Abstract
The POEMS syndrome is associated with elevated vascular endothelial growth factor (VEGF) levels. Several studies have compared serum VEGF levels between POEMS patients and other disease entities showing higher serum VEGF in POEMS syndrome; however, it is unknown whether serum levels are reliable and reproducible given variable platelet release of VEGF. We therefore compared plasma levels of VEGF in 29 patients with POEMS syndrome with those of other disorders (n = 76). We demonstrated that plasma VEGF levels are useful in differentiating POEMS from other plasma cell dyscrasias, neuropathic processes, and multisystem illnesses. Plasma VEGF is also useful in monitoring disease activity after treatment and correlates with clinical improvements better than hematologic response.
Introduction
The POEMS syndrome is a paraneoplastic disorder secondary to an underlying plasma cell dyscrasia. It is clinically manifested by a constellation of polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes (POEMS) as well as several other features, such as thrombocytosis, erythrocytosis, sclerotic bone lesions, Castleman disease, papilledema, extravascular fluid accumulation (peripheral edema, ascites, pleural effusions), fatigue, and clubbing.1 The hallmark of this complex multisystem disease is the presence of both angiogenic and proinflammatory cytokines, such as vascular endothelial growth factor (VEGF), IL-1β, IL-6, and TNF-α.2-5 Although the true mediator of POEMS is yet unknown, VEGF has been used as a surrogate biomarker. To date, most published literature has evaluated serum VEGF levels in POEMS,5-7 and levels are routinely used to aid in the diagnosis and monitoring of patients after treatment. Tokashiki et al suggested that serum VEGF may be the better test as it includes the VEGF level from the platelet compartment as well as the serum.8 However, the theoretical concern with serum VEGF levels is the unpredictable release of platelet-derived VEGF because of ex vivo platelet activation during the clotting process9 as well as the level of thrombocytosis, a common feature in POEMS syndrome. It is well established that serum VEGF levels are 10-50 times higher than plasma VEGF levels in both health and disease.9,10 To determine whether plasma VEGF is a reliable marker for the diagnosis and management of the POEMS syndrome, we evaluated plasma VEGF levels among patients seen at the Mayo Clinic.
Methods
This study was approved by the Mayo Clinic Institutional Review Board. We undertook a retrospective search of our patient database for plasma VEGF levels done as part of routine clinical care at the Mayo Clinic, Rochester, MN between December 5, 2005 and July 31, 2010. Plasma VEGF levels were performed using plasma samples collected in an EDTA tube by the ELISA method. Testing was performed at the Quest Diagnostics Nichols Institute, San Juan Capistrano, CA. Normal values ranged between 31 and 86 pg/mL. Clinical data of these patients were abstracted and analyzed using JMP software (SAS). The Fisher exact test and the Kruskal-Wallis method were used to ascertain differences between nominal and continuous variables, respectively.
