Abstract
Janus kinase-2 (JAK2) conveys receptor-binding signals by several inflammatory cytokines, including IL-6, via phosphorylation of signal transducer and activator of transcription 3 (STAT3). We demonstrate that selective JAK2 inhibition by TG101348 during initial encounters between human T cells and allogeneic monocyte-derived dendritic cells induces durable, profound, and specific T-cell tolerance upon reexposure to the same alloantigens. Subsequent responses by nonalloreactive T cells to stimulation de novo by a pathogenic nominal antigen remain intact. TG101348 also suppresses primed T-cell responses when present only during alloantigen restimulation. TG101348 ablates IL-6/JAK2–mediated phosphorylation of STAT3, but has no off-target effects on IL-2 or IL-15/JAK3/pSTAT5-dependent signaling, which sustain the responses of regulatory T cells (Tregs) and other effector T cells. JAK2 inhibition preserves Treg numbers and thereby enhances the ratio of CD4+ Tregs to CD8+CD25+ effector T cells in favor of Tregs. JAK2 inhibition also reduces the production of IL-6 and TNF-α in allogeneic MLRs, impairing the activation of central and effector memory T cells as well as the expansion of responder Th1 and Th17 cells. While we have reported the limitations of isolated IL-6R-α inhibition on dendritic cell–stimulated alloreactivity, we demonstrate here that JAK2 represents a relevant biologic target for controlling GVHD or allograft rejection without broader immune impairment.
Introduction
GVHD is a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT). Broadly acting immunosuppressants curtail lymphocyte alloreactivity, but they increase infectious complications and can jeopardize the GVL or graft-versus-tumor benefit of the transplantation. An ideal approach to preventing and treating GVHD would limit alloantigen-specific reactivity while preserving immunity against pathogens and malignant cells.
The systemic dysregulation of inflammatory cytokines mediates the pathophysiology of GVHD, especially the acute form.1 Among these cytokines, IL-6 has recently received increased attention because it promotes inflammation by suppressing regulatory T-cell (Treg) development and promoting Th17 expansion.2-7 IL-6 also supports the maturation and activation of human dendritic cells (DCs).8,9
Mouse HSCT models of GVHD have shown that IL-6 induces direct cytopathic damage to the intestinal epithelium. Its neutralization reduces gut pathology and improves survival,10,11 probably because of the primacy of the gastrointestinal tract in amplifying systemic GVHD.12 Targeting IL-6 with mAb or knock-out strategies, however, has resulted in discordant effects on the Treg/Th17 axis in these mouse models.10,11
We have attempted to replicate the immunosuppressive effect of IL-6 inhibition in mice by studying primary human DC:T-cell interactions in vitro with tocilizumab,13 a mAb to the IL-6 receptor-alpha (IL-6R-α) subunit. Tocilizumab achieved the intended on-target effect of blocking IL-6 signaling in both monocyte-derived dendritic cells (moDCs) and T cells. There were no functional consequences, however, for moDC maturation, alloreactive T-cell proliferation, Treg expansion, or allogeneic Th1/Th17 responses in vitro. Therefore, inhibition of IL-6 by isolated receptor blockade would not limit alloreactivity in a human system.
We therefore focused on Janus kinase-2 (JAK2), which relays the signaling function not only of IL-6R-α, but also of other inflammatory cytokine receptors with relevance for allogeneic graft-host interactions.14 The JAKs comprise a family of nonreceptor protein tyrosine kinases, which include JAK1, JAK2, JAK3, and Tyk2. These kinases associate with the cytoplasmic domains of cytokine receptors.14 Upon their own phosphorylation, the JAKs induce downstream phosphorylation of signal transducer and activator of transcription (pSTAT) proteins.14 Activated pSTATs in turn function as transcription factors that mediate cellular differentiation and growth.14
JAK2 mediates T-cell signaling in response to various proinflammatory cytokines, including IL-6, IL-12, and IL-23.14 These cytokines are critical to the development and expansion ofTh1 cells, which use IL-12, and Th17 cells, which use IL-6 and IL-23.2,15,16 Th1 and Th17 cells can in turn induce alloreactive end organ damage in GVHD.17 JAK2 is therefore a principle gatekeeper of alloreactivity and inflammation and it represents an attractive target with which to control GVHD.
