Abstract
We conducted a phase 3 randomized trial comparing 2 different doses of daunorubicin as induction chemotherapy in young adults (60 years of age or younger) with acute myeloid leukemia (AML). Of 383 patients who were analyzed, 189 received standard-dose daunorubicin (SD-DN, 45 mg/m2 per day times 3 days) and 194 received high-dose daunorubicin (HD-DN, 90 mg/m2 per day times 3 days) in addition to cytarabine (200 mg/m2 per day times 7 days) to induce complete remission (CR). The CR rates were 72.0% in the SD-DN arm and 82.5% in the HD-DN arm (P = .014). At a median follow-up of 52.6 months, overall (OS) and event-free (EFS) survival were higher in the HD-DN arm than in the SD-DN arm (OS, 46.8% vs 34.6%, P = .030; EFS, 40.8% vs 28.4%, P = .030). Differences in CR rate and both OS and EFS remained significant after adjusting for other variables (CR, hazard ratio [HR], 1.802, P = .024; OS, HR, 0.739, P = .032; EFS, HR, 0.774, P = .048). The survival benefits of HD-DN therapy were evident principally in patients with intermediate-risk cytogenetic features. The toxicity profiles were similar in the 2 arms. In conclusion, HD-DN improved both the CR rate and survival duration compared with SD-DN in young adults with AML. This study is registered at www.clinicaltrials.gov as #NCT00474006.
Introduction
The current standard treatment for induction of complete remission (CR) in previously untreated adults with acute myeloid leukemia (AML) is a combination of anthracycline and cytarabine.1,2 This induction chemotherapy combination was proposed on consideration of the results of a series of randomized trials. Although ∼ 30%-40% of patients with AML attained CR when either cytarabine or daunorubicin was given as a single agent, higher CR rates (> 50%) could be achieved by combining the 2 drugs. The Cancer and Leukemia Group B established that combination therapy with 3 days of daunorubicin and 7 days of cytarabine was better than a drug combination featuring 2 days of daunorubicin and 5 days of cytarabine and that 10 days of cytarabine treatment yielded results that were no better than those after 7 days of drug use.3,4 Daunorubicin at a dose of 30 mg/m2 per day was inferior to 45 mg/m2 per day in patients younger than 60 years and was less toxic than adriamycin at 30 mg/m2 per day.5 Neither the addition of other drugs3,6 nor the use of intermediate-dose to high-dose cytarabine7-9 improved patient outcomes. Therefore, the most widely used drug combination for induction chemotherapy is daunorubicin 45 mg/m2 per day intravenously for 3 days and cytarabine 100-200 mg/m2 per day, given by continuous intravenous infusion over 7 days.
Several nonrandomized trials have suggested that a higher dose of daunorubicin may be more effective. The Southwest Oncology Group found that the extent of CR was substantially better when daunorubicin was given at 70 mg/m2 per day compared with 45 mg/m2 per day.9,10 Although patients were not randomized, sequential studies by the same cooperative group have appeared, in which only the daunorubicin dose was varied. In a Japanese trial, in which tumor response was used to determine the effects of administered daunorubicin, the optimal drug dose during induction therapy was ∼ 280 mg/m2 (40 mg/m2 per day for 7 days), which is higher than the conventional dose of 45 mg/m2 per day for 3 days.11 In another study, a CR rate of 81% was achieved using 90 mg/m2 per day of daunorubicin.12 The possibility of cardiac toxicity must be considered if an increase in anthracycline dose or dose intensity is planned. However, when the daunorubicin induction dose was increased to 270 mg/m2, given in 3 blocks of 90 mg/m2 each, in a clinical trial with acute lymphoblastic leukemia patients, no early or late cardiac toxicity event was observed.13 A recent randomized trial conducted by the Eastern Cooperative Oncology Group (ECOG) demonstrated that intensification of induction therapy using a high daily dose of daunorubicin (90 mg/m2 per day for 3 days) improved both the CR rate and survival duration compared with those of young adults with AML receiving the standard daunorubicin dose (45 mg/m2 per day for 3 days).14 Beneficial effects of intensification of daunorubicin during induction chemotherapy in AML patients were also demonstrated in another randomized trial in elderly patients.15
We conducted a randomized trial comparing standard-dose daunorubicin (SD-DN, 45 mg/m2 per day) and high-dose daunorubicin (HD-DN, 90 mg/m2 per day) in young Korean adults with AML. The induction scheme was similar to that of the ECOG study, except for the daily cytarabine dose (200 mg/m2 in the present study vs 100 mg/m2 in the ECOG trial) and the infusion time of daunorubicin (continuous intravenous infusion in the present study vs short intravenous infusion over 10-15 minutes in the ECOG trial). Herein, we report the results of our randomized trial; the present work featured a longer follow-up duration than that of the ECOG study.