Results and discussion
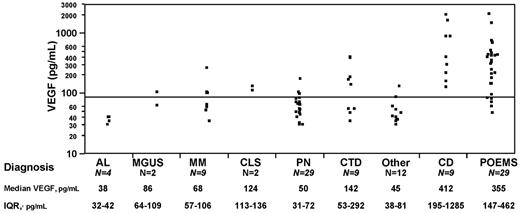
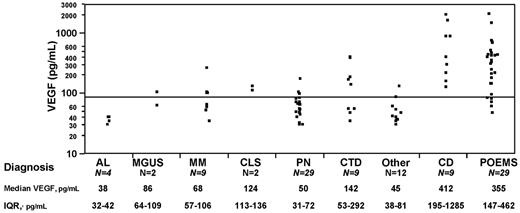
Plasma VEGF levels were performed in 105 patients with POEMS syndrome (N = 29), Castleman disease (N = 9), multiple myeloma (N = 9), monoclonal gammopathy of undetermined significance (MGUS; N = 2), amyloidosis (N = 4), peripheral neuropathy (N = 29), capillary leak syndrome (N = 2), connective tissue disease and/or vasculitis (N = 9), and miscellaneous other diseases, (“other”; N = 12) at the time of diagnosis (Figure 1). Patients with Castleman disease were those patients who had a clonal plasma cell disorder and lymph node biopsy-proven histology (ie, POEMS with Castleman disease). The group with peripheral neuropathy included 20 patients who also had a monoclonal protein (5 with an IgM monoclonal protein), and the remainders were patients with chronic idiopathic demyelinating polyradiculoneuropathy, inflammatory, hereditary, and idiopathic peripheral neuropathy. The group with connective tissue diseases and/or vasculitis included patients with lupus, scleroderma, polymyalgia rheumatica, Moyamoya disease, necrotizing vasculitis, Churg-Strauss syndrome, microscopic polyangiitis, and cerebral vasoconstriction syndrome. The “other” disease group included a patient each with Hodgkin lymphoma, amyotrophic lateral sclerosis, chronic pain syndrome, and neuromuscular hyperexcitability, and all of the other cases were patients who were not diagnosed with a disease entity. Plasma VEGF levels in patients with POEMS syndrome and Castleman disease were markedly elevated compared with patients with other plasma cell dyscrasias (P < .001), peripheral neuropathy (P < .001), and connective tissue disease/vasculitis (P < .009). Twenty patients with peripheral neuropathy, 5 patients with connective tissue disease/vasculitis, and 7 of the others group also had a monoclonal gammopathy. Median plasma VEGF levels for these patients were separately assessed and found to be 53 pg/mL (peripheral neuropathy with MGUS), 58 pg/mL (connective tissue disease/vasculitis with MGUS), and 48 pg/mL (“other” with MGUS). Four patients with POEMS had normal baseline VEGF; 3 of them were either receiving or had received high-dose steroids within 2 weeks for a presumptive diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy. Eighteen patients with neither POEMS nor Castleman disease had a high VEGF. These included both patients with capillary leak syndrome, 5 patients with connective tissue disease/vasculitis, and 2 patients with myeloma who had coexisting connective tissue disease and vasculitis. A patient with multiple myeloma and peripheral neuropathy each had plasma VEGF levels done during hospitalization for acute illness and had levels of 271 ng/mL and 180 ng/mL, respectively. The remaining patients (1 myeloma, 1 MGUS, 2 peripheral neuropathy, and 3 other disease group) had mildly elevated plasma VEGF levels between 88 and 134 pg/mL. Using receiver operating characteristic analysis, the best VEGF cut-off for POEMS diagnosis was 146 pg/mL; however, a cut-off of 200 pg/mL had a specificity of 95% with a sensitivity of 68% in support of a POEMS diagnosis. We conclude that, like serum VEGF,7 plasma VEGF levels are useful in diagnosing POEMS syndrome and Castleman disease and can help differentiate from other plasma cell dyscrasias as well as diseases presenting with similar features (ie, peripheral neuropathy, connective tissue disease, and vasculitis). Elevated plasma VEGF levels have been described in several types of cancer,11 rheumatologic illnesses,12,13 renal diseases,14 and in sepsis.14 The sensitivity of plasma VEGF increases further when used in the appropriate clinical setting, and after accounting for acute illnesses, and medications (corticosteroid use).
Plasma VEGF distributions in patients with POEMS syndrome and related disorders. IQR indicates interquartile range; AL, immunoglobulin light chain amyloidosis; MM, multiple myeloma; CLS, capillary leak syndrome; PN, peripheral neuropathy; CTD, connective tissue disease/vasculitis; and CD, Castleman disease.
Plasma VEGF distributions in patients with POEMS syndrome and related disorders. IQR indicates interquartile range; AL, immunoglobulin light chain amyloidosis; MM, multiple myeloma; CLS, capillary leak syndrome; PN, peripheral neuropathy; CTD, connective tissue disease/vasculitis; and CD, Castleman disease.