TG101348 is a highly specific JAK2 inhibitor with 300-fold higher binding affinity for JAK2 than JAK3.18 The sparing of JAK3 is important, because T-cell effectors require IL-2 and IL-15 and Tregs require IL-2, both of which signal through JAK3/pSTAT5.19-21 Patients with myelofibrosis also tolerated oral TG101348 very well in a recent phase 1 trial, with toxicity restricted to mild anemia and thrombocytopenia.22
Because cytokine dysregulation is a hallmark of GVHD, and JAK2 signaling supports the function of many of these proinflammatory cytokines, we hypothesized that specific JAK2 blockade with TG101348, in contrast to isolated inhibition of IL-6R-α, would keep alloreactive Th1 and Th17 lymphocytes in check when stimulated by allogeneic moDCs. Durable induction of alloantigen-specific tolerance by JAK2 blockade should prove clinically beneficial in GVHD, in which adaptive immunity by alloantigen-nonreactive T cells against other pathogenic antigens is maintained.
Methods
Human leukocytes, culture media, and reagents
PBMCs were isolated over Ficoll-Paque Plus (GE Healthcare Biosciences) from leukocyte concentrates from healthy volunteer donors (New York Blood Center, American Red Cross). HLA-A*0201–restricted blood products were obtained from consenting individuals in agreement with the Declaration of Helsinki and existing tissue procurement protocols approved by the Institutional Review and Privacy Board of Memorial Hospital, Memorial Sloan Kettering Cancer Center (MSKCC).
Complete RPMI 1640 medium (MSKCC Media Prep Core Facility) was supplemented with 10mM HEPES (Sigma-Aldrich), 1% penicillin/streptomycin, 1% l-glutamine (Cellgro; Mediatech), 55μM 2-ME (GIBCO, Invitrogen), and heat-inactivated pooled human serum (PHS; Gemini Bioproducts). PHS 1% vol/vol in complete RPMI 1640 medium was used for cytokine-based cultures to generate moDCs, whereas 10% vol/vol was used for functional assays, including moDC:T-cell MLRs. IMDM (MSKCC Media Prep Core Facility) was supplemented with 10mM HEPES, 1% penicillin/streptomycin, 1% l-glutamine, 55μM 2-ME, and 10% PHS. TG101348 was synthesized by the MSKCC Organic Synthesis Core Facility according to the published chemical structure23 at a stock concentration of 10mM in DMSO. TG101348 was diluted to 1μM in complete RPMI 1640 with either 1% or 10% vol/vol PHS for a final DMSO concentration of 0.01% vol/vol. The working concentration of TG101348 (1μM) was determined by assessing the dose-dependent suppression of T-cell proliferation in an allogeneic MLR (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). JAK inhibitor I (Calbiochem), a nonselective JAK inhibitor, was diluted to 250nM in complete RPMI 1640 with either 1% or 10% vol/vol PHS for a final DMSO concentration of 0.02% vol/vol (supplemental Figure 2).
Fluorochrome-conjugated anti–human mAbs and flow cytometry
For moDCs, FITC-conjugated, PE-conjugated, Alexa Fluor 647 (AF647)–conjugated, allophycocyanin (APC)–conjugated, and PE-cyanine-7 (PE-Cy7)–conjugated mouse anti–human MAbs included anti-CD80, anti-CD83, anti-CD86, anti–HLA-DR, anti–PD-L1, anti–PD-L2, and anti-pSTAT3 (pY705; BD Biosciences) and FITC-conjugated anti-CCR7 (R&D Systems).
For T cells, FITC-conjugated, PE-conjugated, AF647-conjugated, APC-conjugated, and PE-Cy7–conjugated mouse anti–human MAbs included anti-CD3, anti-CD8, anti-CD25, anti-CD45RO, anti-pSTAT3 (pY705), anti-pSTAT5 (pY694), and anti–IFN-γ (BD Biosciences); FITC-conjugated, AF647-conjugated, and APC-conjugated anti-CD3, anti-CD127, anti-human Foxp3, and anti–IL-17a (eBioscience); PE-Texas Red–conjugated anti-CD4 (Invitrogen); and FITC-conjugated anti-CCR7 (R&D Systems).
Fluorochrome-conjugated mouse Igs were used as isotype controls. Cells were acquired with an FC 500 flow cytometer (Beckman Coulter) and analyzed with FlowJo 8.8.7 software.
Cytokine-based generation of moDCs and T-cell purification
Immature moDCs were generated under the aegis of defined, recombinant human cytokines over 5 days, then terminally matured and activated for an additional 48 hours by a standardized combination of inflammatory cytokines and prostaglandin E2 (PGE2), exactly as published.9 T cells were mechanically purified without activation to > 95% from bulk PBMCs using tissue culture plastic nonadherence and elution through nylon wool,9 except where noted.
