Abstract
The Mds1 and Evi1 complex locus (Mecom) gives rise to several alternative transcripts implicated in leukemogenesis. However, the contribution that Mecom-derived gene products make to normal hematopoiesis remains largely unexplored. To investigate the role of the upstream transcription start site of Mecom in adult hematopoiesis, we created a mouse model with a lacZ knock-in at this site, termed MEm1, which eliminates Mds1-Evi1 (ME), the longer, PR-domain–containing isoform produced by the gene (also known as PRDM3). β-galactosidase–marking studies revealed that, within hematopoietic cells, ME is exclusively expressed in the stem cell compartment. ME deficiency leads to a reduction in the number of HSCs and a complete loss of long-term repopulation capacity, whereas the stem cell compartment is shifted from quiescence to active cycling. Genetic exploration of the relative roles of endogenous ME and EVI1 isoforms revealed that ME preferentially rescues long-term HSC defects. RNA-seq analysis in Lin−Sca-1+c-Kit+ cells (LSKs) of MEm1 documents near complete silencing of Cdkn1c, encoding negative cell-cycle regulator p57-Kip2. Reintroduction of ME into MEm1 LSKs leads to normalization of both p57-Kip2 expression and growth control. Our results clearly demonstrate a critical role of PR-domain–containing ME in linking p57-kip2 regulation to long-term HSC function.
Introduction
Maintenance of normal hematopoiesis depends on balanced self-renewal of HSCs and differentiation of blood cell progenitors within a supportive BM environment. Defects in stem cell function can result in a wide range of phenotypes, including cytopenias, dysplasias, and leukemias. HSCs can be functionally divided into those that can reconstitute long-term (> 16 weeks) and those with short-term repopulating ability (3-6 weeks).1 It was inferred early on from studies with 5-fluorouracil (5-FU) that the most primitive, long-term reconstituting cells within the marrow are largely quiescent, whereas the short-term repopulating cells are actively cycling.2,3 Further studies showed that a second injection of 5-FU 2 days after the first can kill off long-term repopulating cells,4 indicating that the stem cell pool enters the cell cycle after depletion of cycling progenitors by the first 5-FU injection.5 More recent studies indicate that immunophenotypic HSCs, Lin−Sca-1+c-Kit+ cells (LSKs), can be separated into actively dividing HSCs and dormant HSCs, with the latter being in a quiescent state but able to be recruited to the active state during stress.6 HSCs with the ability to long-term engraft irradiated recipients reside in the dormant pool. These are uniquely marked by the cell-surface phenotype CD150+/CD48− LSKs or by CD135−/CD34− LSKs. There is good evidence that the relative quiescence of HSCs is due to negative regulation of the cell cycle,7 metabolic activity,8,9 and differentiation10 ; pathways involved in aging,11,12 response to oxidative stress,13 and apoptosis14 play important roles as well.
The Ecotropic proviral integration site 1 (Evi1) gene encodes several zinc-finger protein isoforms and plays a causal role in myeloid neoplasms via its activation as a common site of proviral insertional activation in murine acute myeloid leukemia (AML)15 or as a site of chromosomal rearrangement in human AML.16 Evi1 is part of a larger Mds1-Evi1 complex (or Mecom) locus (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Whereas Evi1 possesses its own transcription start site(s), mRNA transcripts initiating at Myelodysplastic syndrome 1 (Mds1), located 0.5 Mb upstream of Evi1, can splice from exon 2 of Mds1 into exon 2 of Evi1 to encode a larger, 160-kDa MDS1-EVI1 (ME) protein (supplemental Figure 1B-D). Relative to EVI1 proper, ME possesses a 190-aa N-terminal extension encoded by exons 1 and 2 of Mds1 and exon 2 of Evi1 (supplemental Figure 1B). This region contains a 106-aa PRDF1-RIZ (PR) domain (supplemental Figure 1B), found in members of a family of nuclear-regulatory proteins including positive regulatory domain 1 binding factor 1 (PRD1-BF1)17 (also known as B lymphocyte–induced maturation protein 1 [Blimp-1]18 ) and Rb-interacting zinc-finger protein (RIZ).19 The function of the PR domain of ME (also known as PRDM3) remains elusive.
Evi1 has an essential role in hematopoiesis: it is expressed in HSCs,20-23 and disruption of exon 7 of Evi1 (common to both ME and Evi1) results in the absence of functional hematopoietic precursors in the paraaortic splanchnopleural region of mouse embryos.20 Conditional deletion of exon 4 of Evi1 (also common to both ME and Evi1) results in a decreased frequency of HSCs and colony forming cells (CFCs), whereas no change in the frequency of mature myeloid cells or lymphocytes is seen. In addition, these mice demonstrated delayed recovery of HSCs and platelets after a myelosuppressive treatment with 5-FU.24 These results indicate that Evi1 is indispensable for the maintenance of hematopoiesis. However, they do not distinguish between the role of ME and Evi1, because the targeted disruptions result in the loss of both types of transcripts.
In the present study, we used both immunophenotype and functional assays to uncover an essential role for ME in the regulation of HSC quiescence. ME-deficient mice lack quiescent, long-term repopulating cells. Within the LSK population, the percentage of proliferating cells is markedly higher. Both of these features indicate a chronic shift from dormant HSCs to active HSCs. This phenotypic shift is cell autonomous and is associated with an increased number of common granulocyte-macrophage progenitors. These data suggest that ME plays a critical role in maintaining long-term HSC function.
Methods
Mice
The creation of the MEm1, Evi1fl3, and Evi1ko3 alleles is described in supplemental Methods. Tail biopsies of 3-week-old offspring were analyzed by PCR using primers for wild-type (WT) Mds1 and lacZ sequences. All procedures involving mice were approved by the institutional animal care and use committees of all participating institutions.