Methods
Patient population
All patients had been cytologically confirmed to have AML; the level of myeloblasts in bone marrow exceeded 20%. Patients with acute promyelocytic leukemia or chronic myeloid leukemia in the blastic phase were not included. No patients had been previously treated, and all patients were 15-60 years of age. The Karnofsky performance scale was 60 or more, and hepatic and renal function was adequate. Normal cardiac function, with a left ventricular ejection fraction ≥ 50%, was documented on a multigated heart scan or echocardiogram. No significant infection or uncontrolled bleeding was present. Patients were excluded if they had any history of malignancy within the previous 5 years (except for curatively treated cervical carcinoma in situ or basal cell carcinoma of the skin), if they had previously received radiotherapy of the pelvis, or if they had severe coexistent disease. Pregnant or lactating women were also excluded.
The study was approved by the Institutional Review Board of each participating institute. All patients provided informed consent in accordance with the Helsinki Protocol before randomization. We randomly assigned patients to cytarabine plus SD-DN or to cytarabine plus HD-DN.
Treatment
Patients randomized to the SD-DN arm received cytarabine 200 mg/m2 by continuous intravenous infusion over 24 hours daily for 7 consecutive days, and daunorubicin 45 mg/m2 by continuous intravenous infusion over 24 hours daily for 3 consecutive days. Patients randomized to the HD-DN arm received the same dose of cytarabine, using the same treatment schedule; and daunorubicin 90 mg/m2, by continuous intravenous infusion over 24 hours daily for 3 consecutive days. For patients showing persistent leukemia after initial induction chemotherapy, the second course of induction chemotherapy consisted of cytarabine 200 mg/m2 given by continuous intravenous infusion over 24 hours daily for 5 days and daunorubicin 45 mg/m2 by continuous intravenous infusion over 24 hours daily for 2 days. Patients who did not attain CR after the second course of induction chemotherapy were eliminated from the study.
Patients in either group who attained CR received the same consolidation chemotherapy. High-dose cytarabine, consisting of cytarabine 3 g/m2 given by 3-hour intravenous infusion every 12 hours (twice daily) on days 1, 3, and 5, to constitute a total of 6 doses per course, was given over a total of 4 courses. Next, patients received 2 monthly treatments with cytarabine (200 mg/m2 per day via 3-hour intravenous infusion for 5 days) and daunorubicin (45 mg/m2 via rapid intravenous infusion on the first treatment day). For patients who had intermediate- or poor-risk cytogenetic features and for whom appropriate donors were available, allogeneic hematopoietic cell transplantation (HCT) was performed during the first period of CR, as determined by the attending physicians.
Evaluation
Standard cytogenetic techniques were used to karyotype leukemic cells at diagnosis. Patients were classified into 3 risk groups on the basis of karyotype.1 Good-risk status was defined by the presence of abnormalities in core-binding factors [ie, t(8;21)(q22;q22), inv(16)(p13.1q22), or t(16;16)(p13.1;q22)]. Poor-risk status was defined by the presence of inv(3)(q21q26.2); t(3;3)(q21;q26.2); t(6;9)(p23;q34); 11q23 abnormalities except for t(9;11)(p22;q23); −5, del(5q), −7, or 17p abnormalities, or the presence of a complex karyotype (with 3 or more abnormalities). The presence of a normal karyotype, or any other cytogenetic abnormality including t(9;11)(p22;q23), was considered to indicate intermediate-risk status.
