Abstract
Hemophagocytic lymphohistiocytosis (HLH) used to have a dismal prognosis. We report the final results of HLH-94, the largest prospective diagnostic/therapeutic HLH study so far. The treatment includes immunosuppressive and cytotoxic therapy aiming at clinical remission, followed by HSCT in patients with familial, persistent, or recurrent disease. Altogether, 249 patients fulfilled inclusion criteria and started HLH-94 therapy (July 1994-December 2003); 227 (91%) were followed-up for ≥ 5 years. At 6.2 years median follow-up, estimated 5-year probability of survival was 54% ± 6%. Seventy-two patients (29%) died before HSCT, 64 within 1 year, 97% of whom had active disease. In 124 patients who underwent HSCT, 5-year survival was 66 ± 8%; tendency to increased survival (P = .064) in patients with nonactive disease at HSCT. Patients with familial disease had a 5-year survival of 50% ± 13%; none survived without HSCT. Patients deceased during the first 2 months more often had jaundice, edema, and elevated creatinine. Forty-nine patients (20%) were alive without signs of HLH activity and off-therapy > 1-year without HSCT; they presented at older age (P < .001), were more often female (P = .011), and less often had CNS disease (P < .001) or hepatomegaly (P = .007). To conclude, HLH-94 chemoimmunotherapy has considerably improved outcome in HLH. Collaborative efforts are needed to further reduce early mortality, HSCT-related mortality, and neurologic late effects.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) has had a dismal prognosis; in 1983, long-term survival was reported as 4%.1 Since then, and with the introduction of an international treatment collaboration, survival has increased dramatically worldwide.2,3 We present a summary of the long-term outcome of the first prospective international HLH treatment study, HLH-94.
HLH comprises a group of disorders characterized by accumulation of lymphocytes and macrophages, often with phagocytosis by macrophages of blood cells (hemophagocytosis).4 The inherited form, familial hemophagocytic lymphohistiocytosis (FHL), is autosomal recessive and affects mostly infants and young children, but has also been reported in adolescents and adults.5-7 Disease-causing mutations have so far been reported in 4 genes, PRF1,8 UNC13D,9 STX11,10 and STXBP2,11 coding for proteins crucial for lymphocyte cytotoxicity.12 Other genetic syndromes associated with HLH are Griscelli syndrome type 2 (GS2),13 Chédiak-Higashi syndrome (CHS),14 and X-linked lymphoproliferative disorder type 1 and 2 (XLP1, XLP2).15,16 Secondary HLH (sHLH) can be triggered by infections or be related to systemic rheumatologic diseases or malignancies. In the absence of a known family history or a genetically confirmed diagnosis, differentiation between FHL and sHLH is difficult.17
HLH is caused by a deficient down-regulation of the immune response, leading to an unbridled inflammation. Symptoms include fever, hepatosplenomegaly, lymphadenopathy and, if the CNS is affected, neurologic symptoms.18,19 Laboratory work-up typically shows cytopenia, hypofibrinogenemia, and elevated triglycerides, ferritin, transaminases and cytokines. The histopathologic hallmark is accumulation of lymphocytes and macrophages, sometimes involved in hemophagocytosis, in BM, lymph nodes, and spleen, and/or other organs.1,2,4,19 Diagnostic guidelines for HLH were first presented in 199120 (Table 1) and these were used in the HLH-94 study. On January 1, 2004, a modified study protocol (HLH-2004, which is still open) with revised diagnostic criteria was introduced.21
In addition to presenting long-term outcome of patients treated according to the HLH-94 protocol, we descriptively present findings at initial presentation and how these findings differ between subgroups of HLH patients. We further focus on patients deceased during initial therapy and on patients who survived without continuous chemoimmunotherapy or HSCT.
