Abstract
A delicate balance between immunostimulatory and immunosuppressive signals mediated by dendritic cells (DCs) and other antigen-presenting cells (APCs) regulates the strength and efficacy of antiviral T-cell responses. HIV is a potent activator of plasmacytoid DCs (pDCs), and chronic pDC activation by HIV promotes the pathogenesis of AIDS. Cholesterol is pivotal in maintaining HIV envelope integrity and allowing HIV-cell interaction. By depleting envelope-associated cholesterol to different degrees, we generated virions with reduced ability to activate pDCs. We found that APC activation was dissociated from the induction of type I IFN-α/β and indoleamine-2,3-dioxygenase (IDO)–mediated immunosuppression in vitro. Extensive cholesterol withdrawal, resulting in partial protein and RNA loss from the virions, rendered HIV a more powerful recall immunogen for stimulating memory CD8 T-cell responses in HIV-exposed, uninfected individuals. These enhanced responses were dependent on the inability of cholesterol-depleted HIV to induce IFN-α/β.
Introduction
Dendritic cells (DCs) play major roles in initiating and sustaining innate and adaptive immune responses, and are the nexus at which immune stimulation or suppression occurs. Peripheral blood DCs include the lymphoid-derived plasmacytoid DCs (pDCs) and the myeloid DCs (mDCs).1 Both DC subsets act as antigen-presenting cells (APCs) and activate Ag-specific T lymphocytes. The efficiency of T-cell activation depends on appropriate DC stimulation that favors costimulatory molecule expression and cytokine production.1 DC activation is attributable to the recognition of conserved structural motifs of potential pathogens by TLRs, leading to DC maturation into fully competent APCs.1 TLR7 and TLR8 are triggered by single-stranded RNA, whereas TLR9 binds unmethylated CpG-rich DNA, allowing DC activation by most viruses.2 Human mDCs express TLR8, whereas pDCs express TLR7 and TLR9.2 TLR engagement results in the up-regulation of costimulatory molecules CD80 and CD86 and production of IL-12 by mDCs.1,2 These responses favor T-cell activation and CD4 T-cell polarization toward an IFN-γ–secreting Th1 phenotype.1,3,4 Conversely, pDCs mainly produce type I IFN (IFN-α and IFN-β) in response to TLR7/9 ligands.5 IFN-α/β are produced early during viral infections and act as immunostimulatory cytokines favoring APC maturation and as antiviral factors. IFN-α/β exert their antiviral function by activating intracellular restriction mechanisms and through antiproliferative and proapoptotic effects on multiple cell types, including T lymphocytes.6 Type I IFN responses are critical in the early phases of immune responses, but the chronic and systemic activation of pDCs can paradoxically lead to deleterious consequences for the immune system, resulting in inhibition of T-cell proliferation and promotion of cell death.7
The immunosuppressive enzyme indoleamine-2,3-dioxygenase (IDO) is induced in pDCs upon TLR7/9 engagement.8,9 The immunoregulatory activity of IDO, combined with the negative effects of IFN-α/β on T-cell proliferation and survival,6,10 support the hypothesis that pDCs may behave as immunosuppressive DCs rather than classic APCs, particularly in certain chronic pathologic conditions.9,11,12 Prolonged pDC activation during chronic infections may favor pathogen persistence by interfering with Ag-specific T-cell responses that may otherwise efficiently eliminate the infectious agent.12,13 A model for HIV immunopathogenesis has been proposed based on the potential suppressive activity of pDCs.11 HIV is a powerful activator of pDCs, which could contribute to several aspects of HIV immunopathogenesis: (1) IFN-α/β–dependent apoptosis of CD4 T cells14,15 ; (2) induction of immunosuppressive ligands such as programmed death ligand 1 (PDL1) via IFN-α/β16 ; (3) up-regulation of T-cell activation markers by IFN-α/β17,18 ; (4) chemoattraction of CCR5+ CD4 T cells at the infection site, favoring systemic diffusion of the virus19 ; and (5) IDO-mediated suppression of T-cell responses and alteration of the Th17/regulatory T cell balance.18,20,21 Therefore, although IFN-α/β may act as potent inhibitors of HIV replication during the acute phase of infection, prolonged pDC activation during the chronic phase may be harmful for the immune system, dampening anti-HIV effector T-cell responses. Nevertheless, there is no direct demonstration that pDC activation is a specific mechanism adopted by HIV to escape adaptive immune responses.
Both productive infection of CD4+ cells and pDC activation by HIV require the interaction between viral gp120 and cellular CD4.22,23 This specific feature of HIV renders it an ideal model with which to study the effects of virus-induced pDC activation on antigen-specific T-cell responses. Therefore, because the cellular and viral components of the HIV-pDC interaction are known, it is possible to modify the ability of HIV to bind target cells, including pDCs. gp120-CD4 binding is stabilized by interactions involving cellular proteins on both the cell surface and the viral envelope.24,25 The area of virus-cell contact involves a membrane microdomain of the HIV envelope that contains tightly packed cholesterol and subsets of cellular proteins.26 Partial withdrawal of cholesterol by treatment with the starch derivative 2-hydroxy-propyl β-cyclodextrin (βCD) destabilizes the envelope organization, depriving HIV of its ability to infect CD4+ cells in vitro.26,27 Extensive cholesterol withdrawal causes dissociation of the microdomain from the envelope, generating noninfectious permeabilized virions that retain most of the gp120 but have lost the soluble mature form of the gag protein p24 while conserving the majority of unprocessed immature gag polyprotein p55.26 More than 90% of virions are also depleted of RNA by this method.