JAK2 inhibitor treatment of moDCs
The JAK2 inhibitor drug TG101348 (1μM) or DMSO diluent control (0.01%) was added where indicated from days 5-7 during the inflammatory cytokine–induced maturation and activation of moDCs.9 These TG101348 or control pretreated moDCs were harvested, washed, and analyzed for surface expression of CCR7, CD80, CD83, CD86, HLA-DR, PD-L1, and PD-L2 and compared with baseline expression by immature moDCs. Where specified, these pretreated or control moDCs were evaluated for stimulatory potency in primary allogeneic MLRs.
Functional assessment of moDC stimulatory capacity and T-cell proliferative responses
The allogeneic MLR is a standard assay of DC stimulatory potency, which also has relevance to graft-host interactions in allogeneic HSCT. For primary allogeneic MLRs, a fixed number of bulk T cells (105) and varying numbers of cytokine-matured allogeneic moDCs were plated in 3-fold dilutions to yield DC:T ratios of 1:30 to 1:1000. Each ratio was cultured in triplicate microwells of U-bottomed 96-well plates (Costar; Corning) in a final volume of 100 μL of complete RPMI-10%PHS. After 5 days at 37°C, T-cell proliferation was determined by a colorimetric assay according to manufacturer's instructions (CellTiter96 Aqueous One Solution Cell Proliferation Assay MTS; Promega).
Secondary MLRs used T-cell responders from the primary MLRs after a thorough wash and resuspension. These were immediately cultured with fresh moDCs from the original stimulator (first party), but only at a single moDC:T cell ratio of 1:30 and only for 3 days because of the more brisk nature of secondary immune responses. Culture conditions and colorimetric assay readouts were otherwise identical.
There was no pretreatment of the moDC stimulators in these allogeneic MLRs. The JAK2 inhibitor drug, TG101348 (1μM), or DMSO diluent control (0.01%) was added directly to either the primary or secondary MLRs, but not to both. In this way, we could evaluate T-cell responses to secondary restimulation by alloantigen after priming against that same alloantigen in the presence of the JAK2 inhibitor. Conversely, we could assess T-cell responses to secondary restimulation by alloantigen in the presence of JAK2 inhibition after unadulterated priming to the same alloantigen.
Where specified, a separate cohort of T cells primed against alloantigen in the presence of the JAK2 inhibitor was stimulated for 5 days by autologous HLA-A*0201–expressing moDCs (DC:T = 1:30) loaded with GILGFVFTL influenza matrix peptide (fluMP; Beckman Coulter). This allowed assessment of the capacity of these T cells to respond to nominal antigen de novo.
T-cell phenotype and cytokine analysis
T cells and supernatants were harvested from allogeneic MLRs. Supernatants were analyzed for IL-6 and TNF-α using a Human Inflammation Cytometric Bead Array Kit (BD Biosciences) per the manufacturer's instructions. For Treg analysis, T cells were surface stained for CD3, CD4, CD25, and CD127, followed by fixation, permeabilization (eBioscience), and intracellular staining for Foxp3. Tregs were identified by gating on the CD3+, CD4+, and CD25bright cells, followed by determination of Foxp3 expression and absence of CD127.24,25 Surface expression of CCR7 and CD45RO was used to identify alloantigen nonreactive T cells expressing CCR7 and lacking CD45RO, central memory T cells expressing both CCR7 and CD45RO, and effector memory T cells lacking CCR7 but expressing CD45RO after a 5-day allogeneic MLR. The surface expression of CD25 tracked activation within each defined T-cell compartment.
To assess Th1 and Th17 responses, CD4+ T cells were negatively selected from healthy donor PBMCs using an EasySep enrichment kit (STEMCELL Technologies), with > 95% purity by flow cytometry. CD4+ T cells and allogeneic, cytokine-matured moDCs were cocultured in allogeneic MLRs (DC:T = 1:30) with TG101348 or DMSO. After 6 days at 37°C, the CD4+ T cells were harvested and stimulated with PMA (30 ng/mL)/ionomycin 0.5μM (Sigma-Aldrich) in IMDM-10% PHS for 6 hours. GolgiStop (Monensin; BD Biosciences) was added after the first hour of stimulation. The CD4+ T cells were surface stained for CD3, CD4, and CD25, followed by fixation (Cytofix; BD Biosciences) and permeablization with cold methanol (90% vol/vol). Intracellular staining for IFN-γ and IL-17 was then assessed.