Bone marrow examination to evaluate remission status was performed 14 days after induction chemotherapy commenced and on the day on which a patient showed full hematologic recovery. CR was defined according to standard criteria; < 5% blasts in an aspirate sample with marrow spicules; hematologic recovery measured as an absolute neutrophil count ≥ 1000/μL, a platelet count ≥ 100 000/μL, and absence of blasts with Auer rods or extramedullary leukemic involvement.16 The causes of failure of CR induction were divided into 3 categories.16 Treatment failure because of resistant disease was diagnosed in appropriately treated patients who survived ≥ 1 week after completion of the initial course of treatment but for whom the final peripheral blood smear and/or bone marrow sample showed persistent leukemia. Treatment failure attributable to complications of aplasia was diagnosed in patients who survived ≥ 1 week after completion of the initial course of treatment and died while cytopenic, but for whom the final posttreatment bone marrow sample was aplastic or hypoplastic, without evidence of leukemia. Treatment failure was considered to be of an indeterminate cause in patients who died < 1 week after completion of the initial course of treatment; and in patients who died more ≥ 1 week after the completion of treatment, for whom the most recent peripheral blood smears did not show persistent leukemia and who did not undergo bone marrow evaluation after completion of therapy.
Relapse after CR was defined as the reappearance of leukemic blasts in the peripheral blood, or ≥ 5% blasts in the bone marrow, not attributable to any other cause, such as bone marrow regeneration after consolidation therapy; or the appearance of extramedullary leukemic involvement.
Adverse events were graded using the National Cancer Institute Common Toxicity Criteria for Adverse Event Version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf).
Statistical analysis
The present study was a prospective, randomized, nonblinded work evaluating the effects of induction chemotherapy in terms of daunorubicin dose intensity in AML patients, excluding those with acute promyelocytic leukemia. Our calculated target accrual was 300 eligible patients; this number was required to afford a statistical power of 0.8 on a 2-sided (P = .05) level test of survival distribution equality, under the following assumptions: event-free survival (EFS) was exponentially distributed; the 5-year EFS in the lower survival arm (SD-DN) was 20%; and the survival hazard ratio (HR) was 1.75/1.00. The endpoints were CR rate, overall survival (OS), relapse-free survival (RFS), and EFS. OS was calculated for all patients from the date of entry into the study to the date of death from any cause. RFS was calculated only for patients achieving CR, from the date of achievement of CR until the date of relapse or death from any cause. EFS was calculated for all patients from the date of entry into the study to the date of induction treatment failure, relapse from CR, or death from any cause.
The χ2 test was used to compare categorical variables and the Mann-Whitney U-test or Student t test to compare continuous variables. Survival curves were drawn using the Kaplan-Meier method, and differences in survival were compared using the log-rank test with censored data. Multivariate analysis was performed using stepwise multiple logistic regression for CR achievement, and Cox proportional hazards model was used to estimate the various forms of survival.
Results
Patient characteristics
The study commenced in August 2001, and patient accrual was completed in September 2008. A total of 402 adult patients were enrolled from 8 Korean institutes, but 19 patients were eliminated: withdrawal of informed consent in 10, changes of diagnosis in 5, prior treatment for leukemia in 2, and unknown reasons in 2. Thus, the remaining 383 patients (189 in the SD-DN arm and 194 in the HD-DN arm) were included in our statistical analyses (Figure 1).
CONSORT flow diagram. *If patients showed persistent leukemia at interim bone marrow examination (usually 14 days after induction chemotherapy), the second course of induction was given.
CONSORT flow diagram. *If patients showed persistent leukemia at interim bone marrow examination (usually 14 days after induction chemotherapy), the second course of induction was given.