Methods
The HLH-94 treatment protocol
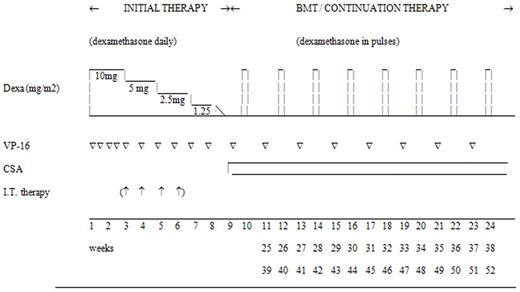
The overall aim of the HLH-94 treatment protocol was to induce and maintain a state of resolution of the disease to ultimately cure primary, persistent, and relapsing forms by HSCT. The protocol included an initial intensive therapy with immunosuppressive and cytotoxic agents for 8 weeks, with the aim to induce remission of the disease activity (Figure 1). This initial therapy included etoposide, which is proapoptotic in HLH,22 twice weekly during the first 2 weeks, and then weekly, in combination with dexamethasone (initially 10 mg/m2 for 2 weeks followed by 5 mg/m2 for 2 weeks, 2.5 mg/m2 for 2 weeks, 1.25 mg/m2 for 1 week, and 1 week of tapering). Intrathecal methotrexate was recommended for patients with progressive neurologic symptoms and/or persisting abnormal cerebrospinal fluid findings. Patients with nonfamilial disease that resolved after 8 weeks could then stop treatment, and restart only if signs of reactivation occurred, to avoid prolonged therapy and HSCT for patients with secondary HLH. For patients with familial, persistent, or relapsing disease, continuation therapy was recommended to keep the patient in remission until an allogeneic HSCT could be performed. The continuation therapy consisted of pulses of dexamethasone in combination with etoposide and cyclosporine A (CSA). HSCT was recommended as soon as a suitable donor was found, and the suggested conditioning included a combination of busulfan, cyclophosphamide, etoposide, and, if the donor was unrelated, antithymocyte globulin (ATG). The HLH-94 was approved by the Histiocyte Society and the Ethics Committee of the Karolinska Institutet. The funding sources had no involvement in any part of design, results, or publication.
Overview of the HLH-94 treatment protocol. BMT: Patients with familial or persistent disease were recommended to go to HSCT as soon as an acceptable donor was available, preferably when the disease was nonactive. The patients without familial or persistent disease were recommended to stop therapy after the initial therapy, and restart in case of reactivation. Dexa: Daily dexamethasone (10 mg/m2 for 2 weeks followed by 5 mg/m2 for 2 weeks, 2.5 mg/m2 for 2 weeks, 1.25 mg/m2 for 1 week, and 1 week of tapering; pulses were 3 days, 10 mg/m2 daily). VP-16: Etoposide 150 mg/m2 IV. IT therapy: Intrathecal methotrexate in patients with progressive neurological symptoms and/or persisting abnormal cerebrospinal fluid findings. CSA: Cyclosporin A aiming at blood levels of 200 μg/L (trough value).
Overview of the HLH-94 treatment protocol. BMT: Patients with familial or persistent disease were recommended to go to HSCT as soon as an acceptable donor was available, preferably when the disease was nonactive. The patients without familial or persistent disease were recommended to stop therapy after the initial therapy, and restart in case of reactivation. Dexa: Daily dexamethasone (10 mg/m2 for 2 weeks followed by 5 mg/m2 for 2 weeks, 2.5 mg/m2 for 2 weeks, 1.25 mg/m2 for 1 week, and 1 week of tapering; pulses were 3 days, 10 mg/m2 daily). VP-16: Etoposide 150 mg/m2 IV. IT therapy: Intrathecal methotrexate in patients with progressive neurological symptoms and/or persisting abnormal cerebrospinal fluid findings. CSA: Cyclosporin A aiming at blood levels of 200 μg/L (trough value).
Presenting features and follow-up information were collected on study-specific forms, completed by the treating hospital at diagnosis and 2 and 6 months after therapy start. Follow-up was then updated annually for patients on therapy and/or with signs of active HLH in the last year. For patients who received transplantations, questionnaires were requested at the time of HSCT, 100 days thereafter, and then yearly. Cutoff for data entry was October 2008.
Inclusion criteria and method
Included patients were < 16 years, started HLH-94 therapy July 1, 1994 to December 31, 2003, had no previous cytotoxic or CSA therapy and no known underlying chronic or malignant disease. They fulfilled all HLH diagnostic criteria (Table 1) or had a familial history in combination with a clinical picture suggestive of HLH.
