI, data are included until the last date with available information on transfusions. This date is indicated as censored for patients who died or who remain RBC-TI at data cut-off. Median duration of RBC transfusion follow-up for all treatment groups combined was 1.55 years (RBC transfusion follow-up for ≥ 1, ≥ 2, and ≥ 3 years was available for 85, 54, and 9 patients, respectively).
Subgroup analysis of erythroid response (lenalidomide 10 mg vs 5 mg)
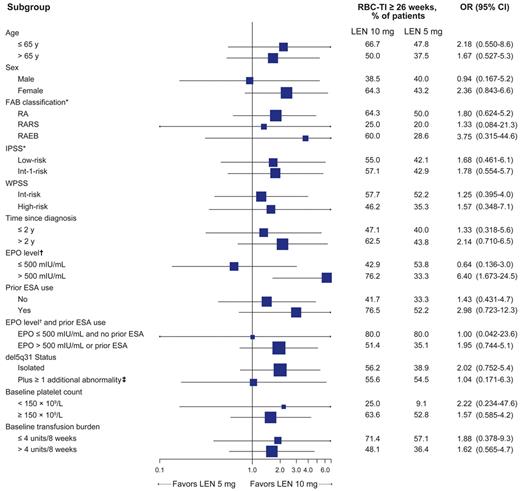
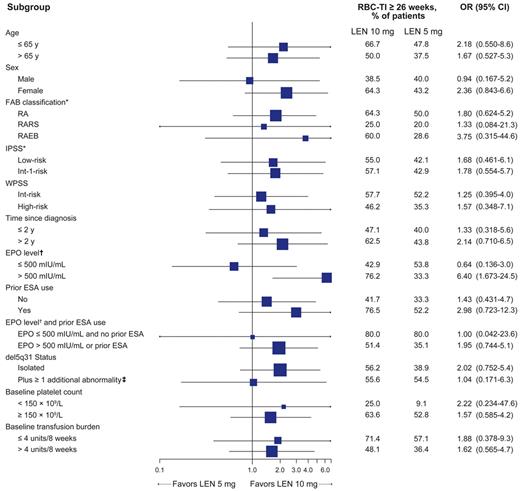
RBC-TI for ≥ 26 week rates (mITT population) favored lenalidomide 10 mg over 5 mg for most subgroups (Figure 3). In 45 lenalidomide-treated patients with baseline EPO levels > 500 mIU/mL, the RBC-TI rate was significantly higher with lenalidomide 10 mg versus 5 mg (76.2% vs 33.3%; P = .004).
Forest plot of subgroup analysis for achievement of RBC-TI for ≥ 26 weeks in patients randomized to lenalidomide (LEN) 10 mg or 5 mg (mITT population). Horizontal bars represent 95% CI.
*Based on central hematologic review.
†EPO level data were missing for 10 patients in the 5 mg group and 6 patients in the 10 mg group.
‡Because of the small number of patients with del5q31 plus ≥ 2 additional abnormalities (n = 5), this group was combined with those with del5q31 plus 1 additional abnormality to allow comparison with the isolated del5q31 group. Among the 5 patients randomized to lenalidomide with del5q31 plus ≥ 2 additional abnormalities, 1 patient in the lenalidomide 5 mg group achieved RBC-TI for ≥ 26 weeks.
OR indicates odds ratio; RARS, RA with ringed sideroblasts; WPSS, World Health Organization classification-based Prognostic Scoring System; and ESA, erythropoiesis-stimulating agent.
Forest plot of subgroup analysis for achievement of RBC-TI for ≥ 26 weeks in patients randomized to lenalidomide (LEN) 10 mg or 5 mg (mITT population). Horizontal bars represent 95% CI.
*Based on central hematologic review.
†EPO level data were missing for 10 patients in the 5 mg group and 6 patients in the 10 mg group.
‡Because of the small number of patients with del5q31 plus ≥ 2 additional abnormalities (n = 5), this group was combined with those with del5q31 plus 1 additional abnormality to allow comparison with the isolated del5q31 group. Among the 5 patients randomized to lenalidomide with del5q31 plus ≥ 2 additional abnormalities, 1 patient in the lenalidomide 5 mg group achieved RBC-TI for ≥ 26 weeks.