The distribution of eligible patients was well balanced between the 2 treatment groups, except in terms of sex (Table 1). The proportion of males was significantly higher in the HD-DN arm than in the SD-DN arm (59.8% vs 47.6%, P = 0.017). Median patient age was 43 years (range, 15-60 years). Seventeen patients had secondary AML; this was therapy-related in 3 and had evolved from myelodysplastic syndrome in 14. Karyotyping results at diagnosis were available for all but 5 patients; 81 patients were at good risk (21.1%), 239 at intermediate risk (62.4%), and 57 at poor risk (14.9%).
Treatment data
Interim bone marrow examination was usually performed 14 days after commencement of the first session of induction chemotherapy, to permit a decision to be made on whether a second round of therapy was required. The proportion of patients who showed a hypoplastic marrow (blasts < 5%) at interim bone marrow evaluation was significantly higher in the HD-DN arm than in the SD-DN arm (75.3% vs 60.3%, P = 0.002; Table 2). A total of 64% of patients attained CR using only one course of induction chemotherapy (56.1% in the SD-DN arm vs 71.1% in the HD-DN arm, P = 0.002). A second course of induction chemotherapy was administered to 110 patients, at a median interval of 17 days (range, 13-125 days) from the start of the first course to that of the second course. On completion of the second course of induction chemotherapy, 52 patients attained CR. Thus, a total of 296 patients (77.3%) achieved CR using either 1 or 2 courses of induction chemotherapy. The CR rates differed significantly between the SD-DN and HD-DN arm (72.0% vs 82.5%, P = 0.014). The main reason for treatment failure in either arm was the presence of resistant disease (73.6% vs 82.4%, P = 0.635). Most patients received postremission therapy. Allogeneic HCT was performed in 46.6% of patients, usually after the second course of high-dose cytarabine. Five patients received autologous HCT, which was off-protocol therapy, but they were included in the survival analyses. Mean number (± SD) of chemotherapy courses, which were delivered as post-remission therapy, was 2.7 ± 1.2 for high-dose cytarabine and 0.5 ± 0.8 for monthly cytarabine plus daunorubicin. The proportion of patients receiving post-remission therapy and the nature of the therapy were similar between the 2 arms (Table 2).
Infection was the most common severe adverse event (grade 3 or higher as rated by National Cancer Institute Common Toxicity Criteria for Adverse Events Version 3.0) both during and after induction treatment. The frequency of severe adverse events was similar in the SD-DN and HD-DN arms (Table 3). No late cardiac toxicity was reported. None of the time to recovery of neutrophil or platelet numbers after induction chemotherapy, transfusion of red blood cells or platelets during induction treatment, or the duration of intravenous antibiotic therapy differed significantly between the 2 arms (Table 3).
Survival data
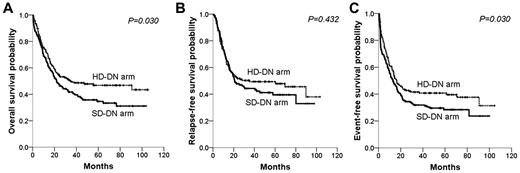
The median follow-up duration for surviving patients was 52.6 months (range, 3.2-105.6 months). During this time, 87 patients failed to achieve CR, 121 relapsed after CR, 40 died without evidence of relapse, and 218 died of any cause. The median OS was 25.1 months (95% confidence interval [CI], 17.0-33.3 months). The probabilities of OS, RFS, and EFS at 5 years were 40.8%, 44.8%, and 34.6%, respectively. The HD-DN arm showed a significantly longer OS and EFS than did the SD-DN arm (OS, 5-year probability, 46.8% vs 34.6%, P = .030; EFS, 40.8% vs 28.4%, P = .030; Figure 2). RFS did not significantly differ between the 2 arms (49.4% vs 39.6%, P = .432; Figure 2). Both the relapses and the deaths in remission among the patients who achieved CR occurred with similar incidences between the 2 arms (HD-DN arm vs SD-DN arm; relapses, 40.0% vs 41.9%, P = .740; deaths in remission, 11.3% vs 16.2%, P = .217).
Survival differences between the standard-dose (SD-DN) and high-dose daunorubicin (HD-DN) arms. (A) Overall survival; (B) relapse-free survival; (C) event-free survival.