Phosphorylation of STAT proteins
JAK2 signaling was evaluated with respect to IL-6 and its receptor, by measuring the degree of STAT3 phosphorylation in response to ligand binding. Resting bulk T cells or immature moDCs (3 × 106 cell/mL) were Serum-starved in complete RPMI at 37°C for 3 hours with either TG101348 (1μM) or DMSO (0.01%). The cells were pulsed with recombinant human IL-6 (rhuIL-6; 5000 IU/mL) or not for 10 minutes. Cells were then fixed (Cytofix; BD Biosciences) and permeabilized with cold methanol (90% vol/vol). T cells or immature moDCs were then stained for CD3 or HLA-DR, respectively, and intracellular pSTAT3 (pY705).
JAK3 signaling was evaluated for IL-2 and its receptor by measuring the degree of STAT5 phosphorylation in response to ligand binding. PBMCs were stimulated with Con A (5 μg/mL; MP Biomedicals) for 48 hours to generate activated T-cell blasts, then harvested and washed with α-methylmannoside (10mM; Calbiochem) to remove the Con A. The blastic T cells were serum-starved in complete RPMI with either TG101348 (1μM) or DMSO (0.01%) at 37°C for 3 hours. The cells were then pulsed with rhuIL-2 (50 IU/mL; Chiron) or not for 10 minutes, followed by fixation (Cytofix; BD Biosciences) and permeabilization with cold methanol (90% vol/vol). T cells were then stained for CD3 and intracellular pSTAT5 (pY694). The IL-2–induced STAT5 phosphorylation index was measured by dividing the percentage of pSTAT5 in the sample by the percentage of pSTAT5 in its respective baseline.
Statistical analyses
Statistical comparisons used a paired, 2-tailed, Student t test and Prism Version 5.02 software (GraphPad). By exception and where stated, an unequal number of replicates across donors required a permutation-based paired t test to determine whether there was a difference between the DMSO group and the TG101348 group. The permutation operation was repeated 2000 times to compute the significance level. P < .05 was deemed statistically significant.
Results
JAK2 inhibition partially impairs the phenotypic maturation of human moDCs
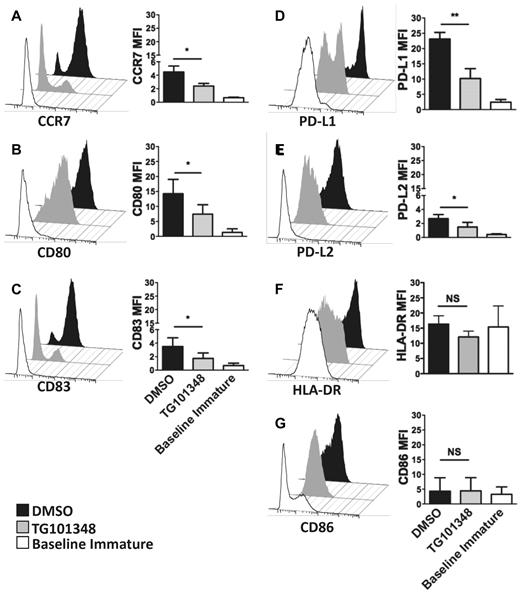
The viability of moDCs treated with 1μM of TG101348 was assessed by TO-PRO-3 iodide exclusion and was similar for both TG101348 (1μM) and DMSO (0.01%) groups at > 80% (supplemental Figure 1B). Inhibiting JAK2 during inflammatory cytokine–induced maturation of moDCs produced an intermediate activation phenotype. The expressions of CCR7, CD80, and CD83 (Figure 1A-C) and the negative immunoregulatory ligands PD-L1 and PD-L2 (Figure 1D-E) were decreased relative to the DMSO control. CCR7 and CD83 were the most affected. In contrast, the expression of CD86 and HLA-DR was similar between the 2 conditions (Figure 1F-G). These findings demonstrated that JAK2 inhibition partially impaired the maturation phenotype of moDCs in response to inflammatory cytokine stimuli.
JAK2 inhibition partially impairs the phenotypic maturation of human moDCs. (A-G) Selected phenotypic markers were assessed 48 hours after maturing moDCs with a combination of inflammatory cytokines (IL-1-β, IL-6, TNF-α, and PGE2)9 in the presence of either DMSO control (0.01%) or the JAK2 inhibitor TG101348 (1μM). Bar graphs show the average mean fluorescent intensities (MFI) ± SD from 4 (A,C,G) and 3 (B,E-F) independent experiments. *P < .05 by paired t test.