Flow cytometry, FDG staining, and Ki67 analysis
FACSCalibur, LSR II, and FACScan instruments (BD Biosciences) were used for flow cytometric analysis and cell sorting. Analysis of all flow cytometry data were done using FlowJo Version 8.5.3 software (TreeStar). Data reflect at least 2 independent experiments, each conducted in triplicate. For isolation of the BM subpopulation, progenitor and HSC populations were identified and purified as described previously.13 Standard LSK analysis involved antibodies for lineage markers: CD11b (Mac-1), Ly6G (Gr-1), B220, CD3, Ter119 (all from BD Pharmingen; Lin cocktail, all conjugated to PE-Cy5), PE-conjugated Sca-1, and APC-conjugated c-Kit. For fluorescein di-β-D-galactopyranoside (FDG) staining, BM cells were harvested from mice, washed with PBS, and stained as follows. Expression of lacZ was assessed through staining with FDG. For immunophenotypic characterization of long-term HSCs (LT-HSCs), Lin− cells were stained with antibodies against c-Kit, Sca-1, CD150, and CD48, or c-Kit, Sca-1, CD34, and CD135 (all from eBioscience). Common lymphoid progenitors (CLPs) were defined as Lin−/c-Kit+/Il7ra+; common myeloid progenitors (CMPs) were defined as Lin−/c-Kit+/CD34+/Fcgr−/Il7ra−; granulocyte-macrophage progenitors (GMPs) were defined as Lin−/c-Kit+/CD34+/FCgr+/Il7ra−. For Ki67 analysis, Lin− cells were stained with antibodies against c-Kit, Sca-1, and Ki67 or an isotype control (BD Pharmingen).
Retrovirus production and infection
MIGR1-ME, MIGR1-EVI1, MIGR1-MDS1, and MIGR1 vectors were separately transfected together with Gag-pol and VSVG to produce retrovirus in 293T cells. Forty-eight hours after transfection, retrovirus supernatants were harvested. LSKs were sorted and cultured in IMDM and 20% horse serum containing 100 ng/mL SCF and 6 ng/mL IL-3 for 16 hours before infection. Retrovirus infections were carried out 3 times for 8 hours each in the presence of 4 μg/mL polybrene, 100 ng/mL SCF, and 6 ng/mL IL-3. Forty-eight hours after infection, those positive infected cells were sorted with green fluorescent protein and cytospun for immunochemistry analysis.
BrdU analysis, HPP-CFC assays, and immunostaining on glass slides
BrdU analysis of HSCs was performed as described previously.25 BrdU (Sigma-Aldrich) was added at 1 mg/mL to drinking water for 3 days. HSCs were sorted, cytospun onto glass slides, and stained and counted using a BrdU detection kit (Roche). High proliferative potential CFC (HPP-CFC) assays were performed as described previously.26,27 Sorted LSKs were cytospun onto glass slides, fixed, and stained with antibodies for p57-Kip2, p21-Cip1, and p27-Kip1, or Ki67 (Abcam), followed by development with diaminobenzidine.
Competitive and noncompetitive BM transplantation
Whole BM was harvested from WT and homozygous mice that had the CD45.2 isotype, and 500 000 of these cells were injected into lethally irradiated CD45.1 C57BL6/SJL mice. Mice were analyzed at 1 and 3 months after transplantation. BM was harvested and analyzed by flow cytometry for the presence of CD45.2+ cells. For competitive transplantations, mixtures of homozygous (CD45.2) and WT (CD45.1) or WT (CD45.2) and WT (CD45.1) BM (200 000 cells of each) were injected into irradiated recipients, and followed over time by flow cytometric analysis of peripheral blood leukocytes using the CD45 isotype.
RNA sequencing
Pools of LSKs were isolated from cohorts of 7 each of age- and sex-matched MEm1/m1 and WT mice as described in “Flow cytometry, PDG staining, and Ki67 analysis.” RNAs were isolated using the RNeasy Plus Kit (QIAGEN). Independent repeats were generated from pools of LSKs purified from different cohorts of 7 each of MEm1/m1 and WT mice. RNA sequencing was carried out in the Functional Genomic Core at the University of Rochester using SOLiD Workflow Automation (Applied Biosystems).
Results
Knock-in of lacZ into Mds1 exon 1
To assess the role of the Mds1 and ME gene products in hematopoiesis, we created a targeted disruption of the Mds1 transcription start site by inserting a promoterless lacZ gene into the first coding exon in the mouse. The structure of this allele, designated Mds1-Evi1tm1ap (herein termed MEm1; supplemental Figure 2) is such that the expression of lacZ is under the control of the Mds1 promoter and the splice donor is deleted. The β-galactosidase protein produced contains an SV40-derived nuclear localization signal. Matings between heterozygous mice generated live-born homozygous MEm1/m1 mice close to the expected Mendelian frequency (supplemental Table 1). MEm1/m1 mice appeared normal at birth, but grew more slowly than WT, attaining a smaller size at adulthood; all developed a progressive kyphosis (S.C.J. et al, unpublished data). MEm1/m1 mice routinely survived to > 10 months of age, but did exhibit an increased mortality, often attributable to a malocclusion that was found uniformly in MEm1/m1 mice. To assess allelism with Evi1, matings of MEm1/+ and Evi1+/− mice28 were performed, which identified no mice carrying both mutations (P < .0001; supplemental Table 2). These data indicate an essential role for the ME transcript during development.
To further characterize MEm1/m1 mice, we assessed Mds1 and ME mRNA transcripts in organs from MEm1/m1 and WT mice. An RNAse protection probe spanning the ME junction was used to quantitate Mds1, ME, and Evi1 mRNA transcripts (supplemental Figure 3). As shown in supplemental Figure 3C and D, negligible levels of Mds1 and ME transcripts were seen in organs from MEm1/m1 mice. However, levels of Evi1 transcripts were normal in MEm1/m1 organs, indicating that the 2 promoters function independently.