Survival differences between the standard-dose (SD-DN) and high-dose daunorubicin (HD-DN) arms. (A) Overall survival; (B) relapse-free survival; (C) event-free survival.
Prognostic factors
The prognostic values of several variables, including treatment group, were assessed by univariate (Table 4) and multivariate analyses (Table 5). Molecular data, such as FLT3, NPM1, or others, were not collected and could not be included in the analyses. Multivariate analysis showed that cytogenetic risk group (for CR rate, OS, RFS, and EFS), Karnofsky performance score (for CR rate, OS, and EFS), age (for OS, RFS, and EFS), uric acid level (for OS, RFS, and EFS), and secondary leukemia (for OS) were independent prognostic factors in terms of clinical outcomes (Table 5). After adjustment for these factors, the HD-DN arm showed significantly better outcomes than did the SD-DN arm in terms of CR rate (HR = 1.802; 95% CI, 1.080-3.005; P = .024), OS (HR = 0.739; 95% CI, 0.580-0.974; P = .032), and EFS (HR = 0.774; 95% CI, 0.600-0.998; P = .048).
Subgroup analysis
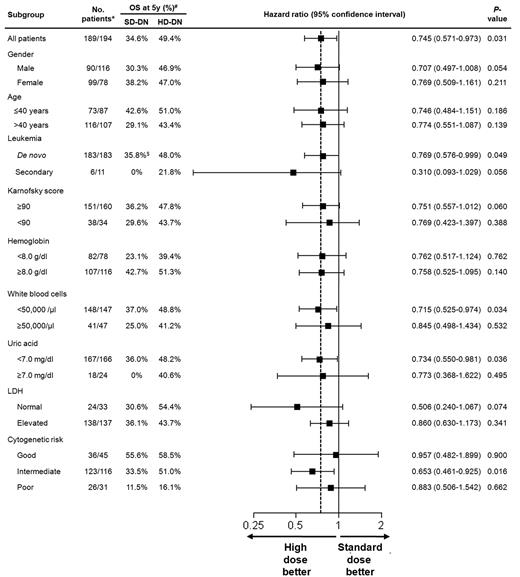
Subgroup analysis was performed using a univariate Cox proportional hazards model to evaluate the effects of daunorubicin dose on OS (Figure 3). All HRs were for patients receiving HD-DN compared with those receiving SD-DN.
Subgroup analysis for OS. A univariate Cox proportional hazards model was used to estimate HRs and the significance of the comparison for OS. The horizontal lines represent 95% CIs for the ratios. All HRs are for patients receiving HD-DN compared with those receiving SD-DN. *Number of patients denotes patients' number for SD-DN/patients' number for HD-DN. #OS probability at 5 years. $Survival probability at 4 years.
Subgroup analysis for OS. A univariate Cox proportional hazards model was used to estimate HRs and the significance of the comparison for OS. The horizontal lines represent 95% CIs for the ratios. All HRs are for patients receiving HD-DN compared with those receiving SD-DN. *Number of patients denotes patients' number for SD-DN/patients' number for HD-DN. #OS probability at 5 years. $Survival probability at 4 years.
