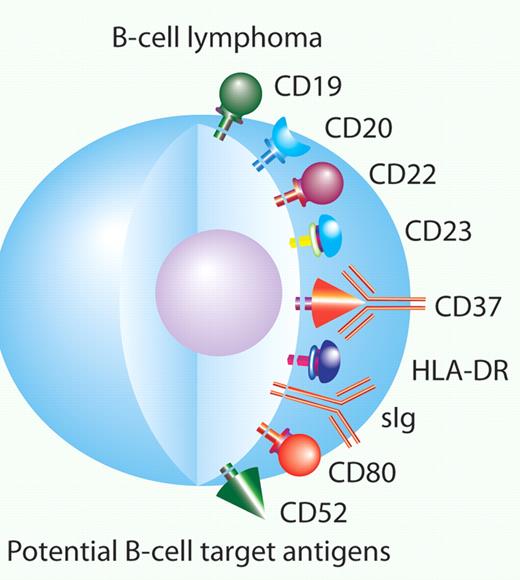
In this issue of Blood, Heider et al describe the preclinical development of a novel Fc-engineered monoclonal antibody targeting the B-cell antigen CD37, an antigen that may have very different properties then CD20.1
In 1997, the first monoclonal antibody for treatment of cancer, rituximab, was approved by the US Food and Drug Administration to treat low-grade lymphoma. It was quickly adopted by clinicians to treat nearly all forms of CD20-positive B-cell malignancies. This use was supported by numerous clinical trials demonstrating its efficacy, including a clear survival advantage when rituximab was combined with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) in patients with diffuse large-cell lymphoma.2 Since rituximab's approval, numerous other monoclonal antibodies have been studied, including anti-CD19, anti-CD20, anti-CD22, anti-CD23, anti-CD80 and anti–HLA-DR antibodies (see figure). These antibodies have provided a few small successes to date. Alemtuzumab has been approved for the treatment of chronic lymphocytic leukemia (CLL). The radioimmunoconjugates 131I tositumomab and 90Y- ibrutumomab tiuxetan have been approved for the treatment of low-grade lymphoma, and ofatumumab was recently approved for refractory CLL. So far none of these antibodies have achieved the widespread use that rituximab enjoys.
CD37 is one of many potential antibody targets for B-cell malignancies. Professional illustration by Paulette Dennis.
CD37 is one of many potential antibody targets for B-cell malignancies. Professional illustration by Paulette Dennis.
Here, Heider and colleagues describe the preclinical development of a novel monoclonal antibody targeting CD37, mAb 37.1.1 The target of this antibody, CD37, is not novel, but it has been forgotten by most for many years. CD 37 is a tetraspanin. It is expressed on mature B cells and very minimally expressed on T cells, monocytes, and macrophages. There is a high level of expression of CD37 across all subtypes of B-cell non-Hodgkin lymphoma. It is also expressed in CLL, hairy cell leukemia, and lymphoplasmacytic lymphoma.3,4 It was used in the late 1980s and early 1990s as the target for radioimmunotherapy. However, interest in CD37 as a target for radioimmunotherapy waned with the development of anti-CD20 antibodies, which were hypothesized to have better targeting and biodistribution.5,6
An important question is the relative importance of CD37 as a target compared with CD20. While there are some minor differences, the 2 antigens are expressed on a similar spectrum of malignancies, and trying to develop an anti-CD37 antibody based on these differences alone would be difficult. Recently there has been a revival in interest in targeting CD37. TRU-16 is a small modular immunopharmaceutical (SMIP) that also targets CD37. It is composed of a single-chain antibody variable region specific for CD37 linked to an immunoglobulin constant domain. It is also being developed for CLL and other B-cell malignancies. In vitro studies investigating the mechanism of action of a TRU-16 suggests there are important differences between CD20 and CD37 as a target.7 These studies indicate the induction of apoptosis via CD37 signaling events are caspase independent and involve tyrosine phosphorylation. This mechanism is very different from that seen with anti-CD20 antibodies and chemotherapy.
In addition to targeting a relatively understudied antigen, mAb 37.1 is of interest because the Fc portion of the antibody has been engineered to increase affinity for Fc-γ RIIIa. There are a variety of ways one might improve the efficacy of an antibody for a particular antigen. Modifying the Fc portion to elicit superior antibody-dependent cellular cytoxicity is one approach that has seen some early success. For instance, GA-101 is an Fc-engineered type II IgG-1 antibody targeting CD20. It has both superior direct cytotoxicity as well as immune effector cell–mediated cytotoxicity in comparison to rituximab.8 GA-101 accomplishes this modification through glycoengineering, a process in which the carbohydrate component of the Fc portion of the antibody is altered to enhance affinity for Fc-γ RIIIa. In the case of mAb 37.1, the improved affinity is accomplished by a point mutation rather then glycoengineering.
Ultimately, whether this antibody will succeed where numerous others have failed is hard to say. However, the combination of a relatively novel target that likely induces apoptosis through pathways different from approved agents combined with improvements in antibody function through Fc engineering is attractive. The clinical developmental pathway for this B-cell antibody or any other therapy for lymphoma is challenging. It is a crowded field with both approved and investigational agents. The next generation of antibodies will have to be substantially better than what is available today to make it through FDA approval. The development of mAb 37.1 into a clinically useful therapy for patients with B-cell malignancies is clearly many years away. Its story is one that will be followed with great interest by laboratory and clinical investigators alike.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■