LacZ expression in the hematopoietic stem and progenitor cells of MEm1/+ mice
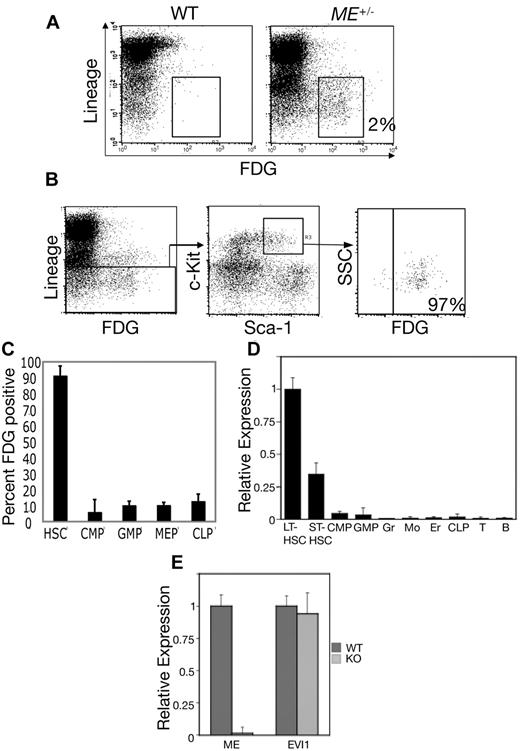
Given that EVI1 plays an important role in hematopoiesis, we assessed the expression of Mds1 in adult hematopoiesis. We performed flow cytometry of BM cells with FDG, a fluorogenic substrate of β-galactosidase. BM was harvested from WT and heterozygous MEm1/+ mice, and cells were stained with FDG along with fluorescently labeled antibodies to Sca-1, c-Kit, and lineage markers (B220, CD3, Mac1, Gr1, and Ter119; Figure 1). ME expression was restricted to only 1%-2% of whole BM cells, with nearly all FDG-positive BM cells expressing very low or undetectable levels of mature hematopoietic differentiation antigens (Figure 1A), indicating that in vivo expression of ME is restricted to immature hematopoietic progenitor cells. In the murine system, all HSCs are contained within the LSK subset of BM cells. To determine whether ME is expressed in this population, we gated first on the LSK population and then determined the levels of FDG expression in this cell subset. As shown in Figure 1B, ∼ 97%-98% of LSKs expressed high levels of β-galactosidase. To further define the expression of ME in the hematopoietic system, we quantitated the percentage of FDG-positive cells among the committed progenitors CMPs, GMPs, megakaryocyte-erythroid progenitors (MEPs), and CLPs, which were identified by established immunophenotypic criteria.13 This revealed again that nearly 100% of HSCs (LSKs) stained with FDG, whereas a very low percentage of committed progenitors stained (Figure 1C), indicating that the ME gene is down-regulated during transit from the stem cell compartment to lineage-committed derivatives.
ME is preferentially expressed in HSCs. (A) Whole BM cells were loaded with FDG and then stained with a cocktail of Cy-chrome-labeled lineage markers (B220, CD3, CD11b, Ly6G, and Ter119). Shown is a 2-dimensional scatterplot of FL3 (y-axis; Cy-chrome fluorescence) compared with FL1 (FDG fluorescence). Enumeration of FL1 channel fluorescence in BM from WT mice reveals minimal staining (< 0.1%), whereas MEm1/+ BM shows that 2% of the cells are positive; the majority (> 90%) are Lin−. (B) Whole BM cells from MEm1/+ mice were loaded with FDG and stained with Cy-chrome–labeled lineage markers as above, as well as with PE-labeled Sca-1 (FL2) and APC-labeled c-Kit (FL4). Gating was performed first on Lin− cells, and then on c-Kit/Sca-1 double-positive cells. Within this population, 97% of the cells were FDG positive. These studies and those in panel A were repeated at least twice with identical results. (C) FDG staining reveals low-level ME expression in committed progenitors. Shown is the percentage of HSCs and committed progenitors that stained positive for β-galactosidase activity using the fluorogenic substrate FDG. HSCs were identified as LSKs, whereas committed progenitors were identified as described by Tothova et al.13 The error bars represent SD based on 3 independent flow cytometry readings of different mice. (D) Quantitative Taqman PCR analysis of RNA from subpopulations of mouse BM cells including LT-HSCs, ST-HSCs, CMPs, GMPs, granulocytes (Gr), monocytes (Mo), erythroids (Er), CLPs, and T and B lymphocytes. Cell purity, as assessed by analytical flow cytometry, was > 97%. Data were normalized first to β-actin, then to expression level in LT-HSCs and are shown ± SD. (E) Quantitative Taqman PCR in isolated LSK hematopoietic progenitor cells demonstrating specific ablation of the ME transcript in LSKs in MEm1/m1 mice (light gray) versus WT (dark gray) mice ± SD. Primers/probe sets detected the nonoverlapping exon 1 of ME and exon 1 of Evi1, respectively.
ME is preferentially expressed in HSCs. (A) Whole BM cells were loaded with FDG and then stained with a cocktail of Cy-chrome-labeled lineage markers (B220, CD3, CD11b, Ly6G, and Ter119). Shown is a 2-dimensional scatterplot of FL3 (y-axis; Cy-chrome fluorescence) compared with FL1 (FDG fluorescence). Enumeration of FL1 channel fluorescence in BM from WT mice reveals minimal staining (< 0.1%), whereas MEm1/+ BM shows that 2% of the cells are positive; the majority (> 90%) are Lin−. (B) Whole BM cells from MEm1/+ mice were loaded with FDG and stained with Cy-chrome–labeled lineage markers as above, as well as with PE-labeled Sca-1 (FL2) and APC-labeled c-Kit (FL4). Gating was performed first on Lin− cells, and then on c-Kit/Sca-1 double-positive cells. Within this population, 97% of the cells were FDG positive. These studies and those in panel A were repeated at least twice with identical results. (C) FDG staining reveals low-level ME expression in committed progenitors. Shown is the percentage of HSCs and committed progenitors that stained positive for β-galactosidase activity using the fluorogenic substrate FDG. HSCs were identified as LSKs, whereas committed progenitors were identified as described by Tothova et al.13 The error bars represent SD based on 3 independent flow cytometry readings of different mice. (D) Quantitative Taqman PCR analysis of RNA from subpopulations of mouse BM cells including LT-HSCs, ST-HSCs, CMPs, GMPs, granulocytes (Gr), monocytes (Mo), erythroids (Er), CLPs, and T and B lymphocytes. Cell purity, as assessed by analytical flow cytometry, was > 97%. Data were normalized first to β-actin, then to expression level in LT-HSCs and are shown ± SD. (E) Quantitative Taqman PCR in isolated LSK hematopoietic progenitor cells demonstrating specific ablation of the ME transcript in LSKs in MEm1/m1 mice (light gray) versus WT (dark gray) mice ± SD. Primers/probe sets detected the nonoverlapping exon 1 of ME and exon 1 of Evi1, respectively.
To complement and extend this analysis, ME expression was quantitated by quantitative PCR in hematopoietic subpopulations including long- and short-term HSCs, CMPs, CLPs, and more mature, committed subfractions. This revealed that the highest levels of ME expression were in LT-HSCs, with a decrease of > 50% in short-term HSCs (ST-HSCs), whereas much lower levels were detected in more mature cells (Figure 1D). These data provide direct evidence that ME is expressed in murine HSCs. Therefore, the expression of the gene is restricted to undifferentiated cells.