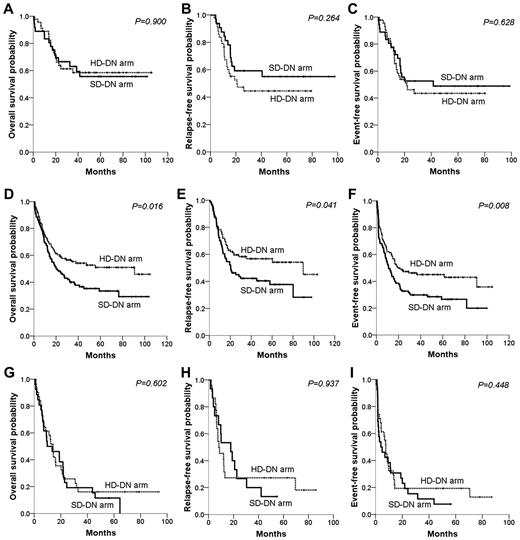
When subgroup analysis according to cytogenetic risk group was performed, the survival benefit of HD-DN treatment compared with SD-DN was most prominent in the intermediate-risk group (Figure 4). The HD-DN arm showed significantly higher OS (51.0% vs 33.5%, P = .016), RFS (56.7% vs 37.7%, P = .041), and EFS (45.1% vs 26.7%, P = .008) than did the SD-DN arm. No significant survival difference between patients of good- or poor-risk status, in the HD-DN and SD-DN arms, was evident. CR rates were somewhat higher in the HD-DN arm than in the SD-DN arm for each cytogenetic risk group, but the differences were not statistically significant (97.8% vs 88.9%, P = .099 for good-risk group; 79.3% vs 70.7%, P = .126 for intermediate-risk group; and 71.0% vs 57.7%, P = .296 for poor-risk group).
Survival differences between the SD-DN and HD-DN arms in terms of cytogenetic risk group. The top row of panels shows data on patients with good-risk cytogenetics; the second row, data for those with intermediate-risk cytogenetics; and the third row, data on patients with poor-risk cytogenetics. (A,D,G) OS. (B,E,H) RFS. (C,F,I) EFS.
Survival differences between the SD-DN and HD-DN arms in terms of cytogenetic risk group. The top row of panels shows data on patients with good-risk cytogenetics; the second row, data for those with intermediate-risk cytogenetics; and the third row, data on patients with poor-risk cytogenetics. (A,D,G) OS. (B,E,H) RFS. (C,F,I) EFS.
Discussion
Our current randomized study is similar to the ECOG work14 in terms of study period, overall scheme (especially that of the induction phase), and results (Table 6). Differences between the 2 trials included the dose of cytarabine and infusion time of daunorubicin used during induction treatment, the cytarabine dose levels, and the duration of reinduction therapy (cytarabine 200 mg/m2 for 5 days and daunorubicin 45 mg/m2 for 2 days in the present study vs cytarabine 100 mg/m2 for 7 days and daunorubicin 45 mg/m2 for 3 days in the ECOG trial), and the scheme of postremission therapy. The randomized ECOG study reported, for the first time, that intensification of daunorubicin dose during induction chemotherapy in AML patients could improve the outcomes of young adult patients, without increasing toxicities. These results were in contrast to the findings of studies featuring cytarabine dose intensification during induction. Such induction has been associated with greater toxicities, but with no improvement in CR rate or survival.7,9 The present study had a longer follow-up duration than that of the ECOG work, and our data confirm the results of the cited study. The use of HD-DN resulted in a significant increase in CR rate and a longer survival duration than that associated with the use of SD-DN. In the ECOG study, the CR rate (57.3%) in the SD-DN arm was lower than in other studies, and the authors of the cited work considered that this might be explained by the enrollment of patients with secondary AML. The CR rate of patients receiving SD-DN in our current study was 72.0%, equivalent to the results of other studies.17 Thus, elevated CR rates might be achieved using HD-DN, regardless of the nature of the study population. On subgroup analysis that considered cytogenetic risk groups individually, the benefits afforded by use of HD-DN were most significant in patients with intermediate-risk cytogenetic features, whereas no difference in outcome was evident between patients with poor-risk cytogenetic features, despite a statistically insignificant improvement in the CR rate (Figure 4). New treatment approaches are needed for patients with poor-risk cytogenetic features.
