Abstract
B-cell chronic lymphocytic leukemia (CLL), an incurable leukemia, is characterized by defective apoptosis. We found that the SET oncoprotein, a potent inhibitor of the protein phosphatase 2A (PP2A) tumor suppressor, is overexpressed in primary CLL cells and B-cell non-Hodgkin lymphoma (NHL) cell line cells. In CLL, increased levels of SET correlated significantly with disease severity (shorter time to treatment and overall survival). We developed SET antagonist peptides that bound SET, increased cellular PP2A activity, decreased Mcl-1 expression, and displayed selective cytotoxicity for CLL and NHL cells in vitro. In addition, shRNA for SET was cytotoxic for NHL cells in vitro. The SET antagonist peptide COG449 inhibited growth of NHL tumor xenografts in mice. These data demonstrate that SET is a new treatment target in B-cell malignancies and that SET antagonists represent novel agents for treatment of CLL and NHL.
Introduction
The SET protein is a potent physiologic inhibitor of protein phosphatase 2A (PP2A)1 that was isolated from a chromosomal rearrangement at 9q34 in a patient with acute undifferentiated leukemia.2 The SET protein is overexpressed in chronic myelogenous leukemia (CML) cells, and SET protein levels are further elevated during blast crisis.3 SET overexpression in CML cells correlates with decreased PP2A activity.3 This indicates that many of the SET oncogenic activities may be manifest through inhibition of PP2A. PP2A plays a role in many cellular processes, including cell cycle regulation, cell proliferation, apoptosis, development, cytoskeleton dynamics, cell motility, and stem cell self-renewal.4 In addition, PP2A is a critical tumor suppressor gene that regulates multiple important oncogenic signal transduction pathways.5-7 PP2A inhibition is essential for cell transformation and tumor formation,8,9 but overexpression of PP2A inhibitory proteins in chronic lymphocytic leukemia (CLL) has not been reported.
Of the nearly 84 000 annual cases of leukemia in the Western world, B-cell CLL is the most common, accounting for ∼ 30% of adult leukemia cases.10 Characterized by accumulation of monoclonal mature B cells,11 the CLL clinical course is heterogeneous, with some patients experiencing an aggressive course that demands early treatment and others experiencing long survival without disease-related symptoms or ever requiring treatment.11 Aberrant apoptosis in CLL cells correlates with arrest either in the G0 or early G1 phases of the cell cycle.12,13 This defective apoptosis in CLL cells is partly the result of aberrant signaling through the Akt kinase and the ERK MAPK pathways, in which phosphorylated-Akt is necessary for survival of the leukemia cells.14,15 The observation of aberrantly activated Akt and downstream pathways in CLL cells also suggests that the normal regulator of these pathways, PP2A, is unable to perform its normal role.
We thus sought to determine whether SET is overexpressed in CLL cells relative to normal B cells. We found that SET is significantly overexpressed in CLL cells and related non-Hodgkin lymphoma (NHL) cell line cells. In freshly isolated CLL patient samples, higher cellular levels of the SET correlated with more aggressive disease requiring earlier treatment. Antagonism of SET using shRNA-mediated knockdown or pharmacologic antagonism with novel cell-permeable SET antagonist peptides induced apoptosis, reduced cellular levels of Mcl-1, and caused death of CLL and NHL cells, but normal B cells were scarcely affected by SET antagonism. We also found that pharmacologic SET antagonism in vivo inhibited growth of B-cell NHL tumor xenografts in SCID mice.
Methods
General
All reagents were from Sigma-Aldrich unless noted otherwise. Anti-SET antibody was from Santa Cruz Biotechnology. Anti–β-actin, total c-Myc, pS62 c-Myc, and Mcl-1 were from Abcam. All primary antibodies were used at a 1:1000 dilution, except for β-actin, which was used at 1:10 000. All secondary antibodies are near-infrared dye conjugated antibodies from LI-COR and were used at 1:10 000. All peptides were synthesized by PolyPeptide Laboratories using standard fluorenylmethoxycarbonyl-based chemistry with acetate at the N-terminus and amides at the C-terminus. Each peptide was > 95% pure.
Normal B-cell and CLL cell preparation
CLL was diagnosed according to the NCI Working Group criteria.16 Healthy volunteers and CLL patients from the Duke University and Durham Veterans Administration Medical Centers were enrolled in Duke University Institutional Review Board–approved research protocols to collect clinical data and blood. Subjects gave signed informed consent before phlebotomy, in accordance with the Declaration of Helsinki. Blood was collected from participants into heparin tubes, and CLL cells were purified as noted before.17 Briefly, CLL cells were isolated using the RosetteSep B cell enrichment cocktail (catalog no. 15024/15064; Stem Cell Technologies) according to the manufacturer's directions. This method yielded CLL cell purity of > 95% CD5+CD19+ B cells as determined by flow cytometry. Normal B-lymphocytes were isolated using the EasySep B-cell enrichment cocktail (catalog no. 15024/15064; Stem Cell Technologies) according to the manufacturer's directions, with a purity of > 94% CD19+ lymphocytes.
IGVH mutation status, CD38 and ZAP70 expression, and interphase cytogenetics were determined as before.17 The length of time to initiation of treatment from the date of diagnosis was defined as the time to treatment (TTT). Individual physicians managing the patients made decisions regarding treatment initiation based on NCI Working Group criteria.16 Some patients' treatments were started before being seen at the Durham Veterans Administration or Duke University Medical Centers. The length of time from diagnosis to death from any cause was defined as overall survival (OS).
Quantitative PCR measurement of SET mRNA
We prepared total RNA from CLL cell pellets using QIAGEN′s RNeasy Mini Kit (catalog no. 74104) following the manufacturer's protocol. cDNA was prepared using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems catalog no. 436881) following the manufacturer's protocol. Quantitative PCR was performed using TaqMan master mix and probes from Applied Biosystems and following the manufacturer's protocol. Fold changes were calculated using ΔΔct method.