JAK2 inhibition partially impairs the phenotypic maturation of human moDCs. (A-G) Selected phenotypic markers were assessed 48 hours after maturing moDCs with a combination of inflammatory cytokines (IL-1-β, IL-6, TNF-α, and PGE2)9 in the presence of either DMSO control (0.01%) or the JAK2 inhibitor TG101348 (1μM). Bar graphs show the average mean fluorescent intensities (MFI) ± SD from 4 (A,C,G) and 3 (B,E-F) independent experiments. *P < .05 by paired t test.
JAK2 inhibition during moDC maturation does not affect moDC-stimulatory potency
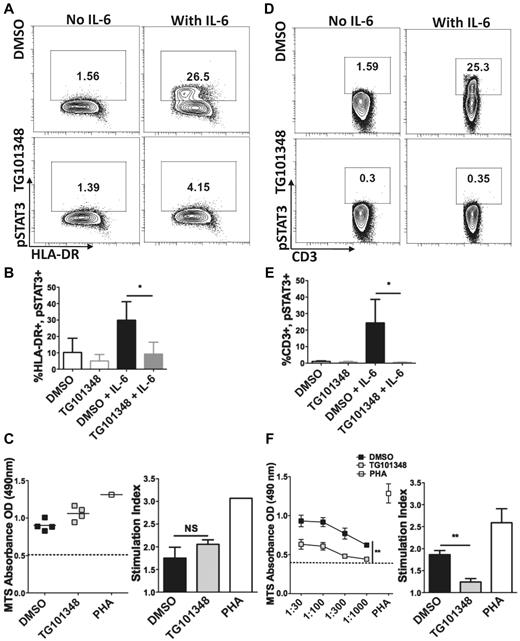
When IL-6 binds its IL-6R-α and gp130 receptor subunits, JAK2 undergoes phosphorylation, which in turn leads to phosphorylation of STAT3.26 We initially investigated the immunosuppressive effects of JAK2 blockade with a nonselective JAK inhibitor. This reagent, JAK inhibitor I, blocked IL-6/JAK2/pSTAT3 signaling and partially impaired the phenotypic maturation of moDCs (supplemental Figure 2). On-target inhibition of JAK2 signaling by the specific inhibitor TG101348 was verified by significant blockade of pSTAT3 (Figure 2A-B).
Pretreatment of moDCs by the JAK2 inhibitor TG101348 blocks IL-6–induced pSTAT3 but does not impair moDC-stimulatory potency. (A) Contour plots depict STAT3 phosphorylation evaluated in immature moDCs with or without rhuIL-6 (5000 IU/mL), treated with the JAK2 inhibitor TG101348 (1μM) or DMSO control (0.01%). (B) Bar graphs depict the means of the gated percentages of moDCs with pSTAT3 in panel A ± SD from 3 independent experiments; *P < .05 by paired t test. (C) Graphs demonstrate T-cell proliferation measured by a colorimetric assay in an allogeneic MLR of T cells stimulated by TG101348 or DMSO-pretreated, cytokine-matured moDCs (DC:T 1:30). Dotted line represents T cells alone. Stimulation index equals optical density (OD) of allogeneic MLR divided by the OD of T cells alone. Scatter plot and bar graph show average of triplicate means from 4 independent experiments ± SEM. NS indicates not significant by paired t test. TG101348 must be present during initial T-cell encounters with allogeneic moDCs to inhibit alloreactive T-cell proliferation. (D-E) IL-6–induced phosphorylation of STAT3 in resting T cells is depicted, mirroring the exact same conditions as used in panels A and B; n = 5; *P < .05 by paired t test. (F) Graphs show T-cell proliferation determined by colorimetric assay in 5-day allogeneic MLRs, with DMSO (0.01%) control or TG101348 (1μM) added directly to the culture once on day 0. Neither the T cells nor cytokine-matured moDCs were pretreated. DC:T ratios vary from 1:30 to 1:1000. Dotted line represents T cells alone. Stimulation indices were calculated for DC:T 1:30 groups, OD of allogeneic MLR divided by OD of T cells alone. Scatter plot and bar graph show average triplicate means ± SEM from 4 independent experiments. **P = .001-.01 by paired t test of area under the curve.