Statistical analyses were performed with SPSS 11.5/SPSS 17.0. Comparison was performed by cross-tabulation for categorical variables (χ2 test or, where frequencies were small, Fisher exact test) and Mann-Whitney tests for continuous variables. Probability of survival was estimated by the Kaplan-Meier method, and differences in survival between groups by the log-rank test. A 2-sided P value of < .05 was considered significant.
Results
Overall results
Included/excluded patients.
Altogether 262 patients fulfilled inclusion criteria. Thirteen patients were excluded because of other diagnoses (GS2, n = 5; CHS, n = 2; metabolic disease, n = 1; malignancy, n = 2; systemic rheumatic disease, n = 3). The remaining 249 patients were recruited from 25 countries: Australia, Argentina, Austria, Canada, the Czech Republic, Denmark, Finland, Germany, Hong Kong, Iceland, Italy, Japan, Korea, The Netherlands, Norway, Oman, South Africa, Saudi Arabia, Spain, Sweden, Switzerland, Turkey, the United Kingdom, the United States of America, and the former Republic of Yugoslavia.
Completeness of data.
Altogether 227 patients (91%) have a follow-up of ≥ 5 years after therapy start provided they survived that long, or a follow-up of ≥ 5 years after HSCT in patients who received transplants. Moreover, follow-up information up to the end of data entry, that is, up to 13.7 years, was available in 177 of 249 (71%) patients. In patients who did not receive transplants with no signs of reactivation 1 year after therapy cessation further follow-up was optional, still 39 of 49 (80%) have a follow-up of at least 5 years.
Major treatment changes.
Major treatment changes were defined as CSA introduced during initial therapy or administration of cytotoxic agents not prescribed in the protocol. Supportive therapy or antiviral agents for EBV, including rituximab, was accepted. All patients with major treatment changes were included in all outcome analyses. Seven patients were shifted to alternative treatment because of persistent disease (n = 5) or HLH reactivation. In addition, 18 patients started CSA treatment already during initial therapy (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In total, 9 (36%) patients with major treatment changes survived.
Presenting features.
The main features at diagnosis are summarized in Table 2. Overall, there was a slight but nonsignificant male preponderance (55%, 95% confidence interval [CI] 49%-61%). The median age at therapy initiation was 8 months (range, 2 days to 15 years); 56% of the patients were younger than 1 year, and 76% younger than 2 years (supplemental Figure 2). In addition to clinical diagnostic criteria, hepatomegaly was the most common clinical finding, present in 95%. Less than half had edema, and approximately one-third had jaundice, rash, or lymphadenopathy. Neurologic symptoms were reported in 33% of the patients before therapy start and a history of recent infection at the onset of HLH symptoms in 45%.
Hemophagocytosis was common in BM (228/246, 92%) but was also reported from spleen (19/26), lymph nodes (12/28), liver (14/41), and cerebrospinal fluid (50/158). Almost all patients had thrombocytopenia (241, 97%) or anemia (220, 88%), whereas only 169 of 246, 69%, had neutropenia. Ferritin levels (normally < 200 μg/L in children > 2 months of age23 ) were > 5000 in 42% of the patients, where 60% had values > 10 000 μg/L; median was 2950 μg/L. Elevated cerebrospinal fluid protein or cell counts were found in 80 of 197 and 82 of 205, respectively, and at least one of these was abnormal in 107 of 203 of the children.
Disease course and outcome.
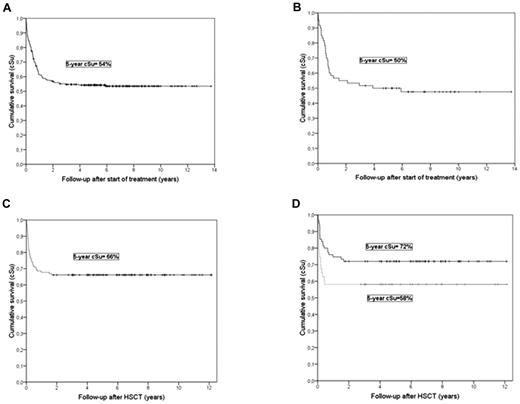
The median follow-up was 6.2 years (range 0.4-13.7). At last follow-up, 135 (54%) children were alive with a 5-year cumulative probability of survival of 54% (95% CI ± 6%). If patients with major treatment modifications were excluded, 5-year survival was 56% ± 6%. The probability of survival is described in detail in Figure 2. Altogether, the therapy induced permanent remission or kept the patient alive until transplantation in 71% (Table 3).