To document the effect of the lacZ insertion on ME and Evi1 expression, RNA transcripts were quantitated in LSKs by quantitative Taqman PCR using nonoverlapping primers and probe sets. This analysis revealed essentially no detectable ME RNA in LSKs from MEm1/m1 mice, whereas Evi1 transcripts were unaffected (Figure 1E). These findings are completely consistent with our RNAse protection data (supplemental Figure 3), and again indicate that the ME and Evi1 transcripts are initiated from distinct promoters.
Knockout of ME results in mild thrombocytopenia and leukocytosis but no anemia nor abnormality in hematopoietic organ size
To investigate whether our MEm1/m1 mice had a hematopoietic phenotype, we performed complete blood counts on a cohort of adult mice (supplemental Figure 4). The homozygous mice showed significantly higher values (P < .01) for total leukocytes and lymphocytes; neutrophils trended higher, but did not reach statistical significance. There were significantly fewer platelets in the homozygous mice relative to the WT, but this was still within the normal range. Red blood cells were normal in number, amount of hemoglobin, size, and shape.
We also assessed spleen, thymus, and liver weights at 8-10 weeks of age in MEm1/m1 mice and compared them with age- and sex-matched WT mice. When normalized for mouse weight, there was no difference in organ weights between MEm1/m1 and WT mice (supplemental Table 3).
Reduced frequencies of ST- and LT-HSCs and increased myeloid progenitors in MEm1/m1 BM
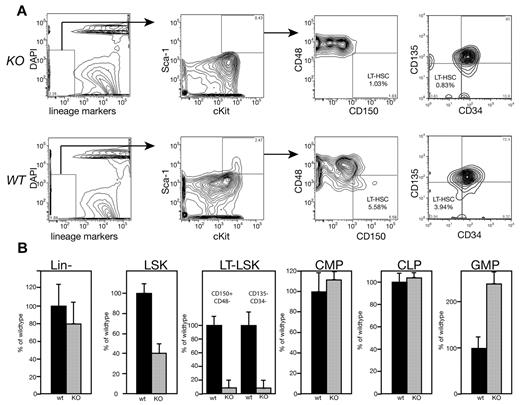
We assessed BM from MEm1/m1 mice by flow cytometry to determine whether any progenitor or stem cell population was present in lower numbers, and this revealed striking differences. Whereas the overall numbers of BM cells per mouse were no different between the mutants and WT, the numbers of Lin− cells were slightly decreased (Figure 2). Within Lin− cells, subpopulations enriched for HSCs were more dramatically decreased in the mutant BM: the number of LSKs in MEm1/m1 BM was 40% of WT, whereas cells with the immunophenotype of LT-HSCs were even lower: both CD150+/CD48− cells29 and CD135−/CD34− cells30 were at 7% of WT (Figure 2). However, committed progenitors were either no different or were more numerous in the MEm1m1 mice relative to WT mice: CMPs and CLPs were both normal, whereas GMPs were > 3 times higher in mutant mice (Figure 2).
Immunophenotyping reveals that MEm1/m1 mice lack long-term repopulating cells. (A) Analytical flow cytometry for immunophenotypic long-term repopulating cells (either CD150+/CD48− or CD135−/CD34−) performed on MEm1/m1 (KO) and WT BM cells as indicated. A representative experiment is shown; essentially identical results were obtained in 2 independent experiments. (B) Yield of Lin− cells ± SD and LSKs ± SD (based on > 30 independent cohorts of mice). LT-LSKs, CMPs, CLPs, and GMPs are indicated; progenitor data are based on 3 independent experiments normalized to WT. Black bars indicate WT BM; gray bars, MEm1/m1 BM.
Immunophenotyping reveals that MEm1/m1 mice lack long-term repopulating cells. (A) Analytical flow cytometry for immunophenotypic long-term repopulating cells (either CD150+/CD48− or CD135−/CD34−) performed on MEm1/m1 (KO) and WT BM cells as indicated. A representative experiment is shown; essentially identical results were obtained in 2 independent experiments. (B) Yield of Lin− cells ± SD and LSKs ± SD (based on > 30 independent cohorts of mice). LT-LSKs, CMPs, CLPs, and GMPs are indicated; progenitor data are based on 3 independent experiments normalized to WT. Black bars indicate WT BM; gray bars, MEm1/m1 BM.
MEm1/m1 mice are deficient in long-term repopulating cells
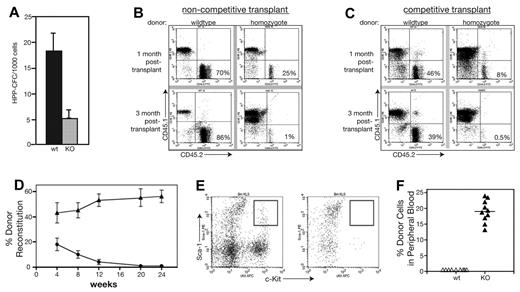
Flow cytometry results suggested that MEm1m1 mice were deficient in LT repopulating cells. To assess the function of these cells, we performed a series of in vitro and in vivo assays for stem cell function. HPP-CFCs are known to represent cells with long-term repopulating ability,31 so we determined their abundance in WT and MEm1/m1 BM, revealing that MEm1/m1 BM possesses less than one-third the number present in WT BM (Figure 3A).
MEm1/m1 mice lack long-term hematopoietic repopulating activities. (A) HPP-CFC assay performed on WT and MEm1/m1 marrow cells. (B) Noncompetitive transplantation of whole BM cells from either WT or from MEm1/m1 mice (CD45.2 isotype) injected into syngeneic CD45.1 recipients. The 2-dimensional scatter plots analyze CD45.1 (recipient) compared with CD45.2 (donor), where the contribution of the donor BM is in the bottom right quadrant. Whereas WT marrow maintains a high percentage of contribution, the MEm1/m1 marrow is not able to persist. (C) Competitive transplantation of WT CD45.1 donor marrow cells together with CD45.2 marrow from either WT (left) or MEm1/m1 (right) donors, transplanted into lethally irradiated CD45.1 syngeneic recipient mice. Flow analysis of peripheral blood at 1 month and 3 months was performed, quantitating CD45.1+ and CD45.2+ cells. The cells of interest are in the bottom right quadrant: WT donors persisted over time, whereas MEm1/m1 cells were not maintained. (D) Summary of competitive transplantation data (n = 9 for each group, error bars indicate SD) on the percentage of donor reconstitution over time. (E) Flow cytometric analysis of BM from recipients at 20 weeks, analyzing for Sca-1 (FL2, y-axis) and c-Kit (FL3, x-axis). Boxed area in top right quadrant represents LSK progenitors, which were present in mice reconstituted with WT BM, but not in recipients reconstituted with homozygous BM. (F) CD45.1 donor engraftment into nonmyeloablated recipients 3 months after injection of 2 × 106 BM cells (n = 10 for each group, error bars indicate SD).