Western blots
Cells were lysed in NP40 buffer (50mM Tris, 0.2% NP40, 150mM NaCl) and the protein concentration of the lysate determined using the BCA assay18 and adjusted to 5 mg/mL total protein. Laemmli protein electrophoresis buffer (4×, 75 μL) was added to the cell lysate (25 μL), and the solutions were heated to 90°C for 5 minutes. Protein solutions were loaded onto gels, separated by SDS-PAGE, and blotted onto nitrocellulose membranes (Bio-Rad). The nitrocellulose membranes were blocked using 5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 3 hours, washed with TBST. The membrane was incubated overnight at 4°C in the primary antibody of one species and a loading control (usually GAPDH) antibody of a second species diluted in SuperBlock.18 We washed membranes with TBST for 1 hour and incubated with the appropriate IRDye 680 or IRDye 800 secondary antibodies to detect the primary and loading control antibodies. The membranes were washed thoroughly, and protein bands were visualized and quantitated (Odyssey Infrared scanner, LI-COR).
Large-scale SET quantification in CLL patient samples
For immunoblot analyses of SET on a larger number of samples, we used anti-I2PP2A (E-15) antibody from Santa Cruz Biotechnology. Frozen patient cells were lysed by freeze-thaw in Mammalian Protein Extraction Reagent (Pierce Chemical) with a protease inhibitor. We used lysates from a single stock of Ramos cells in each individual gel as a control for quantitation across all samples. A total of 50 μg lysate per lane was electrophoresed and blotted onto polyvinylidene difluoride membranes. We used the reversible Ponceau protein staining method as a loading control across lanes.19 Briefly, after transfer, polyvinylidene difluoride membranes were rinsed for 10 minutes in distilled water and incubated in 0.1% Ponceau S solution (Sigma-Aldrich) for 10 minutes, followed by a brief rinse in distilled water. Membranes were imaged using a standard scanner and then rinsed further in distilled water and then in TBST (0.1%) to eliminate the Ponceau stain. After blocking the membranes, we incubated them with antibodies. We processed the blots with an anti–goat IgG-HRP conjugate (Santa Cruz Biotechnology) and SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific). Band densities were quantified and expressed as a ratio of SET band density/Ponceau band density (total proteins) and calculated relative to Ramos lysate SET (arbitrarily established as 100). We dichotomized SET “high” and “low” values using the following cutoffs: SETα 70.3 units, SET β 115 units, total SET(α + β) 74.5 units, and SET α/β ratio of 0.78 units relative to the Ramos cell control.
shRNA-mediated SET knockdown
Lentivirus-expressing shRNA for SET and a noncoding control were from Santa Cruz Biotechnology. Cells (5 × 105/mL) were seeded into 12-well cell culture plates (1 mL/well) in RMPI 1640 media. Polybrene (1 μL, Santa Cruz Biotechnology) and lentivirus (10 μL) were added to each well, and the plate was spun at 90g for 1 hour at room temperature. The plate was then placed at 37°C for 2 hours. Cells were then seeded into 6-well plates, and 2 mL normal growth media was added per well. We treated cells 48 hours after transduction and then harvested 72 hours after transduction for assays and Western blotting.
PP2A activity measurement
Cultured 32D:BCR/Abl CML cells were grown in standard media, treated as indicated, and lysed in NP40 buffer. The protein concentration18 was adjusted to 2 mg/mL protein, and 500 μg of protein was combined with 4 μL of anti-PP2A antibody (1D6; Upstate Biotechnology) and 50 μL of protein-A-agarose beads in 500 μL. The mixture was shaken for 2 hours at 4°C, and then beads were collected by centrifugation. After 4 washes, 50 μL of phosphatase assay buffer (Upstate Biotechnology) was added to the beads, vortexed, and 50 μL of the bead slurry was added to one well of a 96-well plate. A 10mM stock of 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP; Invitrogen) was diluted to 100μM in assay buffer, and 50 μL was added to each well. Fluorescence intensity of the product of cleavage of phosphate from DiFMUP (a synthetic phosphatase substrate) was measured using a plate reader, every 3 minutes, with shaking every 30 seconds over a 30-minute period. Specificity of the phosphatase assay for PP2A was assessed by incubating the immunoprecipitated protein with 25nM okadaic acid (OA), a concentration that selectively for inhibits PP2A. Data presented represent the phosphate release of a sample with the background level of phosphate release of the OA-inhibited control subtracted.
Affinity purification of SET using biotin labeled COG peptides
CLL cells were lysed in NP40 buffer (50mM Tris, 0.2% NP40, 150mM NaCl) by a single freeze-thaw cycle lysate protein concentration adjusted to 5 mg/mL. Streptavidin agarose beads (1 mL) were washed with 10 mL of NP40 buffer, and 0.5 mL of beads was added to 1 mL of extract. Beads were then collected by filtration through a disposable mini-column (Bio-Rad), and the flow-through extract was collected for analysis. After washing with 100 mL of chilled NP40 buffer, the beads were removed from the column, collected by centrifugation, and 75 μL of 4× Laemmli buffer was added. Beads were vortexed and heated to 90°C for 10 minutes to ensure that all proteins were released from the beads. Proteins were separated by SDS-PAGE and Western blotting was used to determine whether the COG133 and COG112 peptides bound to both isoforms of SET from a CLL patient sample.
Annexin-V/propidium iodide assay for apoptosis
Apoptosis was measured using the annexin V–FITC apoptosis detection kit (BD Biosciences PharMingen) according to the manufacturer's instructions.20 Briefly, COG-treated or untreated cells were stained with annexin V-FITC and propidium iodide for 15 minutes in 1 × binding buffer (10mM HEPES, pH 7.4, 140mM NaCl, 2.5mM CaCl2) and analyzed by flow cytometry using a FACSCalibur instrument (BD Biosciences). Data were analyzed using CellQuest Version 3.3 software (BD Biosciences).