Kaplan-Meier survival curves. (A) All eligible study patients treated with HLH-94 (n=249). (B) All patients with an affected sibling (n=60). (C) All patients who received transplants (n=124). (D) Survival related to HLH disease activity at HSCT (nonactive disease: black line; active disease: grey line; the time in both panels C and D is shown as the time from HSCT).
Kaplan-Meier survival curves. (A) All eligible study patients treated with HLH-94 (n=249). (B) All patients with an affected sibling (n=60). (C) All patients who received transplants (n=124). (D) Survival related to HLH disease activity at HSCT (nonactive disease: black line; active disease: grey line; the time in both panels C and D is shown as the time from HSCT).
After the initial treatment of 2 months, 214 (86%) patients were alive, and 122 (59%) of 207 of these had a reported nonactive disease (NAD). Clinically relevant sequelae were reported in 37 (28%) of 133 survivors (Table 3). Neurologic late effects were reported in 19% and ranged from severe mental retardation, cranial nerve paresis, epilepsy, speech delay, learning difficulties and attention-deficit/hyperactivity disorder (ADHD) to noncranial nerve palsy. Non-neurologic late effects were reported in 16%, including nutritional problems and/or growth retardation, hypertension, impaired renal function, obstructive bronchiolitis and hearing impairment. One patient developed acute myelogenous leukemia (AML) 6 months after treatment start, received a transplantation, and survived.
Prognostic factors.
We examined the influence of age at diagnosis, CNS disease, familial disease, and treatment period on the risk for death by univariate analyses. As previously reported, presence of neurologic symptoms and abnormal cerebrospinal fluid at diagnosis is a poor prognostic sign.18 In this group (n = 52), 5-year survival was 40% ± 14% compared with 67% ± 11% in the patients with no neurologic symptoms and normal cerebrospinal fluid (n = 76; P < .01). Furthermore, young age at start of therapy was associated with poor survival (113 patients aged < 6 months at onset of therapy had an estimated 5-year survival of 41% ± 9%, compared with a survival of 65% ± 8% for 136 patients age older than 6 months (P < .001). There was no significant prognostic influence of familial disease, or if treatment was initiated during the first, middle, or last third of the study period.
Subgroup characteristics and results
Familial patients.
The protocol was initiated in 1994, and the first gene mutations causing HLH were reported in 1999. Therefore, “familial” patients were defined as having an affected sibling, and were not included because of genetic findings. Sixty (24%) of 247 patients had an affected sibling (missing data because of adoption, n = 2); for 49 children, this information was available at onset; in 11 a sibling was diagnosed later on. Four sibling pairs have been included. The 60 patients with familial disease were younger (P < .001), more frequently had consanguineous parents (P = .017), less frequently had had a recent infection (P = .044), elevated ferritin (P = .012), or lymphadenopathy (P = .016).
Overall survival and time to response to treatment did not differ in patients with or without a familial history (Table 3). Altogether, 29 (48%) of 60 patients with familial disease were alive at last follow-up, with a median follow-up of 7.5 years (range 3.4-13.7). The 5-year survival was 50% ± 13% (Figure 2B). Seventy-three percent underwent HSCT after a median time of 4.7 months (range 2-38) in the 37 patients with familial disease known at onset, compared with 6.2 months (range 2-39; P = .89) in patients where familial disease was only recognized later or not at all. Among the 7 patients with known familial disease and HSCT performed > 12 months after therapy start, 5 were transplanted with mismatched donors, possibly reflecting difficulties in finding a suitable donor. The 5-year survival after HSCT was 66% ± 14% (median follow-up 6.6 years, range 3.0-12.1). No patient with a familial disease survived without HSCT.