We used a HIV-based in vitro model to study the effect of different levels of pDC activation on APC activity and T-cell responses. We quantitatively depleted cholesterol from the HIV envelope to varying degrees using βCD. Cholesterol depletion was achieved up to and including virus permeabilization. Our results show that cholesterol depletion alone was sufficient to partially relieve HIV-associated immunosuppression. By inducing envelope permeabilization, we generated virions that stimulated APC activation in the absence of immunosuppressive mechanisms. Permeabilized HIV served as a powerful stimulus for HIV-specific T-cell memory responses, demonstrating that HIV directly suppresses antiviral T-cell responses via pDC overactivation.
Methods
A detailed description of the methods used in the present study, including patient characteristics, is provided in the supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Preparation of βCD-treated HIV-1
HIV-1MN (CEMx174 T1) and HIV-1Ada (SUPT1-CCR5 CL.30) were obtained from the AIDS and Cancer Vaccine Program at SAIC-Frederick (National Cancer Institute at Frederick, MD). Inactivation of HIV-1MN and HIV-1Ada with aldrithiol-2 (AT-2) and treatment with βCD were performed as described previously.26,28
Patients and donors
Buffy coats were obtained from the North London Blood Transfusion Service. Whole blood was collected from HIV+ (n = 10) and HIV-exposed seronegative (HESN; n = 10) patients by venipuncture in Vacutainer tubes containing EDTA (BD Biosciences). The study was approved by the institutional review board of the S.M. Annunziata Hospital and written, informed consent was obtained from all subjects in accordance with the Declaration of Helsinki.
Leukocyte isolation and culture
PBLs were isolated by density gradient centrifugation using Histopaque-1077 (Sigma-Aldrich) and cultured at 2 × 106 cells/mL in RPMI 1640 medium (Sigma-Aldrich), 10% FBS (Sigma-Aldrich), and 1% Pen-Strep-Glut (Sigma-Aldrich). HIV was added at 2.5 × 109 RNA copies/mL final concentration. βCD-HIV preparations were used as normalized on the actin content. Cells and supernatants were collected after 18 hours for analysis of IFN-α/β production by ELISA, IDO activity by HPLC, and surface marker expression by flow cytometry.
DyLight-488 HIV preparation and HIV uptake assay
Virus was pelleted at 100 000g for 1 hour at 4°C, resuspended in 0.05M sodium borate buffer, pH 8.5, added to 1 vial of Dylight 488 (DL488), and incubated for 1 hour at room temperature. PBLs from uninfected donors were cultured in the presence of DL488 HIV or βCD-HIV for 18 hours before flow cytometric analysis.
HIV-specific response in HIV+ and HESN
PBLs from HESN and HIV+ patients were cultured at 1 × 106 cells/mL in RPMI 1640 medium (Sigma) containing 10% AB serum (Sigma-Aldrich) for 18 hours with 2.5 × 109 RNA copies/mL of HIV or βCD80-HIV and βCD120-HIV normalized in actin content. To facilitate costimulation, 2 μg/mL of anti-CD28 Abs (R&D Systems) were added. In some experiments, 5 μg/mL of anti-IFNAR2 blocking Abs (Axxora) or 1000 units/mL of recombinant IFN-α2A (rIFN-α2A; Axxora) was added. Brefeldin A (10 μg/mL; Sigma-Aldrich) was added to the cultures after 3 hours of stimulation to block protein secretion. Cytometric analysis was performed using an EPICS XL flow cytometer (Beckman-Coulter). FlowJo software (TreeStar) was used for data analysis.
Statistical analysis
Statistical analyses were performed using SPSS Version 19.0 software. Different conditions were compared using nonparametric Wilcoxon sign-rank test with Hochberg correction for multiple comparisons. Uncorrected P values < .05 are reported in the figures; P values that remained significant after Hochberg correction are indicated with an asterisk.
Results
Viral protein and RNA content of βCD-HIV
AT-2 HIV-1MN treated with 20, 40, and 80mM βCD retained the mature capsid protein p24, whereas HIV-1MN treated with 120 or 160mM βCD showed complete loss of p24 but retention of immature p55 gag polyprotein (supplemental Figure 1A-B). Envelope gp120 was detectable in all virus preparations (supplemental Figure 1C). Therefore, only treatment with 120 and 160mM βCD caused envelope permeabilization and loss of viral capsid protein.26
To account for suboptimal recovery of viral particles after βCD treatment, we compared the levels of actin, a cellular protein retained in the virion even after permeabilization,26 among the different virus preparations (supplemental Figure 1D). Densitometry measurement of actin bands was used to normalize the concentration of untreated and βCD-treated AT-2 HIV-1MN.
AT-2 HIV-1MN treated with 80mM βCD retained approximately 50% of viral RNA, whereas treatment with 120mM βCD resulted in 20- and 10-fold reductions of viral RNA compared with untreated and 80mM βCD80-treated AT-2 HIV-1MN, respectively (supplemental Figure 1E).
Treatment of AT-2 HIV-1Ada with βCD resulted in alterations of viral proteins and RNA content similar to that observed for AT-2 HIV-1MN (data not shown).
Unless otherwise specified, for the biologic assays we used untreated AT-2 HIV-1MN (HIV), cholesterol-depleted but intact AT-2 HIV-1MN treated with 20 or 80mM βCD (βCD20-HIV and βCD80-HIV, respectively), and permeabilized AT-2 HIV-1MN treated with 120mM βCD (βCD120-HIV).