CLL cell culture/cytotoxicity assays
For cytotoxicity assays, 3 × 106 CLL cells/well were cultured in 24-well tissue culture plates in 1.5 mL of Hybridoma SFM (Invitrogen) as described by Levesque et al.21,22 All cultures were incubated at 37°C, 5% CO2 in air. Compounds were applied to CLL cells (0.25 × 106 cells/well in a 96-well plate), and after 72 hours viable cells were assessed using the MTS assay (Pharmacia) to determine the concentration of COG compound that was effective in displaying 50% cytotoxicity for the CLL cells (ED50).22
Xenograft model of tumor growth suppression
Female SCID mice (6-8 weeks old) were injected subcutaneously on left flank with 107 Ramos cells in 200 μL PBS. Mice were monitored daily for tumor growth by palpation. When tumors became large enough for caliper measurement, tumor volumes were calculated as (length × width2) × 0.5, as described by Schliemann et al.23 Once tumors reached 50-100 mm3, we randomly assigned mice to 2 groups, with approximate equalization of tumor volumes between groups. COG449 (5 mg/kg) or a vehicle control was administered by daily subcutaneous injection (5 mL/kg injection volume). At the end of the experiment, tumors were dissected, photographed, and wet weights of each tumor were measured.
Statistical analyses
If quantitative data were normally distributed, single comparisons between groups were made by Student t test; if not, log transformation or nonparametric Mann-Whitney testing was used. When multiple comparisons were made between groups, the data were analyzed using 2-way ANOVA with the Newman-Keuls posttest. Kaplan-Meier curves were used to graphically display OS and TTT relative to SET levels. Statistical significance for TTT and OS was analyzed using the proportional hazards regression and χ2 tests, using an α of .05.
Results
SET is overexpressed in B-CLL cells
We isolated mRNA from freshly prepared B-CLL cells from patients and normal B cells from healthy volunteers and determined SET mRNA levels by quantitative RT-PCR. SET mRNA levels were 11.2 ± 2.7-fold higher in CLL cells than the mean of the normal B cells (P = .03; Figure 1A). Immunoblots from these same samples using an anti-SET antibody demonstrated that both the 290 amino acid α-isoform (accession no. NP_001116293) and the 277 amino acid β-isoform (accession no. NP_003002) that arise from alternative splicing were higher in the CLL cells than in normal B cells (Figure 1B). The total SET(α + β) values were higher in CLL cells (0.048 ± 0.004) than in normal B cells (0.010 ± 0.003, P = .014; Figure 1C). We also evaluated SET expression in the Raji and Ramos cell lines of human B-cell NHL. Unlike CLL cells, these cells proliferate rapidly in vitro and can be more easily genetically manipulated by lentiviral transduction. SET mRNA in Raji cells was 10.5 ± 0.7-fold higher than in normal B cells and 8.2 ± 0.4-fold higher in Ramos cells (P = .0002) than in normal B cells (Figure 1D), which was similar to the overexpression levels observed in the CLL samples. Immunoblot analysis revealed elevated levels of SET protein in both Raji and Ramos cells relative to normal B-cell extracts (Figure 1E). Similar to the CLL cells, both the α- and β- isoforms of SET were overexpressed in the NHL cell lines to comparable degrees, whereas the 277 amino acid β-isoform was more prominent than the α-isoform in normal B-cell samples (Figure 1F).
SET is overexpressed in CLL and NHL. (A) mRNA was isolated from 15 CLL patients and 6 normal B-cell samples, and SET mRNA was quantified by quantitative PCR. (B) Representative immunoblot of 2 normal B-cell extracts (N37 and N42) and 2 CLL cell extracts showing overexpression of both the α- and β-isoforms of SET as indicated in the CLL cells. Vertical lines have been inserted to indicate a repositioned gel lane. (C) SET/GAPDH protein ratios measured for 16 CLL patients and 6 normal B-cell samples used in panel A, showing a significant increase in expression of SET mRNA in B-CLL cells relative to normal B cells. (D) SET mRNA levels from 2 normal B-cell samples and Raji and Ramos cells quantified by quantitative PCR. ***P < .001 (E) Immunoblotting of 2 normal B-cell (N4 and N7) and Raji and Ramos cell extracts. Although SET levels appear higher in the normal samples, it is because of higher-intensity settings for this blot than the normals shown compared with the CLL samples in panel B. (F) Quantitation of the α- and β-isoforms of the SET protein (normalized to GAPDH) for Raji and Ramos NHL cell lines showing that the major increase in SET levels is because of increased α-isoform expression.
SET is overexpressed in CLL and NHL. (A) mRNA was isolated from 15 CLL patients and 6 normal B-cell samples, and SET mRNA was quantified by quantitative PCR. (B) Representative immunoblot of 2 normal B-cell extracts (N37 and N42) and 2 CLL cell extracts showing overexpression of both the α- and β-isoforms of SET as indicated in the CLL cells. Vertical lines have been inserted to indicate a repositioned gel lane. (C) SET/GAPDH protein ratios measured for 16 CLL patients and 6 normal B-cell samples used in panel A, showing a significant increase in expression of SET mRNA in B-CLL cells relative to normal B cells. (D) SET mRNA levels from 2 normal B-cell samples and Raji and Ramos cells quantified by quantitative PCR. ***P < .001 (E) Immunoblotting of 2 normal B-cell (N4 and N7) and Raji and Ramos cell extracts. Although SET levels appear higher in the normal samples, it is because of higher-intensity settings for this blot than the normals shown compared with the CLL samples in panel B. (F) Quantitation of the α- and β-isoforms of the SET protein (normalized to GAPDH) for Raji and Ramos NHL cell lines showing that the major increase in SET levels is because of increased α-isoform expression.