MEm1/m1 mice lack long-term hematopoietic repopulating activities. (A) HPP-CFC assay performed on WT and MEm1/m1 marrow cells. (B) Noncompetitive transplantation of whole BM cells from either WT or from MEm1/m1 mice (CD45.2 isotype) injected into syngeneic CD45.1 recipients. The 2-dimensional scatter plots analyze CD45.1 (recipient) compared with CD45.2 (donor), where the contribution of the donor BM is in the bottom right quadrant. Whereas WT marrow maintains a high percentage of contribution, the MEm1/m1 marrow is not able to persist. (C) Competitive transplantation of WT CD45.1 donor marrow cells together with CD45.2 marrow from either WT (left) or MEm1/m1 (right) donors, transplanted into lethally irradiated CD45.1 syngeneic recipient mice. Flow analysis of peripheral blood at 1 month and 3 months was performed, quantitating CD45.1+ and CD45.2+ cells. The cells of interest are in the bottom right quadrant: WT donors persisted over time, whereas MEm1/m1 cells were not maintained. (D) Summary of competitive transplantation data (n = 9 for each group, error bars indicate SD) on the percentage of donor reconstitution over time. (E) Flow cytometric analysis of BM from recipients at 20 weeks, analyzing for Sca-1 (FL2, y-axis) and c-Kit (FL3, x-axis). Boxed area in top right quadrant represents LSK progenitors, which were present in mice reconstituted with WT BM, but not in recipients reconstituted with homozygous BM. (F) CD45.1 donor engraftment into nonmyeloablated recipients 3 months after injection of 2 × 106 BM cells (n = 10 for each group, error bars indicate SD).
To further assess HSC function in homozygous mice, noncompetitive BM transplantations were performed. Whole BM was harvested from WT and homozygous mice that had the CD45.2 isotype, and 500 000 of these cells were injected into lethally irradiated CD45.1 C57BL6/SJL mice. Mice were analyzed at 1 and 3 months after transplantation. BM was harvested and analyzed by flow cytometry for the presence of CD45.2+ cells. WT donor grafts were successfully maintained in the irradiated recipients at both 1 month and 3 months. Donor cells from homozygous mice were able to provide short-term engraftment of irradiated recipients (Figure 3B); however, they failed to give long-term engraftment of irradiated recipients: MEm1/m1 cells were present at 1 month, but decreased to negligible levels by 3 months (Figure 3B).
Competitive transplantations were also performed, in which mixtures of homozygous (CD45.2) and WT (CD45.1) or WT (CD45.2) and WT (CD45.1) BM (200 000 of each) were injected into irradiated recipients and followed over time by flow cytometric analysis of peripheral blood leukocytes using the CD45 isotype to distinguish cellular genotype. Representative scatter grams show the presence of MEm1/m1 cells at early times and their loss at later times (Figure 3C right), whereas WT cells persisted (Figure 3C left). By 20 weeks, knockout cells were completely lost (Figure 3D). Analysis of BM at this time revealed essentially a complete absence of knockout Sca-1/cKit–positive cells in the recipient, whereas such cells derived from the WT donor were readily identified (Figure 3E). These results indicate that whereas MEm1/m1 mice possess short-term repopulating cells, they are deficient in long-term repopulating cells, which confirms and extends the immunophenotypic analysis results shown in Figure 2.
To further analyze the competitive disadvantage of hematopoietic marrow from MEm1/m1 mice, WT CD45.1 isotype BM cells were transplanted into CD45.2 isotype nonmyeloablated WT or MEm1/m1 mice. In the nonmyeloablative setting, donor cells typically will not engraft, because they cannot outcompete and replace the host hematopoietic cells and this is in fact what we observed when peripheral blood cells were analyzed at 30 months after transplantation of WT CD45.1 cells into normal CD45.2 recipients: no CD45.1 isotype donor cells were detected (Figure 3F). However, when WT CD45.1 cells were transplanted into nonmyeloablated MEm1/m1 mice, the donor cells constituted 20% of circulating leukocytes 3 months after transplantation (Figure 3F), indicating that the host ME-deficient HSCs are effectively outcompeted by incoming WT cells. This experiment also demonstrates that the loss of repopulation capacity of mutant cells is not due to a deficiency in BM homing.
Genetic analysis of the relative roles of endogenous ME and EVI1 isoforms reveals that ME preferentially rescues LT-HSC defects
As documented by Goyama et al, deletion in the mouse of exon 4 of Evi1, which is shared by both ME and Evi1 transcripts, results in a 50% decreased abundance of LSKs in adult BM, as well as the loss of ability to competitively repopulate irradiated recipient mice24 ; this is similar to the phenotype we observed in MEm1/m1 mice. At the protein level, deletion of exon 4 eliminates not only all 3 isoforms of EVI1 (p135, p123, and p103), but also the 160-kDa PR-containing ME protein. Given the similarity of this phenotype to ours, we considered it important to parse the relative contributions of the ME and EVI1 proteins to hematopoietic function, specifically the frequency of HSCs and their potential to develop into committed progenitors and mature blood cells. Because our ME expression data (Figure 1) showed that ME is markedly down-regulated during lineage commitment of HSCs, we considered it unlikely that retroviral transduction of specific cDNAs into HSCs would fully rescue HSC function and developmental potential, because the retroviral LTR would not allow down-regulation of the transduced gene(s) during lineage commitment and differentiation. We decided rather to genetically explore the endogenous roles of the ME and EVI1 isoforms; in this way, the genes, when intact and functional, remain under control of their endogenous transcriptional regulation and retain the ability to form multiple isoforms via alternative splicing (assuming critical exons remain present and intact). We thus created a floxed exon 3 and a knocked-out exon 3 of Evi1 in the mouse, and crossed the former with a mouse strain harboring a tamoxifen-inducible Cre allele (ESR-Cre) to allow for inducible deletion of Evi1 (see supplemental Figure 5). In a first experiment, we assessed the colony-forming capacity of mice lacking either 1 or 2 copies of both ME and Evi1, enumerating both primitive progenitors (HPP-CFCs) as well as the committed progenitors granulocyte-erythroid-monocyte-megakaryocyte (CFU-GEMM), granulocyte-macrophages (CFU-GM), granulocytes (CFU-G), and macrophages (CFU-M) (Table 1). We harvested BM from Evi1+/+;Esr-Cre, Evi1fl3/+;Esr-Cre, and Evi1fl3/fl3;Esr-Cre mice, and assayed colony formation in cytokine-supplemented methylcellulose with and without deletion of ME and Evi1 via ex vivo tamoxifen treatment (Table 1). Whereas the loss of 1 copy of both ME and Evi1 had no effect on colony formation, deletion of both copies resulted in a complete loss of HPP-CFC and a near-complete loss of the multipotent progenitors CFU-GEMM and CFU-GM; these data are consistent with results from Goyama et al, who found a loss in long-term repopulating ability in homozygous deleted mice.24 Interestingly, with deletion of both alleles, the number of unilineage progenitors increases dra-matically: CFU-G and CFU-M both increase 3- to 4-fold (Table 1), indicating that deletion of both ME and Evi1 results in a shift toward a more mature state, and that either one or both play a role in maintaining the stem cell phenotype.