Pretreatment of moDCs by the JAK2 inhibitor TG101348 blocks IL-6–induced pSTAT3 but does not impair moDC-stimulatory potency. (A) Contour plots depict STAT3 phosphorylation evaluated in immature moDCs with or without rhuIL-6 (5000 IU/mL), treated with the JAK2 inhibitor TG101348 (1μM) or DMSO control (0.01%). (B) Bar graphs depict the means of the gated percentages of moDCs with pSTAT3 in panel A ± SD from 3 independent experiments; *P < .05 by paired t test. (C) Graphs demonstrate T-cell proliferation measured by a colorimetric assay in an allogeneic MLR of T cells stimulated by TG101348 or DMSO-pretreated, cytokine-matured moDCs (DC:T 1:30). Dotted line represents T cells alone. Stimulation index equals optical density (OD) of allogeneic MLR divided by the OD of T cells alone. Scatter plot and bar graph show average of triplicate means from 4 independent experiments ± SEM. NS indicates not significant by paired t test. TG101348 must be present during initial T-cell encounters with allogeneic moDCs to inhibit alloreactive T-cell proliferation. (D-E) IL-6–induced phosphorylation of STAT3 in resting T cells is depicted, mirroring the exact same conditions as used in panels A and B; n = 5; *P < .05 by paired t test. (F) Graphs show T-cell proliferation determined by colorimetric assay in 5-day allogeneic MLRs, with DMSO (0.01%) control or TG101348 (1μM) added directly to the culture once on day 0. Neither the T cells nor cytokine-matured moDCs were pretreated. DC:T ratios vary from 1:30 to 1:1000. Dotted line represents T cells alone. Stimulation indices were calculated for DC:T 1:30 groups, OD of allogeneic MLR divided by OD of T cells alone. Scatter plot and bar graph show average triplicate means ± SEM from 4 independent experiments. **P = .001-.01 by paired t test of area under the curve.
Because JAK2 inhibition partially impaired the moDC maturation phenotype, we hypothesized that moDCs pretreated with TG101348 during cytokine maturation would provide an inferior proliferative stimulus in an allogeneic MLR compared with the DMSO control.27 Contrary to that prediction and despite an intermediate activation phenotype, the cytokine-matured moDCs exposed to the JAK2 inhibitor still stimulated robust allogeneic T-cell proliferation (Figure 2C), as evident from the similar OD absorbance and stimulation indices when comparing TG101348 with DMSO control groups.
JAK2 blockade directly inhibits human T-cell responses to alloantigen
As with moDCs, we initially evaluated the nonselective JAK inhibitor I, which blocked IL-6/JAK2/pSTAT3 signaling (supplemental Figure 2). However, unlike specific JAK2 inhibition by TG101348, JAK inhibitor I also suppressed IL-2 signaling through JAK3/pSTAT5 in the treated T cells (supplemental Figure 2G).
We also confirmed on-target inhibition of JAK2 signaling in T cells by assessing IL-6–induced intracellular pSTAT3 with or without TG101348 treatment. The JAK2 inhibitor completely ablated the phosphorylation of STAT3 in the treated T cells pulsed with rhuIL-6 (Figure 2D-E). In addition, the degree of pSTAT3 suppression was greater and more complete in the TG101348-treated T cells compared with the TG101348-treated moDCs.
To determine the effect of JAK2 inhibition on alloreactive T-cell responses, we added TG101348 (1μM) or DMSO (0.01%) directly to the allogeneic MLR on day 0 without pretreatment of either moDCs or T cells, and did not replenish either reagent during the ensuing 5 days of culture. Both conditions maintained similar T-cell viability at > 80% based on TO-PRO-3 iodide exclusion (supplemental Figure 1C). Unlike the previous set of allogeneic MLRs, in which TG101348-pretreated moDCs were used, direct addition of the JAK2 inhibitor to the allogeneic MLR caused a statistically significant decrease in T-cell proliferation (Figure 2F). The stimulation indices in the DMSO control group were nearly double those of the JAK2 inhibitor group, even for the highest DC:T ratio of 1:30. Therefore, the presence of the JAK2 inhibitor during the initial encounters of T cells with alloantigen presented by moDCs proved critical to the substantial reduction in T-cell proliferation.