Non-neurologic late effects were more common in the patients with familial disease than in the other patients (P = .029). In general, the most severe late effects in HLH are neurologic sequelae.24 These late effects were more common in patients with a familial disease (9/29, 31%) compared with patients without familial disease (25/135, 19%), but the difference was not statistically significant (P = .050).
Patients who received transplantations.
Overall, 124 (50%) patients received transplantations after a median time of 6.1 months (range 2-39). Patients who received transplantations were younger (P < .001), less often had edema (P = .001), hypoalbuminemia (P = .014), elevated lactate dehydrogenase (LDH; P = .012) and/or elevated creatinine (P = .004; Table 2).
The conditioning regimen was reported in 118 (95%) of 124 of the patients who received transplantations. The majority (69 patients, 58%) received conditioning with cyclophosphamide, busulfan, and etoposide and/or antithymocyte globuline, but several other regimens were used. In 12 patients, radiation was included in the conditioning.
Overall survival after HSCT was 82 (66%) of 124 (median follow-up 6.5 years [range 1.8-12.1] after HSCT). The 5-year cumulative survival after HSCT was 66% ± 8%: 74% ± 16% with matched related donors (n = 31), 76% ± 12% with matched unrelated donors (n = 46), 61% ± 23% with mismatched unrelated donors (n = 18), and 43% ± 21% with family haploidentical donors (n = 21) (donor type missing n = 8). Ten of 100 patients who received transplantations received a cord blood transplantation (missing information on source, n = 24), and 8 are alive with a median follow-up after transplantation of 4.9 years.
In children with active disease at HSCT (n = 43), 5-year cumulative survival after HSCT was 58% ± 15% compared with 72% ± 10% in patients with no active disease (n = 75; P = .064; disease activity at HSCT missing, n = 6). Sixty patients received transplantations within 6 months after therapy start (36 alive, 60%), 44 between 6 months and 1 year (30 alive, 68%), and 20 later than a year (16 alive, 80%). There was no significant difference of survival between these groups.
In the 42 patients dead after HSCT, the death was transplantation-related in 28 patients and a result of graft failure and HLH reactivation in 8. In 4 additional patients, 3 with multiorgan failure and 1 with a respiratory infection 1.5 years after HSCT, the cause of death could not be further defined. Moreover, 1 patient died of active HLH 2 days after HSCT and 1 from complications of a surgical procedure. The 28 transplantation-related deaths reported were GVHD (n = 5), VOD (n = 5), CMV infection (n = 5), other infections/septicemia (n = 3), respiratory failure/ARDS (n = 4), EBV-driven lymphoproliferative disorder (n = 1), or a combination of these (n = 5). Of the 42 deaths, 29 occurred during the first 100 days after HSCT and 13 later.
Patients who received transplantations had a high proportion of both neurologic late effects (21/82 [26%] compared with 4/53 [8%] in non-HSCT patients, P = .008) and non-neurologic late effects (P = .010; 18/80 [23%] compared with 3/52 [6%] in non-HSCT patients, P = .010).
Patients off therapy/alive without HSCT.
In total 53 patients survived without HSCT. One is still on a low, continuous dose of CSA because of reactivations, and in 3, follow-up is < 1 year after treatment initiation. Forty-nine (20%) of 249 of the children have had no active disease for > 1 year after completion of HLH therapy, and are therefore assumed to have had sHLH. Their median follow-up was 5.3 years (range 1.9-11.6). None of these patients had a familial history of HLH. They were older with a median age of 24 months (range 2-184; P < .001; supplemental Figure 2) and more often female (61%, P = .011). The majority (n = 28, 57%) were reported from Japan, and 52% had a reported history of recent infection. The most frequently confirmed viral trigger was EBV infection (74% of patients with a confirmed infection), but CMV, varicella, hepatitis A, rotavirus, and enterovirus infections were also reported.
Clinically, these patients more often had lymphadenopathy (P = .043), but less often hepatomegaly (P = .007). Neurologic symptoms were significantly less common (P = .006; Table 2). Laboratory work-up more often showed hyponatremia and less often a pathologic cerebrospinal fluid examination (P = .003 and < 0.001, respectively).