Envelope cholesterol depletion affected the ability of HIV to induce IFN-α/β and IDO
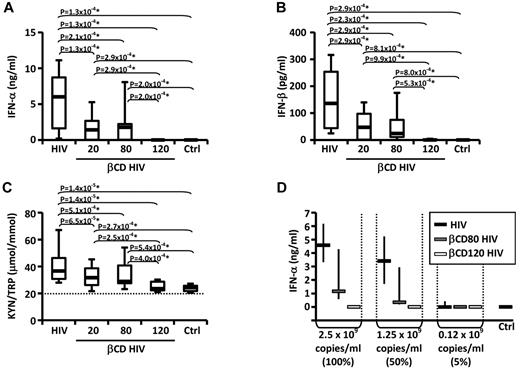
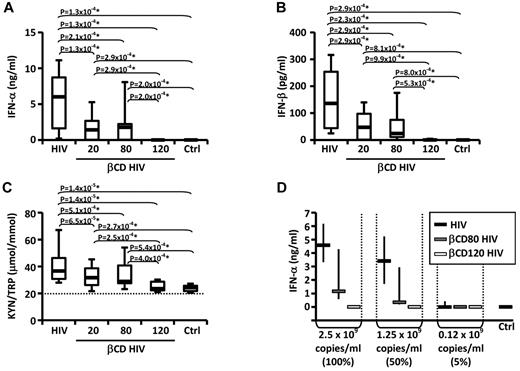
PBLs were cultured in presence or absence of HIV or βCD-HIV for 18 hours. HIV induced the production of IFN-α and IFN-β and increased the KYN/TRP ratio, a measure of IDO enzymatic activity (Figure 1A-C). βCD20/80-HIV showed significantly impaired ability to induce IFN-α, IFN-β, and IDO activity (Figure 1A-C). Permeabilized βCD120-HIV did not induce IFN-α/β and did not stimulate IDO activity (Figure 1A-C).
Envelope cholesterol withdrawal impairs HIV-induced IFN-α production and IDO activity. IFN-α (A) and IFN-β (B) were quantified by ELISA in the supernatants of PBLs from healthy donors (n = 19) cultured with HIV or HIV treated with different concentrations of βCD (20, 80, or 120mM) or medium alone (CTRL). (C) The concentrations of kynurenine (KYN) and tryptophan (TRP) were measured by HPLC (n = 25); the ratio between KYN and TRP is shown; horizontal dotted line indicates the sensitivity threshold of the assay. (D) IFN-α in supernatants from PBLs cultured in the presence of HIV, βCD80-HIV, or βCD120-HIV normalized according to viral RNA concentrations (n = 3). In all graphs, horizontal bars represent median values; in panels A-C, boxes indicate the interquartile range and vertical lines extend to 90th and 10th percentiles. In panel D, vertical lines indicate interquartile range. *P values that remained significant after Hochberg correction for multiple comparisons.
Envelope cholesterol withdrawal impairs HIV-induced IFN-α production and IDO activity. IFN-α (A) and IFN-β (B) were quantified by ELISA in the supernatants of PBLs from healthy donors (n = 19) cultured with HIV or HIV treated with different concentrations of βCD (20, 80, or 120mM) or medium alone (CTRL). (C) The concentrations of kynurenine (KYN) and tryptophan (TRP) were measured by HPLC (n = 25); the ratio between KYN and TRP is shown; horizontal dotted line indicates the sensitivity threshold of the assay. (D) IFN-α in supernatants from PBLs cultured in the presence of HIV, βCD80-HIV, or βCD120-HIV normalized according to viral RNA concentrations (n = 3). In all graphs, horizontal bars represent median values; in panels A-C, boxes indicate the interquartile range and vertical lines extend to 90th and 10th percentiles. In panel D, vertical lines indicate interquartile range. *P values that remained significant after Hochberg correction for multiple comparisons.
The lack of IFN-α/β and IDO induction by βCD120-HIV may have been due to the loss of viral RNA from the virions, resulting in the absence of the TLR7 ligand required for pDC activation. We investigated whether βCD80-HIV and βCD120-HIV induced similar amounts of IFN-α as untreated HIV when the viruses were used at concentrations normalized on viral RNA content. βCD80-HIV and βCD120-HIV showed impaired ability to induce IFN-α even when used at concentrations matching the RNA content of untreated HIV (Figure 1D).
Permeabilized HIV up-regulates the costimulatory molecule CD80 without stimulating cognate immunosuppressive mechanisms in pDCs
We investigated whether the induction of the activation marker CD83 and the costimulatory molecules CD80 and CD86 on pDCs could be modulated by manipulating the HIV envelope (supplemental Figure 2A-B). Exposure of PBLs from uninfected donors to HIV, βCD20-HIV, and βCD80-HIV induced comparable up-regulation of CD83, CD80, and CD86 on pDCs (Table 1 and supplemental Figure 2C-E). Surprisingly, βCD120-HIV induced a statistically significant increase of CD80 on pDCs despite showing no effect on CD83 and CD86 (Table 1 and supplemental Figure 2D). The effect of βCD120-HIV on CD80 expression did not significantly differ from that observed with HIV or βCD20/80-HIV.
PDL1 and the TNF-related apoptosis-inducing ligand (TRAIL) negatively regulate T-cell responses and promote CD4 T cell apoptosis during HIV infection.29,30 HIV and βCD20-HIV induced statistically significant increases in both PDL1 and TRAIL; a similar trend was observed after incubation with βCD80-HIV (Table 1 and supplemental Figure 2F-G). Conversely, permeabilized βCD120-HIV did not cause up-regulation of PDL1 and TRAIL in pDCs (Table 1 and supplemental Figure 2F-G).