In a second experiment, we investigated what happens to colony-forming capacity in compound heterozygous states, which allows one to discern the roles of the different isoforms. Table 2 tabulates the number of functional alleles of ME and Evi1 in each condition, as well as the number of different colony types obtained. Not surprisingly, the addition of a second WT allele of Evi1 to one copy each of ME and EVI1 (the MEm1/fl3;Evi1fl3/+;Esr-Cre genotype without tamoxifen in row 3 of Table 2) yields normal colony numbers. Strikingly, lacking 2 alleles of ME while retaining one copy of Evi1 completely abrogates the ability to form primitive and multipotent progenitors (HPP-CFC, CFU-GEMM, and CFU-GM) and increases the numbers of CFU-G and CFU-M, suggesting that the deficiency in HSC function in Evi1fl3/fl3 BM is due solely to lack of ME rather than EVI1. Haploinsufficiency of Evi1 is an unlikely explanation for this result, because having one copy of both ME and Evi1 allows for normal colony formation (Tables 1–2), demonstrating that genetically “adding back” 1 copy of ME rescues long-term repopulating stem-cell function, whereas adding 1 more copy of Evi1 has less effect. Deletion of both copies of ME while maintaining 2 copies of Evi1 decreases the multipotent progenitors HPP-CFC, GEMM, and GM by 70%, 70%, and 50%, respectively, and increases unilineage progenitors by 3- to 4-fold (Table 2). These data indicate that the increase in unilineage progenitors seen in Evi1fl3/fl3 BM was due to the loss of ME rather than EVI1. These genetic experiments nicely demonstrate that the gene products of the 2 transcription start sites carry out distinct roles in hematopoiesis and help to highlight the essential role of ME in long-term HSC and progenitor cell function.
MEm1/m1 mice HSCs are shifted from the dormant state to the active state
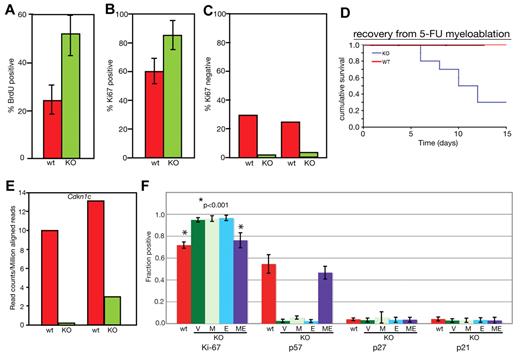
The low number of long-term repopulating cells in MEm1/m1 BM suggested a deficiency in the slowly dividing dormant/quiescent HSC pool. To determine whether this was indeed the case, we performed cell-cycle analysis of MEm1/m1 and WT HSCs. We first compared WT and MEm1/m1 LSKs for the proportion of cells in S/G2/M by BrdU labeling. We fed MEm1/m1 and WT littermates with BrdU at 1 mg/mL in the drinking water for 3 days, harvested their LSKs, enumerated them, and determined the percentage of cells that had incorporated BrdU. This revealed that the percentage BrdU-positive cells was significantly higher in MEm1/m1 HSCs (Figure 4A). Because it is known that BrdU treatment stimulates HSCs to enter the cell cycle, the results can only be considered in a relative manner. We therefore performed Ki67 staining on isolated LSKs from MEm1/m1 and WT mice, cytospun them onto glass slides, and stained them with antibody against Ki67. Because Ki67 is expressed in all cells except those in G0, it provides a good gauge of cells in the cell cycle. We found that 60% of WT HSCs were in the cell cycle, compared with 86% of knockout HSCs (Figure 4B). We supplemented this analysis with flow cytometric assessment of Ki67 gating on LSKs, this time enumerating those in G0 (Ki67 negative). These data confirmed the cytospin results, revealing that 25%-30% of WT LSKs were in G0, compared with 2%-4% of MEm1/m1 LSKs (Figure 4C). These findings clearly indicate that in MEm1/m1 BM a higher percentage of LSKs were in the cell cycle, with a marked decrease in quiescent, G0 LSKs. As confirmation, we assessed the resistance of mice to injection with 5-FU, an agent that kills dividing cells. Both WT and MEm1/m1 mice experienced drops in peripheral blood cell indices after 5-FU treatment (data not shown). However, whereas WT mice rebounded and survived, MEm1/m1 mice failed to do so and 75% of those treated died (Figure 4D). These findings indicate that in MEm1/m1 mice, a higher number of HSCs were in the cell cycle and susceptible to 5-FU treatment.