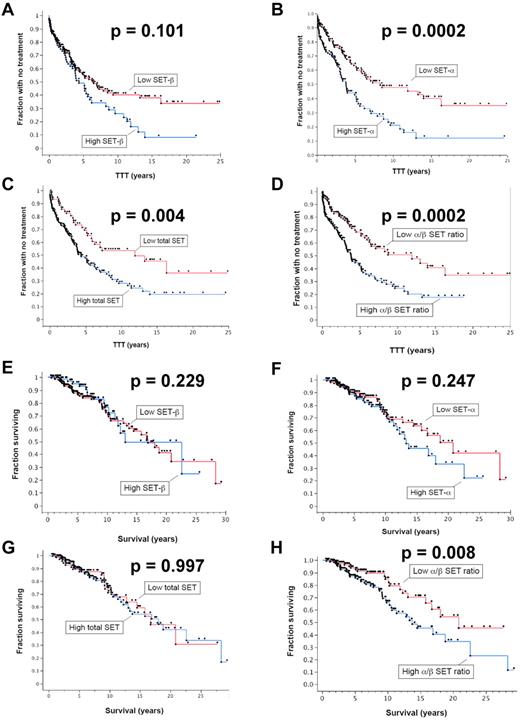
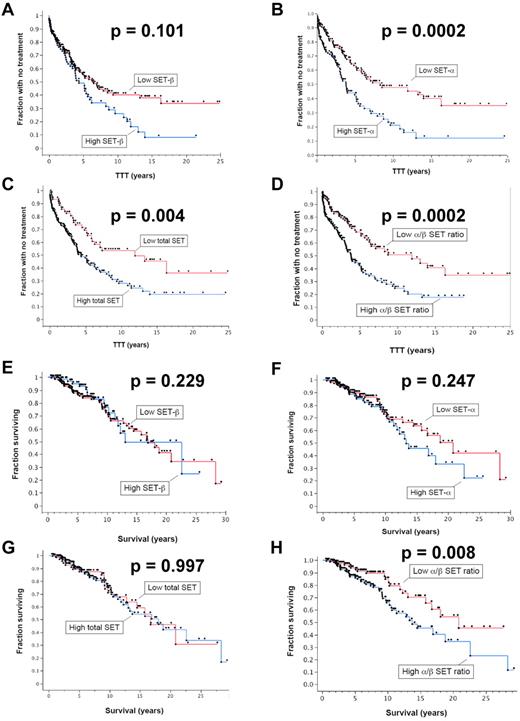
SET levels are predictive of TTT and OS
To determine whether SET levels are indicative of more rapid CLL disease progression, we used Western blotting to quantify the levels of each isoform of the SET protein in cell extracts from 285 patients. Patients were selected for this study based on the availability of stored samples from first diagnosis and patient history. Detailed patient information is given in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We used Kaplan-Meier curves to display differences in time to first treatment (TTT) and OS relative to SET levels (Figure 2). Analysis of the TTT for the patients with high and low levels of the β-isoform of SET showed more rapid progression (shorter TTT) in patients with high SETβ (Figure 2A), but this was not statistically different. However, patients with high SETα (P = .0004; Figure 2B), high total (α + β) SET (P = .0005; Figure 2C), and a high α/β ratio had statistically significantly shorter TTT (P = .019; Figure 2D), indicating that higher SET levels correlated with more rapid progression as signified by the earlier need for therapeutic intervention. The TTT values and statistical parameters are given in Table 1. Patients with elevated SET levels were more likely to have received treatment (n = 140) for the disease than the patients who had not received treatment (n = 145) for SETα (P = .004), for total α + β SET (P = .015), and for α/β ratio (P = .0001).
Cellular SET protein levels correlate with parameters of CLL progression. The time from diagnosis to first needed treatment (TTT) was assessed relative to SET protein levels in CLL cells as determined by quantitative immunoblotting. Patients with high levels of SET were compared with those with low levels of SET for (A) the β-isoform, (B) the α-isoform, (C) the total (α + β) SET, and (D) the numerical ratio of the α-isoform and β-isoform (α/β ratio) (n = 285). Those with high SETα, high total SET(α + β), and high α/β ratios had statistically significantly shorter TTT. The OS for patients with high levels of SET were compared with those with low levels of SET for (E) SETβ, (F) SETα, (G) total SET(α + β), and (H) the numerical ratio of the α-isoform and β-isoform (α/β ratio) (n = 285). The OS was significantly shorter in patients with high α/β ratios of the SET isoforms (n = 285).
Cellular SET protein levels correlate with parameters of CLL progression. The time from diagnosis to first needed treatment (TTT) was assessed relative to SET protein levels in CLL cells as determined by quantitative immunoblotting. Patients with high levels of SET were compared with those with low levels of SET for (A) the β-isoform, (B) the α-isoform, (C) the total (α + β) SET, and (D) the numerical ratio of the α-isoform and β-isoform (α/β ratio) (n = 285). Those with high SETα, high total SET(α + β), and high α/β ratios had statistically significantly shorter TTT. The OS for patients with high levels of SET were compared with those with low levels of SET for (E) SETβ, (F) SETα, (G) total SET(α + β), and (H) the numerical ratio of the α-isoform and β-isoform (α/β ratio) (n = 285). The OS was significantly shorter in patients with high α/β ratios of the SET isoforms (n = 285).