Permeabilized HIV favors monocyte and mDC maturation without inducing immunosuppressive mechanisms
We examined the effect of HIV and βCD-HIV on monocytes in PBLs from uninfected donors in vitro (supplemental Figure 3A-B). Up-regulation of the activation marker CD83 was observed in monocytes after exposure to HIV and βCD20/80-HIV, but not permeabilized βCD120-HIV (Table 1 and supplemental Figure 3C). Conversely, CD80 and CD86 were significantly up-regulated on monocytes after exposure to all HIV preparations (Table 1 and supplemental Figure 3D-E). βCD120-HIV was less potent than HIV and βCD20/80-HIV in up-regulating CD80 and CD86 on monocytes. Similar to pDCs, PDL1 and TRAIL were induced in monocytes by HIV and βCD20/80-HIV, but not βCD120-HIV (Table 1 and supplemental Figure 3F-G).
We also investigated whether HIV and βCD-HIV would modify the expression of MHC class I and costimulatory molecules on mDCs (supplemental Figure 4A-B). As expected, all mDCs stained positive for MHC class I (HLA-ABC) in all conditions tested (supplemental Figure 4C). The mean fluorescence intensity for CD86, but not CD80, was increased in mDCs after incubation with HIV- or βCD-treated HIV (Table 1 and supplemental Figure 4D-E), including permeabilized βCD120-HIV (which was, however, less potent than HIV and βCD20/80-HIV).
When X4-tropic (HIV-1MN) and R5-tropic (HIV-1Ada) βCD–treated HIV were compared, similar results were obtained for IFN-α production, with the exception of lower IFN-α production in response to βCD80-HIV-1Ada compared with βCD80-HIV-1MN (supplemental Figure 5A). This suggests that the lipid composition and envelope organization of the 2 isolates may be slightly different, partly reflecting the different cell lines in which they were grown. βCD-treated HIV-1MN and HIV-1Ada induced similar levels of CD83 on both pDCs and monocytes (supplemental Figure 5A). HIV-1Ada was unable to promote CD80 expression by pDCs independently of βCD treatment (supplemental Figure 5A), but induced CD80 up-regulation in monocytes even after treatment with βCD120, albeit to lower levels than HIV-1MN (supplemental Figure 5A). Finally, HIV-1MN and HIV-1Ada induced comparable up-regulation of CD86 in monocytes, even after treatment with βCD120 (supplemental Figure 5A). These data suggest that, although differences may exist among HIV-1 isolates, the extent of pDC activation by different HIV-1 isolates is dependent on envelope integrity and partial up-regulation of costimulatory molecules on monocytes can be achieved in the absence of IFN-α production by permeabilization via cholesterol withdrawal.
Monocyte activation by HIV was achieved only when total unseparated PBLs were cultured, and not when pDC-depleted PBLs were exposed to the viruses (supplemental Figure 5B). Similar results were obtained for βCD-treated HIV (data not shown), confirming previous studies showing that HIV interacts directly with and activates pDCs, whereas activation of other APC occurs indirectly after exposure to pDC-derived cytokines.31-33
Uptake of HIV and βCD-HIV by APCs
To determine the effect of envelope cholesterol manipulation on HIV uptake by different cell types, we incubated PBLs with DL488-labeled HIV and βCD-HIV (supplemental Figure 6). Cells that acquired HIV were visualized by flow cytometry as DL488+ (supplemental Figure 7A-C). DL488 staining may indicate virions bound to cell surface receptors or virions internalized by either endocytosis or envelope-membrane fusion. Intact HIV was efficiently acquired by pDCs, mDCs, and monocytes after 2 hours of incubation (data not shown). After 18 hours of incubation, approximately 10% of pDCs, 20% of mDCs, and 40% of monocytes stained positive for DL488 (supplemental Figure 7D). Approximately 3% of CD4 T cells were DL488+ after 18 hours, indicating limited reactivity with HIV, whereas > 1.5% of CD8 T cells and B cells stained DL488+ (supplemental Figure 7D).
Preincubation of PBLs with anti-CD4 Abs reduced HIV uptake by pDCs by approximately 50% (supplemental Figure 7E). A 20% reduction in HIV uptake was observed in mDCs and CD4 T cells upon CD4 blockade, whereas anti-CD4 did not significantly inhibit HIV uptake by monocytes (supplemental Figure 7E).
Incubation with DL488-labeled βCD-HIV resulted in significantly lower staining of pDCs, mDCs, and monocytes compared with untreated HIV (Figure 2A-C). There was no difference between cholesterol-depleted intact viruses (βCD20/80-HIV) and permeabilized βCD120-HIV with regard to their uptake by APCs (Figure 2A-C).
Uptake of HIV and βCD-HIV by APCs. Flow cytometric histograms (left) and whisker plots (right) showing staining for DL488 in pDCs (A), monocytes (B), and mDCs (c) from PBLs (n = 10) cultured in presence of DL488-labeled HIV or HIV treated with different concentrations of βCD (20, 80, or 120mM) and unlabeled HIV or medium alone (CTRL). In all histograms, vertical dotted lines indicate thresholds of positive staining based on unlabeled HIV control; 1 example of 10 experiments is shown. In all whisker plots, horizontal bars represent median values; vertical lines indicate interquartile range. *P values that remained significant after Hochberg correction for multiple comparisons.
Uptake of HIV and βCD-HIV by APCs. Flow cytometric histograms (left) and whisker plots (right) showing staining for DL488 in pDCs (A), monocytes (B), and mDCs (c) from PBLs (n = 10) cultured in presence of DL488-labeled HIV or HIV treated with different concentrations of βCD (20, 80, or 120mM) and unlabeled HIV or medium alone (CTRL). In all histograms, vertical dotted lines indicate thresholds of positive staining based on unlabeled HIV control; 1 example of 10 experiments is shown. In all whisker plots, horizontal bars represent median values; vertical lines indicate interquartile range. *P values that remained significant after Hochberg correction for multiple comparisons.