MEm1/m1 mice lack Cdkn1c expression in HSCs. (A) BrdU incorporation. LSKs were isolated from WT and MEm1/m1 mice after 3 days of labeling; cells were harvested, and cytospins were stained for BrdU and counted. Ten independent analyses were performed (P = .0014 by χ2 test). Error bars indicate SD. (B) Ki67 staining on glass slides. LSKs were harvested from WT and MEm1/m1 BM, cytospun onto glass slides, and stained with antibody against Ki67. Data are based on 3 independent analyses; error bars indicate SD. (C) Ki67 staining by flow cytometry. Whole BM from WT and MEm1/m1 mice was stained for the lineage markers c-Kit, Sca-1, and Ki67. Data are the percentage of Ki67-positive cells in the LSK population. Shown are 2 independent experiments. (D) Recovery from myeloablation by 5-FU. Cohorts of 15 MEm1/m1 and 15 WT adult mice were given intraperitoneal injections of 5-FU on day 0, and monitored thereafter. Cumulative survival is charted against time in days. (E) Analysis of Cdkn1c transcript levels in WT and MEm1/m1 LSKs by RNA-Seq; shown are RNA levels (in reads per million mapped reads) in 2 preparations for each genotype, with each preparation consisting of pooled LSKs from 7 mice. (F) Fraction of cells that immunopositive for the cell-cycle markers and regulators indicated, as assessed by diaminobenzidine development of HRP-conjugated avidin/biotinylated antibodies applied to cytospin smears of isolated LSKs from the genotypes indicated after transduction with the viruses indicated: V indicates empty vector; M, MDS1; E, EVI1; and ME, MDS1-EVI1. Error bars indicate SD. *P < .001 by Student t test.
MEm1/m1 mice lack Cdkn1c expression in HSCs. (A) BrdU incorporation. LSKs were isolated from WT and MEm1/m1 mice after 3 days of labeling; cells were harvested, and cytospins were stained for BrdU and counted. Ten independent analyses were performed (P = .0014 by χ2 test). Error bars indicate SD. (B) Ki67 staining on glass slides. LSKs were harvested from WT and MEm1/m1 BM, cytospun onto glass slides, and stained with antibody against Ki67. Data are based on 3 independent analyses; error bars indicate SD. (C) Ki67 staining by flow cytometry. Whole BM from WT and MEm1/m1 mice was stained for the lineage markers c-Kit, Sca-1, and Ki67. Data are the percentage of Ki67-positive cells in the LSK population. Shown are 2 independent experiments. (D) Recovery from myeloablation by 5-FU. Cohorts of 15 MEm1/m1 and 15 WT adult mice were given intraperitoneal injections of 5-FU on day 0, and monitored thereafter. Cumulative survival is charted against time in days. (E) Analysis of Cdkn1c transcript levels in WT and MEm1/m1 LSKs by RNA-Seq; shown are RNA levels (in reads per million mapped reads) in 2 preparations for each genotype, with each preparation consisting of pooled LSKs from 7 mice. (F) Fraction of cells that immunopositive for the cell-cycle markers and regulators indicated, as assessed by diaminobenzidine development of HRP-conjugated avidin/biotinylated antibodies applied to cytospin smears of isolated LSKs from the genotypes indicated after transduction with the viruses indicated: V indicates empty vector; M, MDS1; E, EVI1; and ME, MDS1-EVI1. Error bars indicate SD. *P < .001 by Student t test.
MEm1/m1 HSCs express lower levels of Cdkn1c, which can be normalized by transduction with ME retrovirus
To help explain the striking abnormality in cell-cycle regulation observed in MEm1/m1 HSCs, we performed RNA-Seq analysis on RNA harvested from 2 separate batches of LSKs harvested from MEm1/m1 mice, as well as 2 batches of WT mice. This analysis revealed 296 genes 2-fold higher or more in both MEm1/m1 samples, and 246 genes 2-fold higher or more in both WT samples (supplemental Table 4). Among these differentially expressed genes, there was a relatively limited number encoding cell-cycle regulators (supplemental Table 4). Within this group of genes, the most important regulator was the negative growth regulator Cdkn1c, which encodes p57-Kip2 that preferentially inhibits cyclin E-cdk2, a G1 cyclin complex; this was expressed at markedly lower levels in MEm1/m1 HSCs relative to WT (Figure 4E). Given the unique role of Cdkn1c in maintaining the quiescence of LT-HSCs relative to ST-HSCs,32 the lower level of Cdkn1c expression in MEm1/m1 LSKs is likely an important clue to the mechanism by which ME helps to regulate HSC quiescence. To assess whether reintroduction of ME into MEm1/m1 LSKs could normalize their higher level of cell proliferation and low expression of Cdkn1c, we introduced the gene into MEm1/m1 LSKs via retroviral-mediated gene transfer; in parallel infections, we introduced MDS1 and EVI1 via retrovirus and compared these populations with both WT cells and MEm1/m1 LSKs transduced with vector. Because the low numbers of cells precluded analysis by Western blot, we performed immunostaining of cells on glass slides, and documented the fraction of cells positive for Ki67, p57, p27, and p21 (Figure 4F). This analysis revealed, as before (Figure 4B), that ∼ 60% of WT LSKs express Ki67, indicating that they are cycling, whereas nearly 80% of MEm1/m1 LSKs express Ki67 (Figure 4F; P < .001). We found that transfer of ME via retrovirus led to a significant decrease in the fraction of cells expressing Ki67, similar to that seen in WT LSKs (Figure 4F; P < .001). However, neither transfer of MDS1 nor EVI1 was able to alter cell-cycling status in MEm1/m1 LSKs. As predicted by RNA-seq analysis, p57-Kip2 was expressed in a much lower proportion of MEm1/m1 LSKs relative to WT (Figure 4F) and, remarkably, transfer of ME into MEm1/m1 LSKs resulted in marked up-regulation of p57-Kip2, an effect that was not observed with transfer of either MDS1 or EVI1. These data indicate that the low level of p57-Kip2 and the high cycling rate of MEm1/m1 LSKs were indeed due to the lack of ME.
MEm1/m1 mice do not develop leukemia
Given that knockout of ME leads to decreased expression of Cdkn1c and increased cell cycling, we considered it possible that this may lead to an increased incidence of leukemia. A cohort of 24 MEm1/m1 mice (16 females, 8 males) was allowed to age for > 10 months (10.6-19.2 months), and was observed for the development of any hematopoietic illness or malignancy (supplemental Table 5A). An age-matched WT cohort was also observed. In both cohorts, a few had slightly enlarged spleens, but the majority was within the normal range (range, 50-190 mg; average, 124 mg; normal range, 80-120 mg). Histopathologic analysis including blood smears and H&E-stained tissue sections from complete autopsy revealed no neoplasia. No abnormalities were observed in the blood, lymph tissue, thymus, spleen, or BM. Flow cytometry was performed on spleen tissue for the progenitor marker c-Kit and lineage markers (Mac1, Gr1, B220, TER119, and Thy1), and no significant differences were found between WT and MEm1/m1 mice (supplemental Table 5B). These data indicate that, within the time span examined and without any additional leukemogenic agent (eg, retrovirus or chemical carcinogen), MEm1/m1 mice exhibited no increased susceptibility to leukemia or tumorigenesis.