OR indicates odds ratio; RARS, RA with ringed sideroblasts; WPSS, World Health Organization classification-based Prognostic Scoring System; and ESA, erythropoiesis-stimulating agent.
Predictors of erythroid response
A multivariate analysis based on a logistic regression model showed that factors significantly predictive of RBC-TI for ≥ 26 weeks were lenalidomide treatment (P < .0001 for lenalidomide 10 mg vs placebo; P = .0004 for lenalidomide 5 mg vs placebo), higher baseline platelet count (≥ 150 × 109/L; P = .003), and longer time since MDS diagnosis (> 2 years; P = .05).
Change in hemoglobin levels
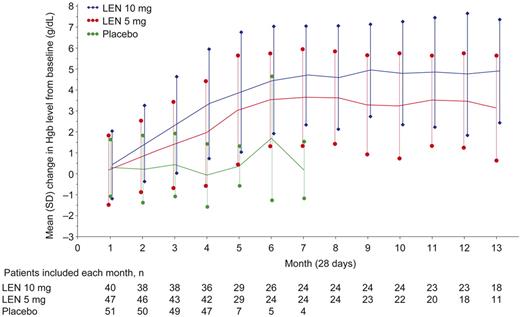
Figure 4 shows changes from baseline in hemoglobin levels (mITT population). Median maximum hemoglobin increases in patients who responded to lenalidomide (RBC-TI for ≥ 8 weeks) were 6.3 g/dL (range, 1.8-10.0 g/dL) with 10 mg and 5.2 g/dL (range, 1.5-8.5 g/dL) with 5 mg.
Mean hemoglobin (Hgb) change from baseline over time by randomized treatment group (mITT population). Data are mean ± SD but were not calculated if the number of patients was ≤ 3. LEN indicates lenalidomide.
Mean hemoglobin (Hgb) change from baseline over time by randomized treatment group (mITT population). Data are mean ± SD but were not calculated if the number of patients was ≤ 3. LEN indicates lenalidomide.
Cytogenetic response and progression
Cytogenetic response rates (complete + partial) in the mITT population were 50.0% and 25.0% in the lenalidomide 10 mg and 5 mg groups, respectively (P = .066). Complete cytogenetic response rates were 29.4% and 15.6% (P = .29). No cytogenetic responses occurred in the placebo group (P < .001 vs both lenalidomide groups). Cytogenetic progression (development of new independent clones as well as additional aberrations together with del5q31) was observed in 8 of 34 lenalidomide 10 mg patients (23.5%; P = .50 vs placebo), 10 of 32 lenalidomide 5 mg patients (31.3%; P = .17 vs placebo), and 5 of 35 placebo patients (14.3%). Similar results were observed in the ITT population (data not shown). Median time to cytogenetic progression was 93 days (range, 85-170 days) in the lenalidomide 10 mg group, 85 days (range, 83-339 days) in the lenalidomide 5 mg group, and 99 days (range, 83-172 days) in the placebo group.
HRQoL
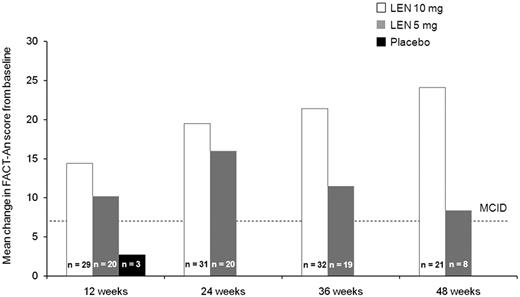
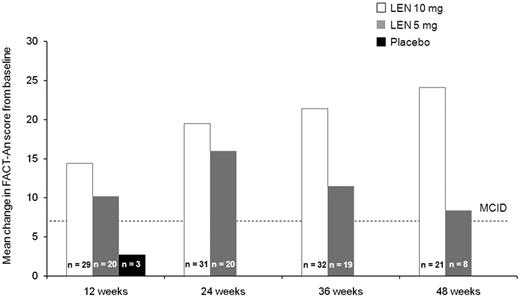
Baseline and week 12 (ie, before crossover) FACT-An scores were available for 71% of randomized patients (lenalidomide 10 mg, n = 48; 5 mg, n = 45; placebo, n = 52). Baseline scores (mean ± SD) were 121.1 ± 21.3, 124.8 ± 25.0, and 121.5 ± 28.0 among the lenalidomide 10 mg, 5 mg, and placebo groups, respectively. Mean change from baseline at week 12 was significantly higher for lenalidomide 10 mg (5.8 vs −2.5; F = 4.25; P < .05) and 5 mg (5.9 vs −2.5; F = 4.18; P < .05) versus placebo. Absolute change from baseline FACT-An scores exceeded 7 points (ie, minimal clinically important difference) among RBC-TI ≥ 26 week responders at weeks 12, 24, 36, and 48 in both lenalidomide groups (Figure 5).