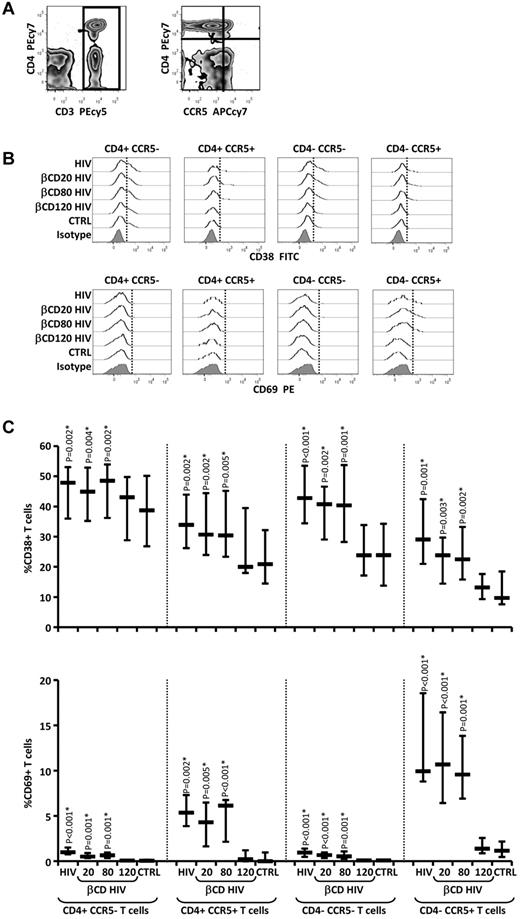
Permeabilized βCD120-HIV does not induce T-cell activation markers
HIV-induced IFN-α/β promotes expression of the T-cell activation markers CD38 and CD69.18 Increased CD38 and CD69 expression is associated with and predictive of HIV disease progression.34-37 Because CCR5+ T cells express high levels of the IFN-α receptor subunit 2 (IFNAR2) and are more sensitive to IFN-α/β signaling,16 we analyzed CD38 and CD69 regulation by HIV and βCD-HIV on CCR5+ and CCR5- T cells (Figure 3A-B). Statistically significant increases in both CD38 and CD69 were observed in all T-cell subsets after exposure to HIV and βCD20/80-HIV but not βCD120-HIV (Figure 3C and supplemental Figure 8). The increase in CD38 expression in response to HIV was significantly higher in CCR5+ compared with CCR5− CD4 T cells, and the increase in CD69 expression was significantly higher in CCR5+ CD4 and CD8 T cells compared with their CCR5− counterparts (supplemental Figure 8). This is consistent with the reported preferential expression of IFNAR2 by CCR5+ T cells.16
Expression of T-cell activation markers after PBMC exposure to HIV and βCD-treated HIV. (A) Flow cytometric contour plots showing detection of T cells (CD3+; left panel) and distinction of T-cell subsets based on CD4 and CCR5 expression (right panel). (B) Flow cytometric histograms of CD38 (top panels) and CD69 (bottom panels) expression on the gated T-cell subpopulations in the different culture conditions (vertical dotted lines indicate thresholds of positive staining based on isotype controls; 1 example of 15 experiments is shown). (C) CD38 (top panel) and CD69 (bottom panel) expression measured as frequency of expressing T cells in the different culture conditions for each T-cell subset (n = 15). Horizontal bars represent median values; vertical lines extend to 75th and 25th percentiles. P values show comparisons with control. *P values that remained significant after Hochberg correction for multiple comparisons.
Expression of T-cell activation markers after PBMC exposure to HIV and βCD-treated HIV. (A) Flow cytometric contour plots showing detection of T cells (CD3+; left panel) and distinction of T-cell subsets based on CD4 and CCR5 expression (right panel). (B) Flow cytometric histograms of CD38 (top panels) and CD69 (bottom panels) expression on the gated T-cell subpopulations in the different culture conditions (vertical dotted lines indicate thresholds of positive staining based on isotype controls; 1 example of 15 experiments is shown). (C) CD38 (top panel) and CD69 (bottom panel) expression measured as frequency of expressing T cells in the different culture conditions for each T-cell subset (n = 15). Horizontal bars represent median values; vertical lines extend to 75th and 25th percentiles. P values show comparisons with control. *P values that remained significant after Hochberg correction for multiple comparisons.