When performing a similar analysis to compare SET levels with OS, patients with high and low levels of the SETβ, SETα, and total SET(α + β) showed no significant difference in OS (Figure 2E-G). However, patients with high α/β ratios had significantly different OS than patients with low ratios of the 2 isoforms (P = .0005; Figure 2H), with differences in median survival indicated in Table 1. CLL patients with high α/β ratios had significantly shorter OS than did patients with low α/β ratios where median survival was 4.1 years shorter in the group with higher α/β ratios (Table 1; P = .008). We also analyzed whether elevated SET levels (SETβ, SETα, total SET(α + β), and α/β ratios) correlated with known prognostic factors, including Rai stage, CD38 expression, IGVH mutational status, Zap-70 expression, and cytogenetic abnormalities (Table 2). We found that elevated SETα and total SET(α + β) levels correlated with increasing Rai stage (P values given in Table 2). Elevated SET α/β ratios correlated significantly with CD38 expression, and elevated SETα, total SET(α + β), and α/β ratios correlated significantly with IGVH mutational status. No significant correlations were observed between SET expression levels and Zap-70 expression or cytogenetic abnormalities. Lack of correlation with cytogenetic abnormalities could relate in part to low numbers of patients in the cytogenetic abnormality subgroups.
Antagonism of SET activates PP2A
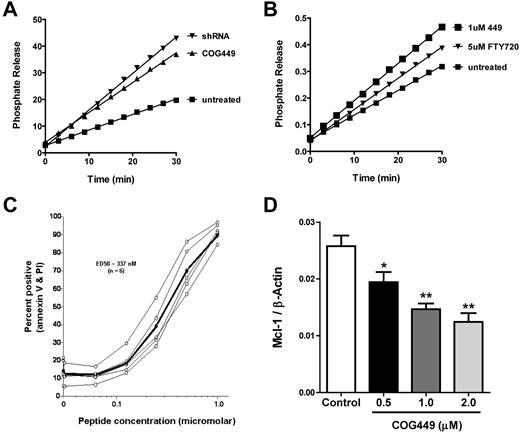
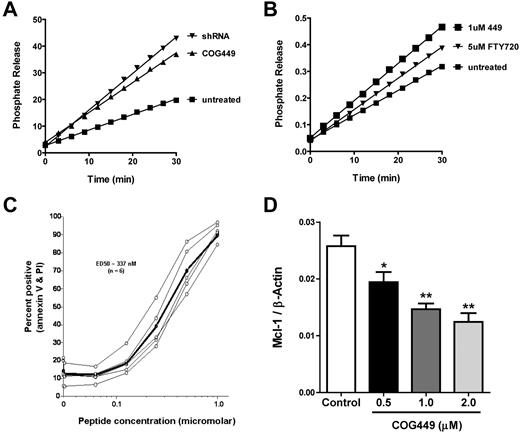
We have previously described peptide antagonists of SET that activate PP2A and lead to dephosphorylation of Akt and the p38 MAPK.24 We confirmed that these peptides bound to both isoforms of SET in cell lysates from CLL samples (supplemental Figure 1). CLL cells generally do not proliferate in vitro, and they are difficult to transfect. Therefore, we measured PP2A activity in 32D:BCR/Abl, cells with elevated SET levels and suppressed PP2A activity.3 We infected these cells with lentivirus encoding a SET-specific shRNA or a control noncoding lentivirus and confirmed reduction of SET by Western blotting (supplemental Figure 2). Cells infected with a control lentivirus were treated with COG449 (a dimerized derivative of COG11225,26 ) or a vehicle control, and PP2A activity was measured. SET antagonism with either SET-shRNA or COG449 increased the activity of PP2A relative to control (Figure 3A). Furthermore, we found that COG449 treatment at 1μM increased the activity of PP2A in these cells to a greater level than did 5μM FTY720 (Figure 3B), a PP2A activator reported by Neviani et al.27
SET antagonism activates PP2A, induces apoptosis, and reduces Mcl-1 levels. (A) SET was knocked down by shRNA to SET after lentiviral transduction in 32D:BCR/Abl cell cultures or treatment with 1μM COG449 followed by lysis with NP40 lysis buffer. PP2A was immunoprecipitated and assayed with the PP2A immunoprecipitation assay kit (Upstate Biotechnology), with the exception of using DiFMUP as a fluorescent substrate. The line for each sample represents the phosphate release from a given sample after subtraction of the OA-inhibited control reactions (P < .05). (B) 32D:BCR/Abl cells were treated with the indicated compounds for 30 minutes followed by lysis with NP40 lysis buffer. PP2A was immunoprecipitated and assayed as in panel A (P < .01). (C) CLL cells were treated with COG449 followed by annexin-V and propidium iodide staining to assess apoptosis and death. (D) Freshly isolated human CLL cells were incubated with the indicated concentrations of COG449 for 24 hours. Cells were lysed, protein lysates subjected to PAGE, and immunoblotted to quantify the Mcl-1 and β-actin ratio. *P < .01 (relative to control). **P < .001 (relative to control).
SET antagonism activates PP2A, induces apoptosis, and reduces Mcl-1 levels. (A) SET was knocked down by shRNA to SET after lentiviral transduction in 32D:BCR/Abl cell cultures or treatment with 1μM COG449 followed by lysis with NP40 lysis buffer. PP2A was immunoprecipitated and assayed with the PP2A immunoprecipitation assay kit (Upstate Biotechnology), with the exception of using DiFMUP as a fluorescent substrate. The line for each sample represents the phosphate release from a given sample after subtraction of the OA-inhibited control reactions (P < .05). (B) 32D:BCR/Abl cells were treated with the indicated compounds for 30 minutes followed by lysis with NP40 lysis buffer. PP2A was immunoprecipitated and assayed as in panel A (P < .01). (C) CLL cells were treated with COG449 followed by annexin-V and propidium iodide staining to assess apoptosis and death. (D) Freshly isolated human CLL cells were incubated with the indicated concentrations of COG449 for 24 hours. Cells were lysed, protein lysates subjected to PAGE, and immunoblotted to quantify the Mcl-1 and β-actin ratio. *P < .01 (relative to control). **P < .001 (relative to control).