Absolute change in FACT-An scores from baseline among patients who achieved RBC-TI for ≥ 26 weeks in the placebo group at week 12 (before crossover) and the lenalidomide (LEN) 5 mg and 10 mg treatment groups at weeks 12, 24, 36, and 48 (safety population). MCID indicates minimal clinically important difference.
Absolute change in FACT-An scores from baseline among patients who achieved RBC-TI for ≥ 26 weeks in the placebo group at week 12 (before crossover) and the lenalidomide (LEN) 5 mg and 10 mg treatment groups at weeks 12, 24, 36, and 48 (safety population). MCID indicates minimal clinically important difference.
Disease progression
In the safety population, median duration of follow-up for AML progression (from date of randomization to AML, death, or last known contact for non-AML survivors, whichever was earliest) was 30.9 months (range, 2.1-56.5 months) in the placebo group, 31.8 months (range, 0.8-59.4 months) in the lenalidomide 5 mg group, and 36.1 months (range, 0.4-57.7 months) in the lenalidomide 10 mg group.
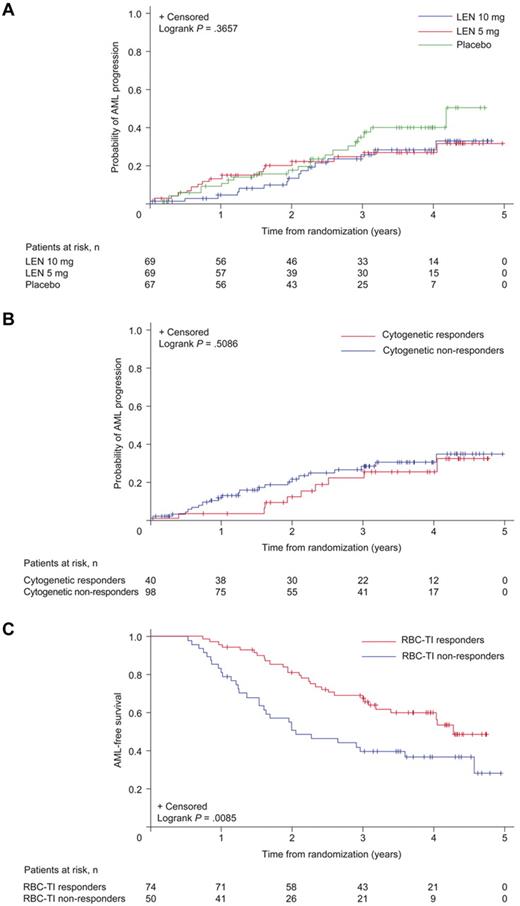
Before crossover at 16 weeks, 2 placebo patients (3.0%), 2 lenalidomide 5 mg patients (2.9%), and no lenalidomide 10 mg patients had progressed to AML. Overall, 52 patients (25.4%) progressed to AML during the double-blind and open-label phases. Of 11 patients randomized to placebo who never received lenalidomide, including 3 patients who completed 52 weeks in the protocol, 4 patients (36.4%) progressed to AML; 17 of 56 patients (30.4%) who initially received placebo and then crossed over to lenalidomide 5 mg progressed to AML, as did 16 of 69 patients (23.2%) in the lenalidomide 5 mg group and 15 of 69 patients (21.7%) in the lenalidomide 10 mg group. Among patients with baseline del5q31 status available, 33 of 135 (24.4%) patients with isolated del5q31, 8 of 38 (21.1%) patients with del5q31 plus 1 additional abnormality, and 8 of 17 (47.1%) patients with del5q31 plus ≥ 2 additional abnormalities progressed to AML. Median time to progression was not reached in the lenalidomide groups (Figure 6A). Cumulative risk of AML for the lenalidomide dose groups combined was 16.8% (95% CI, 9.8-23.7) at 2 years and 25.1% (95% CI, 17.1-33.1) at 3 years.