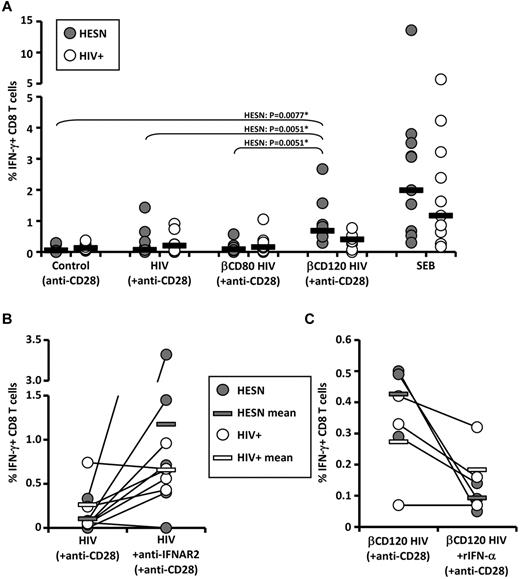
βCD120-HIV elicits memory CD8 T-cell responses in HIV-exposed individuals
We investigated whether βCD120-HIV can serve as a recall antigen in individuals with preexisting immunity against HIV. To control for the confounding factor of endogenous HIV-mediated pathogenesis, we used PBLs from HIV-infected patients (HIV+; n = 10) and from individuals with repeated sexual exposure to HIV without signs of infection, termed HESN (n = 10). PBLs were cultured in presence of anti-CD28 alone (negative control) or in combination with HIV, βCD80-HIV, or βCD120-HIV. Intracellular IFN-γ was measured by flow cytometry in CD4 and CD8 T cells. Staphylococcal enterotoxin B was used as a positive control. βCD120-HIV induced a significantly higher frequency of IFN-γ–producing CD8 T cells in HESN patients compared with both HIV and βCD80-HIV patients, which did not differ from control (Figure 4A and supplemental Figure 9A). HIV+ patients showed a similar trend, but the responses were not statistically significant and less potent than those observed in HESN patients (Figure 4A and supplemental Figure 9A). No HIV-specific responses were observed for CD4 T cells (supplemental Figure 9A), and no increase in IFN-γ–producing CD8 T cells was observed in uninfected and unexposed healthy controls (data not shown). Because the βCD-treated, AT-2–inactivated virus used in this study does not establish the productive infection of target cells, the preferential activation of the CD8 subset suggests a role for cross presentation of extracellular HIV epitopes on class I MHC molecules. To determine whether the different efficiency of HIV and βCD120-HIV as stimuli for HIV-specific CD8 T cells was because of type I IFN production, we stimulated PBLs from HIV+ (n = 5) and HESN (n = 5) patients with HIV in presence or absence of blocking Abs against IFNAR2. In parallel, we tested whether rIFN-α would negatively affect the efficiency of βCD120-HIV as a recall antigen in HESN (n = 3) and HIV+ (n = 3) patients. IFNAR2 blockade resulted in increased IFN-γ responses to HIV in 8 of 10 subjects tested (4 of 5 HESN and 4 of 5 HIV+ patients; Figure 4B and supplemental Figure 9B). Conversely, rIFN-α reduced the IFN-γ responses against βCD120-HIV in 5 of 6 subjects (3 of 3 HESN and 2 of 3 HIV+ patients; Figure 4C and supplemental Figure 9C).
Induction of HIV-specific IFN-γ-producing memory CD8 T-cell responses by cholesterol-depleted HIV. A) Frequency of IFN-γ–producing CD8 T cells in HESN (n = 10) and HIV+ (n = 10) patients after exposure of PBLs to βCD-treated and untreated HIV in the presence of costimulating anti-CD28 Abs. (B) Frequency of IFN-γ–producing CD8 T cells in HESN (n = 5) and HIV+ (n = 5) patients after exposure of PBLs to HIV in the presence or absence of blocking Abs against αIFNAR2. (C) Frequency of IFN-γ–producing CD8 T cells in HESN (n = 3) and HIV+ (n = 3) patients after exposure of PBLs to βCD120-HIV in the presence or absence of rIFN-α. In all plots, gray dots represent HESN patients and white dots represent HIV+ patients (each individual is indicated by a dot). Horizontal bars represent the median values for HESN patients (gray bars in panels B and C) and HIV+ patients (white bars in panels B and C). *P values that remained significant after Hochberg correction for multiple comparisons.
Induction of HIV-specific IFN-γ-producing memory CD8 T-cell responses by cholesterol-depleted HIV. A) Frequency of IFN-γ–producing CD8 T cells in HESN (n = 10) and HIV+ (n = 10) patients after exposure of PBLs to βCD-treated and untreated HIV in the presence of costimulating anti-CD28 Abs. (B) Frequency of IFN-γ–producing CD8 T cells in HESN (n = 5) and HIV+ (n = 5) patients after exposure of PBLs to HIV in the presence or absence of blocking Abs against αIFNAR2. (C) Frequency of IFN-γ–producing CD8 T cells in HESN (n = 3) and HIV+ (n = 3) patients after exposure of PBLs to βCD120-HIV in the presence or absence of rIFN-α. In all plots, gray dots represent HESN patients and white dots represent HIV+ patients (each individual is indicated by a dot). Horizontal bars represent the median values for HESN patients (gray bars in panels B and C) and HIV+ patients (white bars in panels B and C). *P values that remained significant after Hochberg correction for multiple comparisons.
Discussion
DCs play a critical role at the interface between innate and adaptive immunity. Viral pathogens have developed mechanisms of evasion from immune responses, some of which may depend directly on the dynamics of their interaction with DCs. In particular, the preferential induction of immunosuppressive mechanisms during chronic viral infections may be a key factor contributing to the persistence of the viral pathogens. The progressive immunodeficiency caused by HIV represents an extreme example of DC dysregulation and suppression of adaptive immunity.11 We modified the dynamics of HIV interaction with target cells and used this immunopathogenic virus to investigate how pDC function may be molded by viral pathogens to inhibit, rather than enhance, adaptive T-cell responses.
Partial cholesterol depletion reduced the pDC-activating potential of HIV. This was not solely because of the reduced amount of viral RNA, because the defect in IFN-α induction was not corrected when βCD80-HIV and βCD120-HIV were normalized against untreated HIV for RNA content. Two nonmutually exclusive explanations may account for this phenomenon: (1) IFN-α induction may depend on the average RNA content per viral particle rather than the total amount of RNA, with RNA-depleted competing with RNA-containing virions for interaction with pDCs; and (2) βCD treatment may have impaired the functionality of the envelope microdomain and altered the ability of HIV to interact with pDCs. The reduced uptake of βCD-HIV by pDCs argues in favor of the second hypothesis. Recent evidence suggests that the ability of HIV to cause persistent pDC activation may depend on its retention in the early endosomal compartment.38 It is possible that cholesterol removal may have altered the intracellular trafficking of HIV, thereby reducing its ability to activate pDCs. Because the quality and the strength of the virus-cell interaction depend on both cellular and viral molecules on the HIV-1 envelope, it is not surprising that different HIV-1 isolates grown in different cell lines also showed different abilities to activate pDCs.