JAK2 inhibition maintains baseline proportions of nonalloreactive, central, and effector memory T cells while reducing activation within the central and effector memory compartments responding to alloantigen
We evaluated whether JAK2 inhibition altered the relative proportions of nonalloreactive T cells, central memory T cells, and effector memory T cells after moDC stimulation in an allogeneic MLR to which TG101348 or DMSO was added once on day 0 only. We also investigated whether JAK2 blockade reduced activation, based on CD25 expression within these particular T-cell compartments. Nonresponding T cells expressed CCR7 and lacked CD45RO, central memory T cells expressed both CCR7 and CD45RO, and effector memory T cells lacked CCR7 but expressed CD45RO. The surface expression of CD25 tracked relative activation within each defined T-cell compartment.28,29
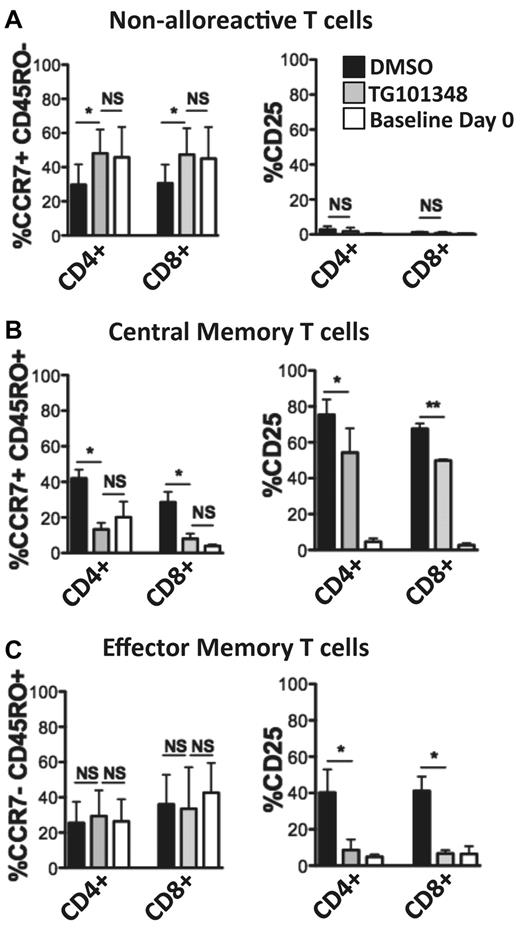
TG101348-treated allogeneic MLRs maintained baseline proportions of CD4+ and CD8+ nonalloreactive, central memory, and effector memory T cells (Figure 3). In contrast, the DMSO control allogeneic MLRs demonstrated a relative decrease in CD4+ and CD8+ nonresponders, which was paralleled by a proportional increase in central memory T cells (Figure 3A-B). JAK2 inhibition suppressed the expression of CD25 within the central and effector memory populations for both the CD4+ and CD8+ T cells (Figure 3B-C). Whereas the percentage of effector memory T cells was similar among the TG101348- and DMSO-treated allogeneic MLRs, this compartment demonstrated the greatest reduction of CD25 expression in response to selective JAK2 inhibition for both CD4+ and CD8+ T cells (Figure 3C). These experiments show that JAK2 blockade suppresses activation in T cells responding to allogeneic moDCs while maintaining proportions of nonalloreactive, central memory, and effector memory T-cell populations similar to that of unstimulated T cells.
JAK2 inhibition maintains baseline proportions of nonalloreactive and central and effector memory human T cells, while reducing activation in the central and effector memory compartments. (A-C) The effect of JAK2 inhibition (TG101348 1μM) compared with DMSO control (0.01%) on CD4+ or CD8+ T cells responding to allogeneic moDCs was assessed with respect to nonalloreactive, central memory, and effector memory T-cell populations developing in 5-day allogeneic MLRs at a DC:T ratio of 1:30. Drug or control was added once on day 0. The percentage of CD25 expression, a marker of lymphocyte activation, for each CD4+ or CD8+ T-cell compartment is shown as a bar graph on the right. Bar graphs show means ± SD from 3 independent experiments; *P < .05; **P = .001-.01; NS, not significant by paired t test.
JAK2 inhibition maintains baseline proportions of nonalloreactive and central and effector memory human T cells, while reducing activation in the central and effector memory compartments. (A-C) The effect of JAK2 inhibition (TG101348 1μM) compared with DMSO control (0.01%) on CD4+ or CD8+ T cells responding to allogeneic moDCs was assessed with respect to nonalloreactive, central memory, and effector memory T-cell populations developing in 5-day allogeneic MLRs at a DC:T ratio of 1:30. Drug or control was added once on day 0. The percentage of CD25 expression, a marker of lymphocyte activation, for each CD4+ or CD8+ T-cell compartment is shown as a bar graph on the right. Bar graphs show means ± SD from 3 independent experiments; *P < .05; **P = .001-.01; NS, not significant by paired t test.
JAK2 inhibition increases the Treg:effector T-cell ratio in human allogeneic MLRs
Whereas CD25 is a marker of effector activation, it is also highly expressed on Tregs.30 Given the dramatic reduction of CD25 expression on CD4+ and CD8+ T cells exposed to TG101348, we verified that JAK2 inhibition permitted the development of allogeneic Tregs in vitro. Treg yields from allogeneic MLRs were very limited, which precluded isolation and confirmation of functional suppression in add-back experiments. We therefore relied instead on their rigorous identification as CD3+CD4+CD25brightFoxp3+ lymphocytes lacking CD127, which is a widely accepted phenotypic profile for human Tregs (Figure 4A).24,25 The alloreactive CD8+ effector T cells were identified by gating on CD3+CD4− cells and then evaluating CD25 expression (Figure 4A).