Their median treatment duration was 4 months (range 41 days–24 months). After 2 months of therapy, NAD was reported in 76%, compared with 54% in the other patients (P = .007). A state of NAD without reactivation was reached in 71% after 2 months, and in 94% within 6 months. Persisting splenomegaly was rare compared with the other patients (P < .001), as well as persisting bicytopenia (P = .026) and hyperferritinemia (P = .003).
The proportion of patients with late effects was 8%; less than in the other patients (P < .001). Four patients had neurologic late effects (developmental delay = 3 and/or seizures = 2).
Deceased patients.
Overall, 114 (46%) patients died; 72 did not receive transplantations. Sixty-four (89%) of these died within the first year (Figure 3, supplemental Figure 1). Among the 8 deaths that occurred later than 1 year after onset of therapy, 2 were because of progressive disease without available HSCT donor, 1 refrained from HSCT because of severe neurologic disease and 5 had relapse, 4 of whom had CNS relapse.
Cause and time of deaths occurring in patients who did not receive transplantation within the first year of treatment (n=64). The majority of patients died with or from active HLH ( ). The causes of death in 4 patients who did not have active HLH at death (■) were: fatal bleeding following liver biopsy (n=1, 7th week); septicemia where HLH status at death is not known (n=1, 2nd week), and cause of death not stated (n=2, 1st and 38th weeks). One patient who died with or from active HLH is not represented in the graph, since the exact death date is missing.
). The causes of death in 4 patients who did not have active HLH at death (■) were: fatal bleeding following liver biopsy (n=1, 7th week); septicemia where HLH status at death is not known (n=1, 2nd week), and cause of death not stated (n=2, 1st and 38th weeks). One patient who died with or from active HLH is not represented in the graph, since the exact death date is missing.
Cause and time of deaths occurring in patients who did not receive transplantation within the first year of treatment (n=64). The majority of patients died with or from active HLH ( ). The causes of death in 4 patients who did not have active HLH at death (■) were: fatal bleeding following liver biopsy (n=1, 7th week); septicemia where HLH status at death is not known (n=1, 2nd week), and cause of death not stated (n=2, 1st and 38th weeks). One patient who died with or from active HLH is not represented in the graph, since the exact death date is missing.
). The causes of death in 4 patients who did not have active HLH at death (■) were: fatal bleeding following liver biopsy (n=1, 7th week); septicemia where HLH status at death is not known (n=1, 2nd week), and cause of death not stated (n=2, 1st and 38th weeks). One patient who died with or from active HLH is not represented in the graph, since the exact death date is missing.
We have analyzed the 35 patients who died during the first 8 weeks of initial therapy in more detail (Table 2). These patients had a larger proportion of consanguinity (35%, P = .025) compared with other patients. Moreover, they more often had jaundice (P = .001), edema (P = .005), and an affected creatinine (P = .001; Table 2). The time reported from onset of symptoms to treatment did not differ in the group of patients deceased during the first 8 weeks compared with other patients.
Altogether 32 (94%) of 34 of the patients deceased during initial treatment died with active disease (missing info on cause of death = 1). Multiorgan failure was specified in 6 patients and septicemia in 14. During the subsequent 4 months, another 17 patients who did not receive transplantations died, all with or because of active HLH. During the second half of the first year, 12 patients who did not receive transplantations died, 11 with active HLH (cause of death missing n = 1). In sum, 64 patients who did not receive transplantations died during the first year; 60 (97%) of 62 died with active HLH disease (missing data = 2; Figure 3).
Discussion
The long-term results of the first prospective study on HLH confirm that over half of patients may be cured. Altogether, HLH-94 induced permanent remission or kept the patient alive until a transplantation in 71% of the patients.