Discussion
Previous studies on targeted mutations of Evi1 involved either insertion into exon 720,28 or deletion of exon 4.24 Because exons 4 and 7 are used by both EVI1 and ME transcripts, these mutations abrogate the production of both isoforms, and therefore the resultant phenotype cannot be unambiguously ascribed to one of the transcripts or both. Therefore, a precise mechanism for the observed defects is difficult to establish. In our knockout, EVI1 transcript levels were normal, allowing us to assess, in isolation, the contribution of ME transcripts to hematopoiesis.
We hypothesize that a critical role of ME in HSCs is to help maintain the quiescent state, and that it does so through up-regulation of Cdkn1c transcription. The mechanism by which Cdkn1c is down-regulated in MEm1/m1 HSCs is unclear. It is likely that this effect is indirect, because, at least in leukemic cells, ChIP-Seq data fail to reveal any peaks of EVI1 binding in the vicinity of Cdkn1c (del Campo J et al, unpublished data). Transcriptional up-regulation of Cdkn1c in HSCs by TGF-β has been documented,33 and EVI1 is known to interact directly with SMAD proteins that function downstream of the TGF-β receptor.34,35 To determine whether TGF-β–mediated up-regulation of Cdkn1c was blocked in MEm1/m1 HSCs, we isolated both WT and MEm1/m1 LSKs and treated them with 5 ng/mL TGF-β for 8 hours, and then analyzed the levels of Cdkn1c transcript by quantitative PCR. In both WT and MEm1/m1 LSKs, this treatment resulted in marked up-regulation of Cdkn1c (data not shown); therefore, it is unlikely that Cdkn1c levels are low in MEm1/m1 LSKs solely because of a block in TGF-β signaling.
The phenotype in our mice is due to the lack of ME alone, because we have demonstrated that EVI1 transcript levels are normal. The major difference between EVI1 and ME is the presence in ME of a 106-aa N-terminal “PR” domain with homology to the SET (for SuVar3-9, Enhancer of split, and Trithorax) family of chromatin modifiers with histone methyltransferase (HMT) activity.36,37 Structural analysis has revealed that the SET domain consists of 12 β sheets separated into an NH2-SET (N-SET, β sheets 1-5) and COOH-SET (C-SET, β sheets 6-12) domains with an unconserved intervening-SET (i-SET) domain in between. A catalytic pocket that binds the target lysine is caged by 4 β sheets, whereas the binding of S-adenosylmethionine (SAM) is dependent on the i-SET domain. A key residue for enzymatic activity is a tyrosine residue (Y287) at the top of the pocket. To further address the possibility of this PR domain possessing HMT activity, we performed in silico structural analysis by “threading” the primary amino acid sequence of the PR domain of ME onto the coordinates of a crystallized SET protein38 using ONO software. This revealed that whereas the catalytic pocket is present in ME, it is larger and lacks the critical Y residue. In addition, the i-SET domain, on which SAM binding depends, is absent (data not shown). Consistent with this in silico analysis, the PR domain of ME possesses neither SAM binding (our unpublished data) nor HMTase activity39 (and our unpublished data). It is tempting to speculate that the PR domain of ME acts as a protein-docking domain without enzymatic activity, and some evidence for this has been reported40 ; however, this was not within the context of HSCs and therefore, the function of the PR domain of ME within the HSC compartment will be an important focus of future studies.
Analysis of human AML reveals that cases with overexpression of EVI1 with a loss of ME expression have a particularly poor prognosis41 ; such cases tend to be associated with either inv3(q21;q26.2) or t(3;3)(q21;q26.2) relative to other 3q abnormalities.42 These cases were associated at a higher frequency with monosomy 7 and N-RAS mutations. Furthermore, Cdkn1c functions as a tumor-suppressor gene.43 From these data, one can imagine that the loss of ME may lead to increased tumors. To investigate this, we aged a cohort of MEm1/m1-deficient mice for tumors, and found no increase in tumors of any type. These data indicate that the loss of ME does not by itself result in increased susceptibility to cancer. However, some of the features of ME loss in our model bear resemblance to myelodysplastic syndrome44 in that there is increased proliferation and increased apoptosis (data not shown), together with decreased platelets (supplemental Figure 3). However, we did not observe overt dysplasia. It may be that the loss of ME expression, when combined with overexpression of EVI1 (or loss of 7q or mutation at N-RAS), leads to a more overt dysplastic or malignant phenotype.
In summary, we have provided important clarification of the role of Mecom in HSC regulation, clearly illustrating an essential role for the ME isoform in the maintenance of LT-HSCs via cell-cycle regulation. Furthermore, we have implicated Cdkn1c as a potential, though likely indirect, downstream target of ME. These data constitute an significant advance in our understanding of the maintenance of LT-HSCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Fripp Sigurdsson for providing a probe for human MDS1; Alyssa Mills, George Steele-Perkins, Sharon Lin, Katie Brock, and Kristina Owens for technical assistance; and Nancy Berliner, Diane Krause, Craig Jordan, Jim Palis, and Kathleen McGrath for helpful comments.
This work was supported by the National Institutes of Health (CA67013, CA81216, CA112188, and CA120313), the American Cancer Society (RPG 99-204-01), and the Aplastic Anemia & MDS International Foundation.
National Institutes of Health
Authorship
Contribution: Y.Z., S.S.-S., K.L.-G., S.C.J., L.C., G.C., C.A.W., F.C., and A.S.P. designed and performed the experiments; and Y.Z., F.C., K.L.-G., and A.S.P. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yi Zhang, Department of Pathology and Laboratory Medicine, University of Rochester, Box 626, 601 Elmwood Ave, Rochester, NY 14642; e-mail: yi_zhang@urmc.rochester.edu; Fernando Carmargo, Harvard Stem Cell Institute, Childrens Hospital, 300 Longwood Ave, Karp 8213, Boston, MA 02115; e-mail: camargo@fas.harvard.edu; and Archibald S. Perkins, Department of Pathology and Laboratory of Medicine, University of Rochester, Box 626, 601 Elmwood Ave, Rochester, NY 14642; e-mail: archibald_perkins@urmc.rochester.edu.