Time to AML progression. Results are presented for the safety population: (A) by randomized treatment group, (B) by cytogenetic response (complete + partial) in patients randomized to lenalidomide (LEN), and (C) by a landmark (6-month) analysis of AML-free survival by RBC-TI for ≥ 8 weeks in patients randomized to LEN. The placebo group includes 56 patients (83.6%) who had not achieved at least minor response by week 16 and were therefore crossed over to lenalidomide 5 mg.
Time to AML progression. Results are presented for the safety population: (A) by randomized treatment group, (B) by cytogenetic response (complete + partial) in patients randomized to lenalidomide (LEN), and (C) by a landmark (6-month) analysis of AML-free survival by RBC-TI for ≥ 8 weeks in patients randomized to LEN. The placebo group includes 56 patients (83.6%) who had not achieved at least minor response by week 16 and were therefore crossed over to lenalidomide 5 mg.
Figure 6B shows time to AML progression by cytogenetic response in patients randomized to lenalidomide. Lenalidomide responders (RBC-TI for ≥ 8 weeks) had longer AML-free survival than nonresponders (6-month landmark analysis; Figure 6C; P = .0085). Time to AML progression was similar between patients included in and excluded from the mITT population (P = .3149).
During the double-blind phase, 3 placebo (4.5%), 2 lenalidomide 5 mg (2.9%), and 2 lenalidomide 10 mg (2.9%) patients progressed from refractory anemia (RA) to RA with excess blasts (RAEB), 1 patient progressed from RAEB in transformation to AML (placebo), and 1 from RAEB to AML (lenalidomide 5 mg).
Survival
The median duration of OS follow-up was 35.9 months (range, 2.1-56.5 months) in the placebo group, 35.5 months (range, 1.9-59.4 months) in the lenalidomide 5 mg group, and 36.9 months (range, 0.4-57.7 months) in the lenalidomide 10 mg group.
At data cut-off, 101 patients had died, including 10 patients within 30 days of their last dose: 4 of 67 (6.0%) placebo patients (infection, disease progression, myocardial infarction), 4 of 69 (5.8%) in the lenalidomide 10 mg group (cerebral hemorrhage because of MDS progression, septic shock [2 cases], AML), and 2 of 69 (2.9%) in the 5 mg group (pulmonary embolism [PE], pneumonia). The case of PE was suspected by the investigator to be related to lenalidomide; the patient had prior history of PE, developed leukemia, and had deep vein thrombosis (DVT) and PE in the setting of acute leukemia.
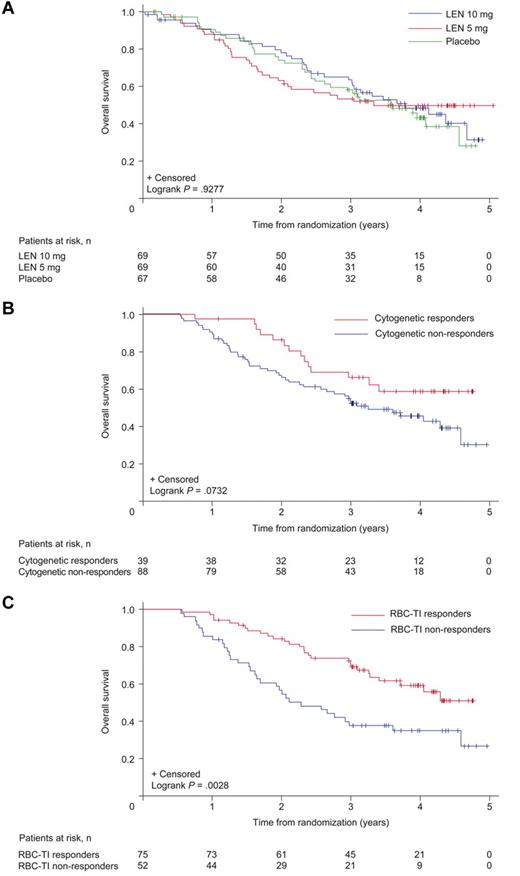
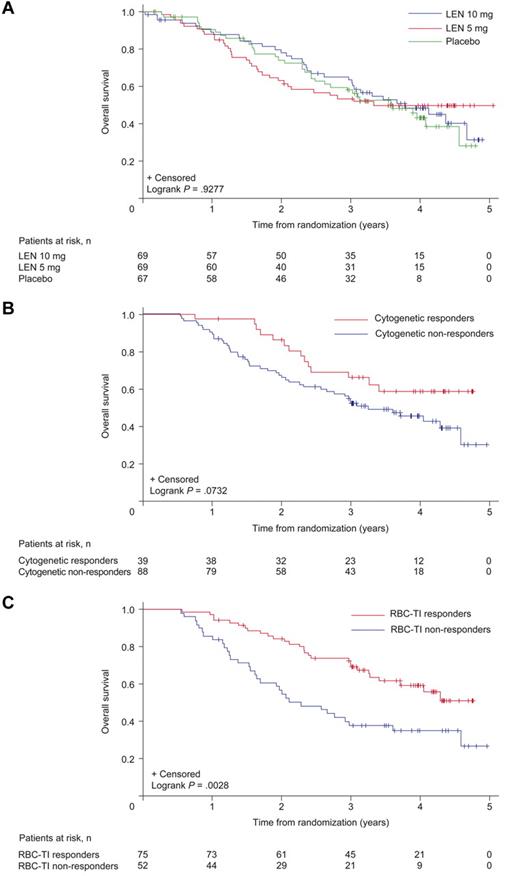