Given the results of both the present study and the ECOG work, HD-DN (90 mg/m2 per day) for 3 days should be the future standard of care for induction of patients with AML, at least those of intermediate-risk cytogenetic groups. However, it is not known whether a dose of 90 mg/m2 per day is superior to a dose of 45 to 90 mg/m2 per day. For example, a dose of 60 mg/m2 per day has been used in some studies,18 but this dose level has not been evaluated in work using randomized comparisons. It is also necessary to compare the effects of HD-DN with that of other agents, especially idarubicin. In a pediatric randomized trial that compared daunorubicin at 60 mg/m2 per day with idarubicin at 12 mg/m2 per day, no significant difference in CR rate or EFS was evident.19 A randomized trial conducted by the French Acute Leukemia Association showed that CR rates were higher when idarubicin was used at 12 mg/m2 per day compared with daunorubicin at 80 mg/m2 per day in elderly patients (50-70 years of age) with AML, but that OS, EFS, and relapse incidence were similar.20 The Japan Adult Leukemia Study Group conducted a randomized study comparing daunorubicin 50 mg/m2 per day for 5 days and idarubicin 12 mg/m2 per day for 3 days in young adults (15-64 years of age) with AML. There was no significant difference in either CR rate or survival.21 Future studies should explore whether HD-DN (90 mg/m2 per day) is superior to daunorubicin at 60 mg/m2 per day or idarubicin at 12 mg/m2 per day in young adults with AML.
Postremission chemotherapy scheme in the present study consisted of 4 courses of high-dose cytarabine (cytarabine 18 g/m2 per course) followed by 2 monthly courses of cytarabine (5 days) plus daunorubicin (1 day). The role of high-dose cytarabine consolidation was evidenced in a randomized trial conducted by the Cancer and Leukemia Group B,22 whereas there have been some debates on clinical effects of monthly myelosuppressive maintenance in AML.23 In the present study, monthly courses of cytarabine plus daunorubicin were given to similar proportion of the patients in each arm, and it is difficult to assess the impact of the maintenance therapy. Recently, maintenance therapy with novel agents, such as hypomethylating agents, is being investigated in AML.24 The postremission therapy scheme was significantly different between the present study and the ECOG trial.14 In the present study, allogeneic HCT was considered in patients with intermediate- or poor-risk cytogenetics if a suitable donor was available and it was performed usually after the second course of high-dose cytarabine. In contrast, the ECOG trial planned allogeneic HCT in patients with intermediate- or poor-risk cytogenetics, or high leukocyte counts (≥ 100 000/μL) if a suitable related donor was available. Allogeneic HCT was performed without any other postremission therapy. Other patients received 2 courses of high-dose cytarabine and proceeded to autologous HCT.
Patient safety is an important concern if HD-DN is to be used; we were especially careful to monitor any increase in cardiac toxicities or prolonged myelosuppression. Neither acute nor late cardiac adverse events increased in extent on use of HD-DN, in agreement with previous observations.12,13 Recovery of neutrophil and platelet numbers after myelosuppression, transfusion requirements during induction, and duration of intravenous antibiotic administration were similar in the 2 groups (Table 3). Number of deaths in the first CR without evidence of leukemia relapse was not significantly different between the 2 groups. Our results thus suggest that the use of HD-DN does not increase the frequency of either short- or long-term adverse events.
In conclusion, HD-DN improved both the CR rate and survival duration, without any increase in toxicity, compared with use of SD-DN, when used to treat young adults with AML. In such patients, the daunorubicin dose should be > 45 mg/m2 per day when the drug is used in induction therapy.
The online version of this article includes a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the clinicians and the investigators of the 8 institutes who entered their patients into this randomized trial and provided the data for the study.
Authorship
Contribution: Je-Hwan Lee designed and performed the project, collected and interpreted the data, and wrote the manuscript; and Y.-D.J., H.K., S.H.B., M.K.K., D.Y.Z., J.-L.L., G.W.L., Jung-Hee Lee, J.-H.P., D.-Y.K., W.-S.L., H.M.R., M.S.H., H.J.K., Y.J.M., Y.-E.J., and K.-H.L. performed the research, collected the data, and approved the final manuscript.
Conflict-of-interest disclosure: K.-H.L. has served as a consultant for Otsuka Pharmaceuticals. The remaining authors declare no competing financial interests.
A complete list of Cooperative Study Group A for Hematology participants appears in the supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online article.
Correspondence: Je-Hwan Lee, Department of Hematology, Asan Medical Center, Asanbyeongwon-gil 86, Songpa-gu, Seoul 138-736, Korea; e-mail: jhlee3@amc.seoul.kr.