Antagonism of SET induces apoptosis, reduces Mcl-1 levels, and is cytotoxic to malignant B cells in vitro and in vivo
Based on the increased activity of PP2A after SET antagonism using COG449, we evaluated the induction of apoptosis in primary CLL cells after treatment with COG449. CLL cells from 7 patients were treated with COG449 or medium control for 2 hours. After treatment, cells were stained with annexin-V and propidium iodide and analyzed by flow cytometry. We observed a dose-dependent increase in annexin-V/propidium iodide staining with an ED50 of 330nM (Figure 3C). To analyze the modulation of antiapoptotic proteins in CLL cells, we measured the effect of COG449 treatment on the antiapoptotic Bcl-2 family member Mcl-1 and observed a dose-dependent decrease in the cellular level of Mcl-1 (Figure 3D).
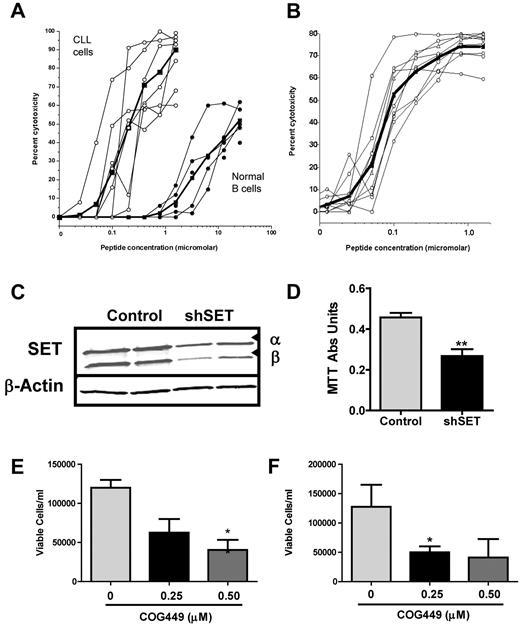
Based on the induction of apoptosis in CLL cells, we tested several analogs of COG449 for cytotoxicity against CLL cells freshly isolated from a number of patients and against normal B cells from healthy volunteers (supplemental Table 2). Cells were cultured with the agents for 72 hours, and the viable cells were assessed for cytotoxicity using the MTS assay. COG445 was cytotoxic to CLL cells isolated from patients with an ED50 of 110nM (Figure 4A). In stark contrast, the EC50 for cytotoxicity in the B cells from normal volunteers was found to be nearly 2-log units higher at ≥ 10 000nM. Similarly, COG449 was cytotoxic with an ED50 of 103nM (Figure 4B). We also analyzed the effect of shRNA-mediated knockdown of SET on the growth of B-cell lymphoma cell lines. Knockdown of SET in Raji cells after infection with a SET-specific shRNA lentivirus resulted in a reduction of SET levels by ∼ 50% (Figure 4C). This SET knockdown reduced the growth after 72 hours (P = .009; Figure 4D). Antagonism of SET with COG449 also inhibited growth of Raji and Ramos NHL cells in vitro (Figures 4E-F). To determine whether this cytotoxicity was modulated by PP2A, we treated Ramos cells with 1nM OA (PP2A inhibitor) and found that this concentration of OA was not cytotoxic. However, treatment of the Ramos cells with 1μM COG449 produced a robust cytotoxic effect that could be partially counteracted by treatment with OA (supplemental Figure 3).
SET antagonism is cytotoxic to CLL cells and inhibits NHL cell growth in vitro. (A) Dose-response curves for COG445 treatment of CLL cells from 7 patients or normal B cells from 5 volunteers. (B) Dose-response curves for COG449 treatment on CLL cells from 7 patients. (C) Raji NHL cells were transduced with SET shRNA or control lentivirus, and knockdown was assessed by Western blotting from 2 samples of each. (D) Growth of Raji cells from panel C was assessed 72 hours after knockdown using a tetrazolium assay and demonstrated that SET antagonism inhibited growth. **P < .01. (E) Growth inhibition of Raji cells treated with the indicated levels of COG449 for 72 hours. (F) Growth inhibition of Ramos cells treated with the indicated levels of COG449 for 72 hours. *P < .05
SET antagonism is cytotoxic to CLL cells and inhibits NHL cell growth in vitro. (A) Dose-response curves for COG445 treatment of CLL cells from 7 patients or normal B cells from 5 volunteers. (B) Dose-response curves for COG449 treatment on CLL cells from 7 patients. (C) Raji NHL cells were transduced with SET shRNA or control lentivirus, and knockdown was assessed by Western blotting from 2 samples of each. (D) Growth of Raji cells from panel C was assessed 72 hours after knockdown using a tetrazolium assay and demonstrated that SET antagonism inhibited growth. **P < .01. (E) Growth inhibition of Raji cells treated with the indicated levels of COG449 for 72 hours. (F) Growth inhibition of Ramos cells treated with the indicated levels of COG449 for 72 hours. *P < .05
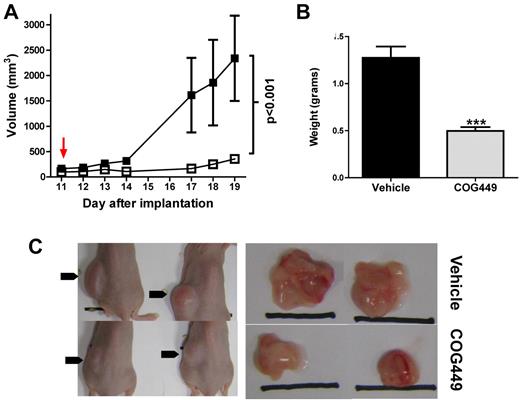
Based on the cytotoxicity of SET antagonism in vitro, we sought to determine whether pharmacologic antagonism of SET with COG449 could reduce growth of cancerous B cells in vivo using a murine model. To determine whether the transgenic TCL-1 model28 was a good candidate for testing of SET antagonist peptides, we isolated malignant CD5+ cells from the spleen of a TCL-1 mouse and performed Western blotting to determine whether SET was overexpressed in these mice. We found that, unlike human CLL cells, SET was not overexpressed in the TCL-1 mouse leukemia-like cells (supplemental Figure 4). Therefore, we used a murine xenograft model with human Ramos NHL cells that overexpress SET. Female SCID mice were subcutaneously injected with 107 cells into the left flank, and tumor growth was monitored daily by palpation and caliper measurement until tumors reached ∼ 150 mm3 on day 11. Mice were randomly assigned to 2 groups so that initial tumor size was approximately equal between groups. Daily treatment with COG449 (5 mg/kg, subcutaneous injection into the right shoulder area) or a vehicle control was initiated and measurements were performed by an experimenter who was blinded to the treatment agents (Figure 5A). At day 19 (a time when control tumor volume reached maximum volume allowed for humane reasons), mice were killed. Tumors were dissected, weighed (Figure 5B), photographed (Figure 5C), and segmented for pathologic examination. Statistical analysis by 2-way ANOVA indicated that tumor growth was significantly inhibited by COG449 (P = .0008) and final tumor mass was significantly lower in COG449-treated animals (P = .0009, t test). Disaggregated cells from 1 portion of the tumors were analyzed by flow cytometry and shown to be human B cells.