Median rates of OS were 42.4 months (95% CI, 31.9 to not reached), ≥ 35.5 months (95% CI, 24.6 to not reached), and 44.5 months (95% CI, 35.5 to not reached) in the placebo, lenalidomide 5 mg, and 10 mg groups, respectively (Figure 7A). Three-year OS for the lenalidomide groups combined was 56.5% (95% CI, 49.5%-63.4%).
Duration of OS. Results are presented for the safety population: (A) by randomized treatment group, by 6-month landmark analyses of OS, (B) by cytogenetic response (complete + partial), and (C) by RBC-TI (≥ 8 weeks) in patients randomized to lenalidomide (LEN). The placebo group includes 56 patients (83.6%) who had not achieved at least minor response by week 16 and were therefore crossed over to LEN 5 mg.
Duration of OS. Results are presented for the safety population: (A) by randomized treatment group, by 6-month landmark analyses of OS, (B) by cytogenetic response (complete + partial), and (C) by RBC-TI (≥ 8 weeks) in patients randomized to lenalidomide (LEN). The placebo group includes 56 patients (83.6%) who had not achieved at least minor response by week 16 and were therefore crossed over to LEN 5 mg.
Cox proportional hazards model for AML-free survival and OS: 6-month landmark analysis
The Cox proportional hazards model showed that in the combined lenalidomide groups, RBC-TI for ≥ 8 weeks was associated with a 42% reduction in the relative risk of AML progression or death (P = .048) and a 47% reduction in the relative risk of death (P = .021; Table 4). Higher baseline ferritin levels, older age, and higher transfusion burden were associated with a significantly increased risk of AML progression or death.
Safety
The most common grade 3 or 4 AEs during the double-blind phase were myelosuppression and DVT (Table 5). Grade 3 or 4 neutropenia and thrombocytopenia generally occurred within the first 2 cycles and subsequently decreased. Grade 3 or 4 DVT was reported in 4 (5.8%), 1 (1.4%), and 1 (1.5%) patients in the lenalidomide 10 mg, 5 mg, and placebo groups, respectively. In 5 of 6 cases, DVT occurred within 3 months of treatment initiation (range, 1-12 months). Hemoglobin levels at DVT onset were 7.0 to 16.1 g/dL. Overall, 3 of 4 patients with DVT in the lenalidomide 10 mg group were aged 65 years of age or older, including 1 with a history of venous thromboembolism and a hemoglobin level of 16.1 g/dL at DVT onset. Among lenalidomide-treated patients with DVT, 3 of 4 patients in the 10 mg group and 0 of 1 in the 5 mg group were responders. Other grade 3 or 4 AEs included infection (10 mg: 16%; 5 mg: 9%) and febrile neutropenia (10 mg: 1%; 5 mg: 3%); no grade 3 or 4 bleeding was reported.