The HLH-94 protocol has provided a major improvement in survival for HLH patients compared with previous experience. In a review by Janka 1983, 96 of 101 HLH patients died within 1 year.1 Prolonged disease remission was first induced with etoposide,25 later confirmed by reports on prolonged remission induced by combination regimens based on epipodophyllotoxins combined with corticosteroids and intrathecal methotrexate.26,27 However, FHL typically relapses in all patients and permanent cure requires HSCT.23,28-30 Aricò et al reported data on 122 HLH patients with a 5-year survival of 21% after therapies including HSCT.31 With regard to sHLH, Imashuku et al reported 82 patients with presumed sHLH treated in Japan 1986-1995 with a 4-year-survival of 57%,32 and also found that immunochemotherapy such as HLH-94 effectively can control the majority of EBV-related sHLH.33,34 Treatment with ATG-based immunotherapy for FHL, performed in 1991-2005 in a single-center setting with 38 patients, gave an overall survival of 21 patients (55%). In the absence of a direct comparison within the same study, comparative conclusions can only be tentative.35,36 The rationale for using etoposide in HLH is strongly based on the primary role of defective cytotoxic cells and defect apoptosis induction37 in the disease pathogenesis; it has been reported that etoposide normalizes deficient apoptosis induction in IL-2–activated lymphocytes from HLH patients.22 Our current data with a 5-year survival of 54% ± 6% in 249 patients correspond well with our HLH-94 interim study report with a 3-year survival of 55% ± 9% in 113 patients, using the same inclusion criteria as in this report.3 Notably, despite the longer follow-up and larger cohort of patients in the present study, all patients who successfully received transplants remained free of HLH, and no patient who did not receive a transplant had recurrence of disease > 1 year after HLH therapy termination.
In the absence of a genetic marker or a known familial disease, it is impossible to clinically differentiate familial from sHLH. In this study, initiated 5 years before the first FHL-causing mutation was described, genetic information was not collected. We used the presence of an affected sibling as a proxy for familial disease. Patients who did not receive transplants, who were alive with no disease activity for > 1 year after therapy termination, were presumed to have suffered sHLH. To investigate clinical distinctions between these groups, we present how they differ at presentation of disease. Patients with presumed sHLH were older (median age 24 months compared with 8 months in the remaining patients, P < .001), but because the range was from 2 months to 134 months, young age alone cannot be used as an indication for HSCT. In addition, these patients were significantly less likely to have hyponatremia (P = .003), neurologic symptoms (P = .006), and cerebrospinal fluid pleocytosis (P < .001), and more likely to have lymphadenopathy at presentation. Female sex (61%) was significantly more frequent (P = .011), as previously reported by Imashuku et al in patients with EBV-HLH.17 On follow-up, late effects were less reported in these patients (P < .001). Patients who died during initial therapy had an increased presence of edema and jaundice, previously described as findings later in the course of disease.1 In addition, they more often had elevated creatinine, possibly reflecting multiorgan failure, and neuroradiologic pathology (Table 2). Early deaths were mainly because of septicemia or multiorgan failure. Separation between treatment- and disease-related deaths in these patients is difficult because these deaths can be caused by both HLH and the treatment. However, nearly all patients who did not receive a transplantation and who died during the first year had signs of active HLH, indicating that intensification of initial therapy may be beneficial, as is currently being tested in the HLH-2004 protocol.
The patients who received transplantations less often presented with elevated LDH, hypoalbuminemia, and/or elevated creatinine (Table 2). They more seldom had edema, possibly mirroring a less advanced disease making them more likely to survive until transplantation. One patient developed AML, possibly secondary to etoposide treatment, and received a transplantation in NAD status. The 5-year survival after HSCT was similar following matched related and matched unrelated donor transplantations. In children with active disease at HSCT, the 5-year survival after HSCT was 58% ± 15% compared with 72% ± 10% in patients with NAD, (P = .064) indicating that patients responding well to initial pre-HSCT therapy fared best, but that some persisting HLH activity should not automatically exclude HSCT.24
In the entire patient cohort, we find a slight (55%) male preponderance. This could imply a genetic heterogeneity of the disease, possibly because of a yet unknown FHL subgroup of X-linked inheritance, alternatively that undiagnosed XLP1 or XLP2 patients with associated hemophagocytic syndrome have unknowingly been included. However, there is now evidence that XLP and other inherited hemophagocytic syndromes such as GS2 and CHS also can be successfully treated with the HLH-94 treatment protocol.38
The most important late effects of HLH are CNS complications, and the frequency and manifestations of CNS disease in 193 patients treated by the HLH-94 protocol have previously been reported.18 Only patients with information on of cerebrospinal fluid examination at diagnosis were included in these analyses, and CNS disease, as defined by abnormal cerebrospinal fluid findings (52%) and/or neurologic abnormalities (37%) at diagnosis, was 63%. Neurologic late effects were reported at last follow-up in 15%, and CNS disease before therapy start was shown to be a risk factor for adverse outcome. We here report similar figures, with 33% of patients having neurologic symptoms before therapy start, abnormal cerebrospinal fluid in 53%, and any of these signs of CNS disease in 127 (67%) of 203. The rate of late effects was 19% at last follow-up.