Surprisingly, partial cholesterol removal had very limited impact on CD83, CD80, and CD86 up-regulation by pDCs. Furthermore, our data indicate that IFN-α/β production and IDO activity can be dissociated from APC activation. Therefore, permeabilized βCD120-HIV was not inert with regard to its effect on APCs, because it selectively stimulated costimulatory molecules even in the absence of IFN-α/β and immunosuppressive and proapoptotic mechanisms such as IDO, PDL1, and TRAIL. This suggests a spectrum of pDC activation that leads first to the up-regulation of costimulatory molecules, followed by the induction of a proapoptotic and antiproliferative mechanism (Figure 5).
Double-threshold model for pDC stimulation and its effect on the course of viral infections. (A) During acute infections or the acute phase of most chronic infections, pDCs are activated beyond the threshold at which they exert antiviral activity, but pDC stimulation is rapidly reduced to maintain APC activity in the absence of proapoptotic and antiproliferative mechanisms. This allows the development and preservation of efficient T-cell responses that clear or control the infection. (B) If pDC activation is not controlled at the end of the acute phase (as during HIV infection), antiproliferative and proapoptotic mechanisms are kept active and undermine the maintenance of memory T-cell responses, favoring viral persistence.
Double-threshold model for pDC stimulation and its effect on the course of viral infections. (A) During acute infections or the acute phase of most chronic infections, pDCs are activated beyond the threshold at which they exert antiviral activity, but pDC stimulation is rapidly reduced to maintain APC activity in the absence of proapoptotic and antiproliferative mechanisms. This allows the development and preservation of efficient T-cell responses that clear or control the infection. (B) If pDC activation is not controlled at the end of the acute phase (as during HIV infection), antiproliferative and proapoptotic mechanisms are kept active and undermine the maintenance of memory T-cell responses, favoring viral persistence.
The mechanism by which permeabilized HIV achieves partial pDC activation without stimulating IFN-α/β production is still obscure. It is possible that residual viral RNA present in the βCD120-HIV preparation is a sufficient stimulus for APCs to up-regulate only surface molecules with a lower activation threshold. An alternative possibility is that other viral components, such as host-derived proteins or unique oligosaccharides on viral proteins, may participate in APC activation independently of the TLR7/8-RNA interaction.
Activation of monocytes was directly dependent on the presence of pDCs. However, βCD120-HIV was capable of stimulating CD80/86 up-regulation in monocytes even without inducing IFN-α/β production by pDCs. It is possible that pDC-derived cytokines other than type I IFN, such as TNF-α, stimulate monocyte maturation into APCs. Alternatively, cell-cell interactions between activated pDCs and monocytes may result in the expression of costimulatory molecules by the latter after incubation with permeabilized HIV.
Limited information is available on the relative efficiency of different cells in interacting with and taking up HIV. Using DL488-labeled HIV, we found that only pDCs, mDCs, monocytes, and CD4 T cells acquired HIV, which is consistent with the expression of the main HIV receptor CD4. Although DL488 staining of target cells does not distinguish between HIV binding to its cellular receptor and internalization via endocytosis or envelope-membrane fusion, the relatively high level of HIV uptake by pDCs, mDCs, and monocytes compared with CD4 T cells is consistent with the endocytotic activity of APCs. Conversely, resting CD4 T cells are poorly endocytotic and are not efficiently infected by HIV unless they have entered the cell cycle.
Surprisingly, CD4 blockade inhibited HIV uptake by pDCs but only partially interfered with HIV uptake by mDCs and CD4 T cells and had no effect on monocytes. Two opposing explanations could account for the different sensitivity of different cell types to CD4 blockade: (1) interaction with CD4 may be more important for HIV uptake by pDCs, whereas other APCs may rely on alternative mechanisms; or (2) the strength or the stability of CD4-gp120 interactions, which is affected by accessory proteins, may differ among cell types, being weaker and easier to block in pDCs than in other cells.
Cholesterol depletion at all levels modified the susceptibility of HIV to be taken up by pDCs, mDCs, and monocytes. Nevertheless, βCD20/80-HIV and permeabilized βCD120-HIV were acquired by APCs at a similar level, which is consistent with the APC-activating effect of βCD120-HIV. This suggests that partial cholesterol depletion is as efficient as permeabilization in impairing the ability of HIV to interact with the target cells. Therefore, although βCD20/80-HIV retained the structural integrity of the envelope, its functionality may be severely compromised by the alteration of the lipid content.
Consistent with its inability to induce IFN-α/β, βCD120-HIV did not cause CD38 and CD69 up-regulation on T cells. However, βCD120-HIV was more potent than HIV in stimulating IFN-γ–producing memory CD8 T cells, suggesting that phenotypic activation may not mirror functional T-cell activity. βCD20/80-HIV exhibited a reduced ability to stimulate IFN-α/β and IDO, which was, however, sufficient to exert immunosuppressive activity on HIV-specific T-cell responses, similar to intact HIV. Conversely, βCD120-HIV was completely deprived of the ability to induce IFN-α/β, IDO, TRAIL, and PDL1, but retained appreciable amounts of immature capsid protein. Permeabilized βCD120-HIV was taken up by APCs and, in the absence of pDC-mediated immunosuppressive mechanisms, proved to be more potent than intact HIV in stimulating HIV-specific T cells. The recovery of CD8 T-cell responses with anti-IFNAR2, together with the inhibition of responses against βCD120-HIV with rIFN-α, confirm a direct role for type I IFN in HIV-mediated immunosuppression.