Lenalidomide dose reductions (per protocol) were required because of AEs in 38 patients (55.1%) and 36 patients (52.2%) in the lenalidomide 10 mg and 5 mg groups, respectively. Dose interruption was reported in 32 patients (46.4%) and 20 patients (29.0%), respectively. The most common reasons for lenalidomide dose reductions were neutropenia (10 mg, 33.3%; 5 mg, 27.5%) and thrombocytopenia (10 mg, 21.7%; 5 mg, 11.6%). The most common reasons for dose interruptions were neutropenia (10 mg, 23.2%; 5 mg, 11.6%) and thrombocytopenia (10 mg, 13.0%; 5 mg, 11.6%). A second dose reduction was required in 11 patients (14.5%) in the lenalidomide 10 mg group and 5 patients (7.2%) in the lenalidomide 5 mg group.
Median times to first dose reduction or interruption were 27 days (range, 10-269 days) and 43 days (range, 7-215 days) in the lenalidomide 10 mg and 5 mg groups, respectively. Median durations of first dose interruption were 14 days (range, 1-161 days) and 11 days (range, 1-64 days), respectively. The median daily doses of lenalidomide at cycle 4 (before crossover) were 6.4 mg and 2.5 mg in the 10 mg and 5 mg groups, respectively, and 5.0 mg in the 10 mg group and 2.5 mg in the 5 mg group thereafter.
G-CSF or GM-CSF was used to prevent or reduce neutropenia in 39 patients (56.5%) in the lenalidomide 10 mg group and 38 patients (55.1%) in the 5 mg group and was not associated with median lenalidomide dose or response.
Discontinuation from double-blind treatment because of AEs was reported in 6 patients (8.7%) in the lenalidomide 10 mg group, 12 patients (17.4%) in the 5 mg group, and 3 patients (4.5%) in the placebo group. Discontinuations because of neutropenia were reported in 1 patient (1.4%) in each of the lenalidomide groups.
Discussion
In this phase 3, double-blind, placebo-controlled study (MDS-004), lenalidomide effectively induced RBC-TI in patients with lower-risk MDS and del5q31 when given at a dose of 10 mg/day for 21 days or 5 mg/day for 28 days, of 28-day cycles. Significantly more patients achieved RBC-TI for ≥ 26 weeks (primary endpoint) with lenalidomide 10 mg or 5 mg (56.1% and 42.6%, respectively) than placebo (5.9%), independent of response criteria and study population. This is consistent with a previous single-arm phase 2 study (MDS-003; N = 148), in which 67% of patients achieved RBC-TI (IWG 2000)13 with lenalidomide (10 mg/day or for 21 days of each 28-day cycle),12 and confirms the efficacy of lenalidomide in this setting. The placebo response was similar to previous reports14,18,19 and underscores the limitations of the IWG criteria.
RBC-TI (≥ 8 weeks) was achieved rapidly (onset of response occurred during cycle 1 in 48.8% of patients), which was comparable to the MDS-003 study (median time to onset of response 4.6 weeks).12 Responses appeared durable in the current study, with the median duration of response not reached (median follow-up, 1.55 years). In a pooled analysis of 4 phase 2 trials (MDS-001, MDS-002, MDS-003, and PK-002) in 168 patients, the median duration of response was 115 weeks.20
In the current study, baseline platelet count (≥ 150 × 109/L) was a significant predictor of RBC-TI for ≥ 26 weeks (P = .003), confirming MDS-003 results.12 Thus, baseline platelet count could be useful in selecting patients for lenalidomide.
Cytogenetic response rates with lenalidomide 10 mg on days 1 to 21 (50.0%) and lenalidomide 5 mg on days 1 to 28 (25.0%) were lower than that observed with lenalidomide 10 mg on days 1 to 28 in the MDS-003 study (73%).12 These results suggest that there may be a dose-response relationship. In addition, there were earlier and more frequent central cytogenetic follow-up investigations in the MDS-004 study (weeks 12, 24, and every 24 weeks thereafter) compared with the MDS-003 study (week 24), which may have contributed to the difference in cytogenetic response rates reported.