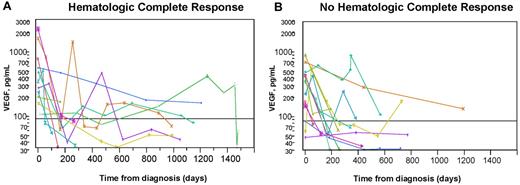
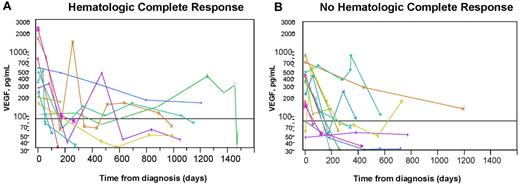
We also evaluated the use of plasma VEGF in the follow-up of patients with POEMS. Again, all published experience with serial VEGF levels among patients with POEMS syndrome is with serum levels.15-18 Twenty-five patients with POEMS syndrome and/or Castleman disease had plasma VEGF levels done at baseline and on at least 2 separate occasions. Hematologic response to treatment was defined according to the International Myeloma Working Group criteria.19 Based on these criteria, a complete response (CR) was defined as negative immunofixation in serum and urine with disappearance of any plasmacytomas and > 5% plasma cells in the bone marrow. Therapies included autologous stem cell transplantation in 18 patients (with a CR in 9), radiation therapy in 5 (with a CR in 1), and 2 patients treated with combination chemotherapy (one patient received rituximab, cyclophosphamide, and prednisolone, and the other patient received 3 different chemotherapy regimens with lenalidomide/dexamethasone, bortezomib, and cyclophosphamide) with no CRs. All patients who had treatment showed clinical improvement and a reduction in plasma VEGF, but only 10 (40%) achieved a CR. The respective median baseline, day 180, and last follow-up plasma VEGF measurements for the 10 CR and 15 non-CR patients were: 378 and 349 pg/mL, 131 and 75 pg/mL, and 60 and 65 pg/mL. There was no significant difference in plasma VEGF levels on day 180 or at last follow-up between patients who had achieved a CR or not. Plasma VEGF levels correlated with the clinical outcome but not with the depth of hematologic response (Figure 2). It is noteworthy that the one patient who was treated with bevacizumab at relapse (Figure 2B green cross) had normalization of his VEGF, as would be expected with an anti-VEGF antibody but had clinical worsening and eventual death.
Serial plasma VEGF levels in patients with POEMS syndrome after treatment. The horizontal line represents normal level of VEGF. Most patients had a significant drop in plasma VEGF after treatment and clinical improvement regardless of whether they did (A) or did not (B) achieve a hematologic CR. Clinical course defined for those who either did not drop into the normal range or had vacillating levels. (A) Blue dot represents marked clinical improvement despite continued modest elevation; gold cross, presumed spurious because no intervention after transplantation other than protracted (2-year) prednisone taper for resolving adrenal insufficiency; fuchsia cross, presumed spurious value 1 year after peripheral blood stem cell transplantation (no further interventions made and VEGF improved); and green cross, clinical improvement after transplantation, but plasma VEGF level rose, initially with stable clinical symptoms. Three months after the rise in VEGF, he developed florid POEMS symptoms. Treatments, including lenalidomide, corticosteroids, cyclophosphamide, and finally bevacizumab, yielded no clinical improvement, and he died of his illness 5 months after relapse. Aqua diamond represents clinical improvement but persistent pleural effusion and adrenal insufficiency after peripheral blood stem cell transplantation. (B) Aqua triangle represents initially treated with lendalidomide (1 cycle not tolerated), followed by 8 cycles of bortezomib with initial improvement but then worsening status (and VEGF), so given high-dose cyclophosphamide with resultant reduction in VEGF and continued clinical improvement. Salmon cross represents after high-dose chemotherapy with autologous stem cell transplantation, persistent FDG uptake, high VEGF, so adjuvant radiation given 16 months into diagnosis; blue triangle, after high-dose chemotherapy with autologous stem cell transplantation, he had clinical improvement but developed idiopathic posttransplantation inflammatory arthritis, which persisted for nearly one year; and gold triangle, clinical improvement, but still painful neuropathy and erectile dysfunction 31 months after autologous stem cell transplantation. Because of rising VEGF and slight worsening of positron emission tomography scan, he was started on lenalidomide and dexamethasone. Subsequent VEGF measurement were not yet made. PET indicates; and SUV, standardized uptake value.