HSCT was recommended for patients with familial, persistent, or recurrent disease, and conventional myeloablative conditioning was suggested in HLH-94. For the future it would be valuable to further improve the 5-year cumulative survival after HSCT, which overall was 66% ± 8% in the current study. There are now single-center reports of improved post-HSCT survival by reduced-intensity conditioning (RIC).39,40 To which extent various forms of RIC can improve post-HSCT survival in larger patient cohorts remains to be evaluated, as well as which RIC approach that is most suitable in various patient settings. Because HLH-94 was designed before the identification of any disease-causing mutations, the protocol itself had to be designed to assist to distinguish between patients who needed from those that did not need HSCT, which was the reason for the tapering of steroids over the first 8 weeks and for stopping therapy after 8 weeks in patients with NAD without a known family history of HLH. Now disease-causing mutations can be identified in a large proportion of patients with familial HLH,41 which should be reflected in the design of future treatment protocols, such as by less steroid tapering during the last part of the initial therapy in patients with verified primary HLH. Moreover, analytic studies of clinical outcome predictors could be valuable in the future to assist in treatment decisions.
To conclude, the HLH-94 treatment protocol has meant a major improvement in the survival of HLH patients. It is effective in inducing remission and cure in 54% of the patients. Moreover, altogether 71% of the patients were successfully treated by HLH-94 so that they either received transplants or achieved long-term remission without HSCT (Table 3). The HLH-94 international collaboration has led to a greater awareness of HLH and its treatment, making earlier diagnosis and therapy possible. Still, efforts need to be made to improve survival further, and to reduce late effects. Because early fatalities represent the most frequent single cause for treatment failure, early identification of poor responders can be beneficial for further treatment improvements. Even though it is difficult to differentiate between treatment-related and disease-related deaths because both the disease and the treatment may lead to neutropenia and severe infections, we know that most patients who die do so with active HLH. Therefore, prompt diagnosis by modern genetic and functional assays of patients with primary forms of HLH and rapid HSCT could be possible ways to reduce pre-HSCT morbidity, by reducing the time at risk for disease reactivation. Similarly, novel treatments reducing disease activity are also worth considering. A tool to distinguish patients with sHLH could be useful to avoid overtreatment in this subset of patients. Identification of prophylactic measures for CNS disease and a suitable salvage therapy are other future goals, as are improved HSCT survival and reduced HSCT sequelae.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors express their sincere gratitude to all reporting clinicians. For excellent help gathering and organizing data, they thank their data managers Martina Jalava Löfstedt and Elisabet Berglöf.
This work was supported by grants provided by the Swedish Childhood Cancer Foundation, the Swedish Research Council, the Cancer and Allergy Foundation of Sweden, Queen Silvia's Jubilee Fund, the Märta and Gunnar V Philipson Foundation, the Histiocytosis Association of America, Sällskapet Barnavård, and Stockholm County Council (ALF).
Authorship
Contribution: J.I.H. initiated the HLH-94 study, which was designed by M.A., R.M.E., A.H.F., H.G., S.I., S.L., D.W., G.J., and J.I.H., with J.I.H. as principal investigator: these individuals all also served as subcenter coordinators; H.T. performed the data analysis for this paper with support from A.C.H.; and H.T. wrote the manuscript, which was finalized by J.I.H. All authors reviewed the paper and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the the Histiocyte Society HLH-94 clinical study group appears in the supplemental Appendix.
Correspondence: Helena Trottestam or Jan-Inge Henter, Childhood Cancer Research Unit, Q6:05, Karolinska University Hospital Solna, SE-171 76 Stockholm, Sweden; e-mail: Helena.Trottestam@ki.se or Jan-Inge.Henter@ki.se.