SET antagonism inhibits NHL cell growth in vivo. (A) Tumor volumes of Ramos cell tumor xenografts in SCID mice after in vivo treatment with 5 mg/kg COG449 (□) or lactated Ringer solution control (■) by subcutaneous injection. The injections were initiated on day 11, once tumors reached palpable sizes of 50-100 mm3. (B) Final tumor mass for treated and untreated Ramos tumors harvested on day 19 after implantation. ***P < .001 by t test. (C) Representative mice and their tumors.
SET antagonism inhibits NHL cell growth in vivo. (A) Tumor volumes of Ramos cell tumor xenografts in SCID mice after in vivo treatment with 5 mg/kg COG449 (□) or lactated Ringer solution control (■) by subcutaneous injection. The injections were initiated on day 11, once tumors reached palpable sizes of 50-100 mm3. (B) Final tumor mass for treated and untreated Ramos tumors harvested on day 19 after implantation. ***P < .001 by t test. (C) Representative mice and their tumors.
Discussion
Studies of a limited number of samples from CML patients and patients with other tumors have reported increased SET levels in isolated malignant cells.2,3 We sought to determine whether the SET oncoprotein was overexpressed in CLL cells relative to normal B cells and to quantify the effect of SET overexpression on parameters of disease. We observed that SET was significantly overexpressed in CLL and NHL cells relative to normal B cells and that the level of overexpression in patient-derived CLL cells correlated with disease severity. In addition, we noted that novel SET antagonist peptides were selectively cytotoxic for CLL and NHL cells in vitro and that COG449 inhibits growth of human NHL xenografts in immunosuppressed mice in vivo.
Two isoforms of SET arise from differential splicing, and their expression is driven by distinct promoters for the α- and β-isoforms. As in CLL, we observed SET overexpression in the Raji and Ramos cell lines of Burkitt lymphoma relative to normal B cells. Although normal B cells predominantly express the β-isoform of SET, we noted that both the α- and β-isoforms were expressed at similar levels in CLL and NHL cells. These isoforms differ only in the N-terminal portion of the protein in which the α-isoform has a 37-amino acid N-terminal region that arises from exon 1 of the SET gene, whereas the β-isoform has a 24-amino acid N-terminal region that arises from exon 2 of the SET gene. The remaining 253 amino acids are identical in each isoform and arise from exons 3 to 9.29 Although the mechanisms that regulate expression of each of these isoforms have not been fully elucidated, Asaka et al reported that the Sp1 transcription factor selectively binds to the SET promoter region upstream of Exon 1 and drives transcription of the α-isoform.29
We found that patients with high SET levels have more aggressive CLL disease as determined by the requirement for earlier therapeutic intervention. Indeed, we found that patients with elevated SET levels were more likely to have received treatment for the disease than the patients who had not received treatment. To our knowledge, our work is the first to document that cellular SET levels significantly relate to disease severity or progression. Furthermore, we found that patients with higher numerical ratios of the α-isoform to β-isoform of SET have significantly reduced OS than do patients with lower numerical ratios or with lower absolute levels of the α-isoform. Again, this is the first time that SET levels have been linked to OS for any form of cancer. These data suggest that the activation of pathways that lead to overexpression of the α-isoform may also activate pathways that are associated with shorter OS. We also found that higher SET levels are found in unmutated IGVH CLL cells relative to mutated cells (P = .0006 for SETα and P = .002 for α/β ratio), and in CD38-positive CLL cells relative to CD38-negative cells (P = .004 for α/β ratio). This corresponds to a previous report in which expression profiling revealed elevated SET mRNA in unmutated IGVH CLL samples relative to those with mutated IGVH.30 This is consistent with the worse prognosis for patients with unmutated IGVH relative to those with mutated IGVH, and worse prognosis in those with high CD38 expression. Finally, we observed that SETα and total SET levels correlated with increased Rai stage (P = .017 and P = .033, respectively). The observation that elevated levels of SET, a PP2A inhibitor, correlate with worse outcome corresponds mechanistically to the observation that the 11q22–23 deletion confers a more aggressive CLL phenotype.31 The 11q22–23 deletion results in deletion of the PPP2R1B gene that encodes the Aβ constant regulatory subunit of PP2A and leads to reduced PP2A activity in these cells.32 We were unable to show a significant correlation between SET levels and cytogenetic abnormalities, including 11q deletions; however, it is unclear whether this relates to the low number of patients with a given cytogenetic abnormality evaluated in this study.