Serial plasma VEGF levels in patients with POEMS syndrome after treatment. The horizontal line represents normal level of VEGF. Most patients had a significant drop in plasma VEGF after treatment and clinical improvement regardless of whether they did (A) or did not (B) achieve a hematologic CR. Clinical course defined for those who either did not drop into the normal range or had vacillating levels. (A) Blue dot represents marked clinical improvement despite continued modest elevation; gold cross, presumed spurious because no intervention after transplantation other than protracted (2-year) prednisone taper for resolving adrenal insufficiency; fuchsia cross, presumed spurious value 1 year after peripheral blood stem cell transplantation (no further interventions made and VEGF improved); and green cross, clinical improvement after transplantation, but plasma VEGF level rose, initially with stable clinical symptoms. Three months after the rise in VEGF, he developed florid POEMS symptoms. Treatments, including lenalidomide, corticosteroids, cyclophosphamide, and finally bevacizumab, yielded no clinical improvement, and he died of his illness 5 months after relapse. Aqua diamond represents clinical improvement but persistent pleural effusion and adrenal insufficiency after peripheral blood stem cell transplantation. (B) Aqua triangle represents initially treated with lendalidomide (1 cycle not tolerated), followed by 8 cycles of bortezomib with initial improvement but then worsening status (and VEGF), so given high-dose cyclophosphamide with resultant reduction in VEGF and continued clinical improvement. Salmon cross represents after high-dose chemotherapy with autologous stem cell transplantation, persistent FDG uptake, high VEGF, so adjuvant radiation given 16 months into diagnosis; blue triangle, after high-dose chemotherapy with autologous stem cell transplantation, he had clinical improvement but developed idiopathic posttransplantation inflammatory arthritis, which persisted for nearly one year; and gold triangle, clinical improvement, but still painful neuropathy and erectile dysfunction 31 months after autologous stem cell transplantation. Because of rising VEGF and slight worsening of positron emission tomography scan, he was started on lenalidomide and dexamethasone. Subsequent VEGF measurement were not yet made. PET indicates; and SUV, standardized uptake value.
We have demonstrated that plasma VEGF levels are useful both for diagnosing and monitoring patients with POEMS syndrome. As in the case of the utility of serum VEGF,2,6,7,20 plasma VEGF is neither 100% sensitive nor specific for the syndrome but can play an important role in making the diagnosis. Moreover, we have shown that plasma VEGF levels correlate better with clinical response than with CR. Although we have not directly compared serum VEGF with plasma VEGF in this study, and knowing that platelets are a major source of VEGF in serum samples in POEMS,21 we contend that plasma VEGF is at least as good, if not better, test. We therefore conclude that plasma VEGF may be used as an option to serum VEGF to aid in the diagnosis of POEMS and Castleman disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kate Johanns for maintaining the Dysproteinemia Database.
Authorship
Contribution: A. D'Souza abstracted the data, performed statistical analysis, and wrote the manuscript; S.R.H., F.B., M.M., M.Q.L., M.A.G., R.A.K., S.K., P.R.G., J.A.L., S.J.R., S.Z., D.D., T.E.W., and S.V.R. assisted in data collection and manuscript preparation; A. Dispenzieri designed the concept, analyzed and interpreted the data, and wrote the manuscript; and all authors reviewed the final draft of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angela Dispenzieri, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: dispenzieri.angela@mayo.edu.