Treatment with lenalidomide improved HRQoL (FACT-An); improvements were apparent at week 12 and were significantly greater with lenalidomide (both groups) than placebo. Notably, longitudinal assessment among RBC-TI for ≥ 26-week responders demonstrated clinically meaningful improvements in HRQoL through week 48.
Overall, AML progression was reported in 30.4% of patients who were randomized to placebo but crossed over to lenalidomide and 23.2% and 21.7% of patients who received lenalidomide 5 mg and 10 mg, respectively. The 2- and 3-year crude cumulative AML progression rates (calculated from randomization) in the lenalidomide dose groups combined were 16.8% and 25.1%, respectively. These rates appear to be higher than those reported in a previous observational study (calculated from diagnosis); however, this observational study was performed before active treatments were available, and most elderly patients did not undergo regular bone marrow aspirate sampling or investigation for disease progression.21 In another observational study of 299 patients, the actuarial risk of AML progression (calculated from diagnosis) at 5 years was 38.8%,22 which appears comparable to the present study. When the AML progression rate was calculated for the 144 patients with 5q− syndrome (World Health Organization criteria), the actuarial risk of AML progression at 5 years was 17.1%.22 It is difficult to compare 5q− syndrome patients with those in the present study as the MDS-004 study did allow recruitment of all subgroups of del5q patients, including those with additional chromosomal abnormalities and medullary blasts of up to 10%. Moreover, all patients were RBC transfusion-dependent, a well-known adverse prognostic factor in MDS for both OS and AML progression.6
The median duration of OS was 42.4 months, not reached, and 44.5 months in patients randomized to placebo, lenalidomide 5 mg, and 10 mg, respectively. Achievement of RBC-TI for ≥ 8 weeks in patients randomized to lenalidomide was associated with a reduced relative risk of AML progression or death. Consistent with previous studies,1,6 higher baseline ferritin level, older age, and higher transfusion burden were associated with an increased risk of AML progression or death. Other studies have reported shorter survival in patients with greater cytogenetic complexity and increased bone marrow blast count.1,22,23 However, in our study, patient numbers in these high-risk groups were too few to significantly differentiate them from those with less severe findings at baseline.
This is the first randomized, placebo-controlled study to evaluate 2 doses of lenalidomide in a predominantly elderly patient population, and provides the opportunity to further examine the safety profile of lenalidomide in lower-risk MDS patients with del5q31; this has proved consistent with previous reports.12 The incidence of AEs was similar for both lenalidomide doses. The most common drug-related AEs were neutropenia and thrombocytopenia, which generally occurred within the first 2 cycles and decreased thereafter. In contrast to the MDS-003 study, where 3 deaths because of neutropenic infection were possibly treatment related,12 there were no treatment-related deaths because of neutropenic infection in MDS-004. This difference may be attributed to improved monitoring of neutropenia and management of febrile neutropenia, the dose reduction rules implemented since MDS-003, and possibly the use of G-CSF or GM-CSF. Rates of discontinuation because of neutropenia or thrombocytopenia were low compared with the proportion of patients who experienced these events. This suggests that cytopenias were effectively managed with dose reduction or treatment interruption and supportive care.
Treatment was discontinued because of AEs in 8.7% of patients in the lenalidomide 10 mg group, 17.4% in the 5 mg group, and 4.5% in the placebo group. In the MDS-003 study,12 20% of patients discontinued because of AEs (primarily neutropenia or thrombocytopenia). The overall incidence of DVT was 3.6% with lenalidomide (10 mg, 4 patients, 5.8%; 5 mg, 1 patient, 1.4%) versus 1.5% with placebo, consistent with the MDS-003 study (3%).12 Therefore, lenalidomide monotherapy does not appear to be thrombogenic in lower-risk MDS patients with del5q31.