Inhibition of PP2A, which can be achieved through overexpression of SET, plays a key role in maintaining an antiapoptotic state. Phosphorylation of signaling kinases is typically associated with enhanced kinase activity toward downstream kinases in the pathway to propagate the signal. In normal cells, kinases and phosphatases play opposite roles in the maintenance of responses to external signals and the return to a basal-like, normal state. Therefore, regulation of kinase signaling pathways can largely be viewed as a balance between the activating kinases and the deactivating phosphatases. In the case of CLL, malignant CLL cells are maintained through defective apoptosis that is linked to activation of the Akt kinase and the ERK pathways. Akt phosphorylation at serine 473 after BCR engagement is required for survival of CLL cells.14 Negative regulation of both the Akt33,34 and ERK35 pathways is accomplished by PP2A-mediated removal of the activating phosphate. Akt activity in CLL cells is further enhanced through aberrant expression of TCL1, which binds to Akt and amplifies its kinase activity, conferring an aggressive CLL phenotype.36 In human CLL cells, Akt activation leads to regulation of proteins that are essential for cell survival through inactivation of proapoptotic proteins. Many proapoptotic proteins are activated by PP2A-mediated dephosphorylation to shift the cellular state toward apoptosis.37,38 Akt activation has also been linked to post-translational stabilization of antiapoptotic proteins, such as Mcl-1 through inhibition of glycogen synthase kinase-3β (GSK3β).15
CLL cells overexpress both Bcl-2 and Mcl-1,39 members of the antiapoptotic Bcl-2 family. High levels of Bcl-2 and Mcl-1 correlate with poor response to fludarabine therapy in patients.40 Mcl-1 levels increase after BCR engagement, and stimulation of the BCR may promote selection of neoplastic B-cell clones.41 CLL cells from patients with high Mcl-1 levels and low BAX levels (and thus high Mcl-1/BAX ratios) have significantly shorter TTT and lower OS than patients with lower Mcl-1:BAX ratios.42 In addition to CLL, Mcl-1 overexpression has been reported in B-cell NHL patients, in whom expression levels correlate directly with tumor grade.43 These data suggest that Mcl-1 may be the most significant antiapoptotic protein associated with B-cell malignancies.44 Cellular levels of Mcl-1 are controlled by transcription and post-translational stabilization. Maurer et al demonstrated that Mcl-1 levels decrease under conditions that reduced Akt activation.45 They also noted that Mcl-1 is phosphorylated at S159, a conserved GSK3β phosphorylation site, and that the destabilization after decreased activation of Akt could be counteracted by treatment with a GSK3β inhibitor. After phosphorylation of Mcl-1 at S159, the Mcl-1 is ubiquitinated and then degraded by the proteosome.46 GSK3β is inhibited by Akt-catalyzed phosphorylation at serine-9.47 This provides a mechanistic explanation for the observation that Mcl-1 levels are inversely correlated with GSK3β activity.48 We found that pharmacologic antagonism of SET results in reduced cellular levels of Mcl-1 and induction of apoptosis. Because PP2A removes the inactivating phosphate at serine-9 of GSK3β,47 the reduction in cellular Mcl-1 can be linked to PP2A activation. We have previously reported dephosphorylation of both Akt and GSK3β in an OA-sensitive manner by COG112, the parental peptide of COG449.49 Our observation matches the report of Liu et al that PP2A activation by FTY720 also resulted in a reduction of Mcl-1 levels in CLL cells.50
SET antagonism in these CLL cells induced apoptosis and cytotoxicity, whereas normal B cells with low SET expression are much less susceptible to SET agonist-induced cytotoxicity. We also found that pharmacologic or genetic antagonism of SET inhibits NHL cell growth in vitro. Importantly, we also noted that SET antagonism inhibits in vivo growth of Ramos cell NHL tumor xenografts in SCID mice at doses that show no adverse effects on the mice. Taken together, our data show, for the first time, that SET levels correlate with CLL disease severity and progression and that SET-mediated inhibition of PP2A activity is required to maintain the antiapoptotic state of CLL cells. Furthermore, SET-mediated inhibition of PP2A is required for inhibition of apoptosis and proliferation of NHL cells. Our data demonstrate that SET is a new treatment target in B-cell malignancies and that SET antagonists represent novel agents for treatment of CLL and NHL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute (D.J.C. and J.B.W.), the Bernstein Family Leukemia Research Fund (J.B.W., D.R.F., and M.C.L.), the Veterans Affairs Research Service (J.B.W.), and the Leukemia & Lymphoma Society (J.B.W.).
National Institutes of Health
Authorship
Contribution: D.J.C. and J.B.W. designed experiments, planned the research, obtained funding for the research, analyzed data, and prepared the manuscript; Y.C., J.O., K.M.M., J.N., A.D.V., B.K.G., and D.R.F. performed research and analyzed data; M.C.L., J.P.G., L.F.D., C.M.C., and J.O.M. provided patient samples; and M.P.V., D.R.F., and M.C.L. analyzed data and edited the manuscript.
Conflict-of-interest disclosure: D.J.C. and M.P.V. are shareholders and employees of Oncotide Pharmaceuticals. D.J.C., M.P.V., J.O., and J.N. are employees of Cognosci Inc. The remaining authors declare no competing financial interests.
Correspondence: Dale J. Christensen, Oncotide Pharmaceuticals, 79 T. W. Alexander Drive, PO Box 110606, Research Triangle Park, NC 27709; e-mail: dchristensen@oncotide.com.