The different responsiveness of HESN and HIV+ patients is likely to be the consequence of the immunocompromised status of the latter. Indeed, CD8 T cells from HIV+ patients also responded poorly against the Staphylococcal enterotoxin B–positive control. However, the reported enhanced immune responsiveness of HESN patients to HIV antigens should also be considered.39 Genetic polymorphisms at endoplasmic reticulum aminopeptidases may favor the presentation of distinctive peptide repertoires to CD8 T cells in HESN patients.40 Furthermore, the cross-presentation of epitopes from soluble extracellular HIV antigens on MHC-I, which is the likely mechanism driving CD8 T-cell responses in our in vitro system, may be enhanced in HESN patients, as suggested by the elevated expression of the heat-shock protein receptor CD91.41 This and other immunologic features may render HESN patients more prone to efficiently respond to HIV.
The negative effect of HIV-induced IFN-α/β on HIV-specific CD8 T-cell responses is consistent with the increasing body of evidence suggesting an immunomodulatory role for type I IFN on T-cell–mediated immune responses. The immunostimulatory effect of IFN-α/β may be associated with the simultaneous triggering of immunoregulatory mechanisms mediated by different interferon-stimulated genes (ISGs). Imbalances of these mechanisms have been studied in murine models of systemic and prolonged administration of TLR7 or TLR9 agonists, which resulted in immunocompromised phenotypes.7,42 In addition, antigen-specific T-cell responses are enhanced in mice when TLR9 ligands are administered locally together with the immunogen, but IDO-mediated suppression of T cells prevails when the same agonist is administered systemically.43 We hypothesized that chronic and systemic activation of pDCs by HIV may cause protective ISGs to be overcome by their immunomodulatory counterparts, dampening efficient antiviral responses and driving HIV disease progression.11 This view is in agreement with recent data showing that nonpathogenic SIV infection of sooty mangabeys or African green monkeys produces a sizeable induction of ISGs, which rapidly contracts at the end of the acute phase.44,45 Conversely, the initial burst of ISGs is maintained beyond the acute and through the chronic stage of pathogenic SIV infection in rhesus macaques.44,45
The ability of HIV to suppress memory T-cell responses via pDC activation is an example of hijacking a pathway classically associated with protective innate immunity, and represents an important hurdle for HIV vaccine–induced T-cell responses. We demonstrated here that even in HESN subjects, who are thought to possess partial immunologic protection against HIV infection,39 memory HIV–specific T-cell responses are severely restrained in vitro by HIV unless the virus is deprived of its pDC-activating capacity. This immunologic phenomenon would also likely occur at the time of viral challenge in vaccinated individuals, who would test positive for T-cell responses against HIV-derived proteins or peptides. Therefore, pDC activation and accumulation at the site of HIV infection occurs within hours of the viral challenge, and alterations in the migratory dynamics of pDCs are observed from the time of primary infection,19,33 suggesting that this cascade of events after pDC activation may be initiated very rapidly after exposure to HIV. We recently proposed that the immunopathogenic mechanisms triggered by HIV at its very first contact with the immune system may rapidly disable vaccine-induced T-cell responses.46
We propose a double-threshold model of pDC activation during viral infections (Figure 5). Low-level APC activation, characterized by up-regulation of costimulatory molecules, can be achieved with minimal stimulation (first threshold), whereas a more potent stimulation is necessary to trigger the innate antiviral responses mediated by IFN-α/β (second threshold). This high level of pDC activation may be achieved in acute viral infections or in the acute phase of most chronic viral infections, during which it contributes to limiting viral replication and promoting the induction of primary T-cell responses (Figure 5A). The transient nature of pDC stimulation causes a contraction of innate responses below the second threshold, allowing the maintenance of APC activation, which favors T-cell responses and clearance or long-term control of the virus (Figure 5A). Conversely, certain chronic infections may not permit the contraction of pDC responses below the second threshold, causing pDC activation beyond the acute and into the chronic phase and the overlap of newly developed T-cell responses with deleterious cytostatic and cytotoxic activities mediated by IDO and IFN-α/β (Figure 5B).
HIV may be the archetypal example of this phenomenon, having specific molecular features that favor its interaction with and activation of pDCs. We were able to reduce or abolish this viral advantage by altering the lipid organization of the envelope, which is critical for HIV binding to its target cells. Therefore, whole HIV can be turned into a powerful recall immunogen by depleting its immunopathogenic potential.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Wellcome Trust award (085164/Z/08/Z to A.B. and C.M.R.) and by National Institutes of Health grants (1R01MH087233-01A1 and 2P01MH070306-06 to D.R.G. and V.N.A.).
National Institutes of Health
Wellcome Trust
Authorship
Contribution: A.B. and D.R.G. designed the experiments, analyzed the data, and wrote the manuscript; C.M.R. designed and performed the experiments and analyzed the data; S.D. performed the experiments and analyzed the data; V.N.A., M.B., L.P., B.T., and D.F. performed the experiments; F.M. and S.L.C. recruited the patients; and G.M.S. and M.C. designed the experiments and provided intellectual advice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Adriano Boasso, Immunology Section, Imperial College, Chelsea and Westminster Hospital, 369 Fulham Road, London, SW10 9NH, United Kingdom; e-mail: a.boasso@imperial.ac.uk; or Dr David Graham, Molecular and Comparative Pathobiology, Johns Hopkins School of Medicine, 733 N Broadway, BRB 835, Baltimore, MD 21205; e-mail: dgraham@jhmi.edu.