In conclusion, in this phase 3, randomized, placebo-controlled study in patients with IPSS Low- or Intermediate-1-risk MDS with del5q31, both lenalidomide doses (5 mg and 10 mg) resulted in significant RBC-TI and cytogenetic responses and were generally well tolerated with a manageable safety profile. RBC-TI was durable and was associated with improvements in hemoglobin levels and HRQoL and reduced risk of death. There was no obvious increase in AML progression with lenalidomide treatment, but continued follow-up is needed. Anticipation of hematologic AEs and use of dose modifications and supportive care as needed should help achieve optimal clinical benefit from lenalidomide. Lenalidomide 10 mg was associated with a better erythroid response rate in patients with increased EPO levels (76.2% vs 33.3%; P = .004), a trend toward a higher cytogenetic response rate (50.0% vs 25.0%; P = .066), and more durable HRQoL improvements, without increasing the incidence of AEs. These findings support the use of a starting dose of 10 mg, with subsequent dose reductions or interruptions if needed.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Guntram Büsche and Hans Kreipe for contributions to central reference diagnostics, Gudrun Goehring for contribution to cytogenetic reference diagnostics, Jay Backstrom for contributing to the analysis and interpretation of data, Nancy Brandenburg for performing statistical analysis for the health-related quality of life data. Nikki Moreland, a medical writer from Excerpta Medica, provided drafts and editorial assistance during preparation of this manuscript supported by funding from Celgene Corporation. The authors are fully responsible for content and editorial decisions for this manuscript.
This research was sponsored by Celgene Corporation.
Authorship
Contribution: P.F., E.H.-L., A. Giagounidis, D.B., R.K., and J.F. designed the research; P.F., A. Giagounidis, D.S., O.B.-R., G.M., M.M., P.M., P.t.B., G.S., C.d.C., A.G.-B., L.N., U.P., M.L., B.Q., M.C., A. Ganser, B.S., D.B., and E.H.-L. performed research and collected data; C.A. performed central reference diagnostics and central marrow biopsy review; B.S. performed central cytogenetic reference diagnostics; T.F. performed statistical analysis; P.F., A. Giagounidis, and E.H.-L. wrote the manuscript; and all authors had access to primary trial data and were involved in analyzing and interpreting the data.
Conflict-of-interest disclosure: P.F. has received honoraria and research funding from Celgene, Ortho Biotech, Roche, Novartis, Cephalon, Epicept, Amgen, and Merck. A. Giagounidis is a consultant for Celgene and Novartis and is on the advisory committee and speaker's bureau for Amgen, GlaxoSmithKline, and Johnson & Johnson. D.S. is a consultant and is on the advisory committee and speaker's bureau for Celgene and Novartis, and is a consultant and is on the advisory committee for Amgen. O.B.-R. has received research funding from Celgene and Roche. G.M. has received honoraria and research funding and is on the advisory committee for Celgene. M.M. has received clinical trial support and honoraria and is on the advisory committee and speaker's bureau for Celgene, and holds equity in Bio GAL. P.M. is on the advisory committee for Celgene, Alexion, Amgen, and Novartis. P.t.B. received research funding and honoraria from Celgene and served on advisory committees and speakers bureau for Celgene. G.S. and C.d.C. are on the advisory committee for Celgene. A.G.-B. is a consultant for Celgene. L.N. has received honoraria from Celgene. U.P has received honoraria and served on advisory committees for Celgene. B.Q. has received research funding from Celgene. A. Ganser is a consultant for Celgene, Novartis, and Genzyme and is on the advisory committee and speaker's bureau for Amgen, GlaxoSmithKline, Johnson & Johnson, and Genzyme. D.B has received honoraria and served on advisory committees for Celgene, Novartis, and Amgen. B.S. has done diagnostic work for Celgene. R.K. and J.F. are employees of, and hold equity in, Celgene. T.F. is an employee of Celgene. E.H.-L. has received research funding from Celgene. The remaining authors declare no competing financial interests.
A complete list of the members of the MDS-004 Lenalidomide del5q Study Group appears in the online supplemental Appendix.
Primary double-blind data from MDS-004 were presented in abstract form at the 51st Annual Meeting of the American Society of Hematology, New Orleans, LA, December 5-8, 2009.
Correspondence: Pierre Fenaux, Service d'Hématologie Clinique, Hospital Avicenne, AP-HP, Université Paris XIII, 125 route de Stalingrad, 93009 Bobigny, France; e-mail: pierre.fenaux@avc.aphp.fr.