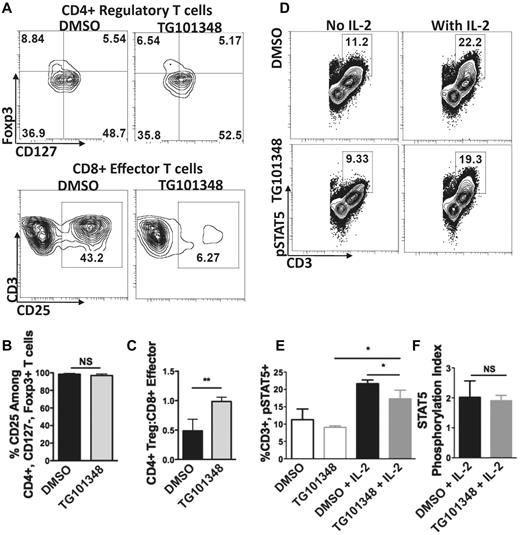
JAK2 inhibition increases the Treg:effector T-cell ratio in human allogeneic MLRs and does not impair IL-2 signaling. (A) Representative contour plots of Tregs (gating on CD3+CD4+CD25bright and then assessing for absence of CD127 and presence of Foxp3)24,25 and CD8+CD25+ effector T cells identified in allogeneic MLRs with DMSO (0.01%) or the JAK2 inhibitor TG101348 (1μM). Five day allogeneic MLRs used DC:T cell ratio of 1:30. Drug or control was added once on day 0. (B) Percent expression of CD25 among the Tregs identified in the DMSO (0.01%) or TG101348 (1μM)–treated allogeneic MLRs. Bar graphs show means ± SD from 5 independent experiments; **P = .001-.01; NS, not significant by paired t test. (C) Effect of JAK2 inhibition on the ratio of Tregs to CD8+CD25+ effector T cells in allogeneic MLRs compared with DMSO. Bar graphs show means ± SD from 5 independent experiments; **P = .001-.01; NS, not significant by paired t test. (D-F) Representative contour plots and bar graphs depict STAT5 phosphorylation in Con A–stimulated T cells with or without rhuIL-2 (50IU/mL) treated with TG101348 (1μM) or DMSO (0.01%). The IL-2–induced STAT5 phosphorylation index was measured by dividing the percentage of pSTAT5 in the sample by the percentage of pSTAT5 in its respective baseline. Bar graphs show means ± SD from 3 independent experiments; *P < .05; NS, not significant by paired t test.
JAK2 inhibition increases the Treg:effector T-cell ratio in human allogeneic MLRs and does not impair IL-2 signaling. (A) Representative contour plots of Tregs (gating on CD3+CD4+CD25bright and then assessing for absence of CD127 and presence of Foxp3)24,25 and CD8+CD25+ effector T cells identified in allogeneic MLRs with DMSO (0.01%) or the JAK2 inhibitor TG101348 (1μM). Five day allogeneic MLRs used DC:T cell ratio of 1:30. Drug or control was added once on day 0. (B) Percent expression of CD25 among the Tregs identified in the DMSO (0.01%) or TG101348 (1μM)–treated allogeneic MLRs. Bar graphs show means ± SD from 5 independent experiments; **P = .001-.01; NS, not significant by paired t test. (C) Effect of JAK2 inhibition on the ratio of Tregs to CD8+CD25+ effector T cells in allogeneic MLRs compared with DMSO. Bar graphs show means ± SD from 5 independent experiments; **P = .001-.01; NS, not significant by paired t test. (D-F) Representative contour plots and bar graphs depict STAT5 phosphorylation in Con A–stimulated T cells with or without rhuIL-2 (50IU/mL) treated with TG101348 (1μM) or DMSO (0.01%). The IL-2–induced STAT5 phosphorylation index was measured by dividing the percentage of pSTAT5 in the sample by the percentage of pSTAT5 in its respective baseline. Bar graphs show means ± SD from 3 independent experiments; *P < .05; NS, not significant by paired t test.
In contrast to prior experiments in which JAK2 inhibition reduced CD25 expression among the T-cell memory and effector populations, CD25 expression by CD4+CD127−Foxp3+ Tregs was comparable in allogeneic MLRs exposed to TG101348 or DMSO control (Figure 4B). TG101348 not only supported Treg survival, but JAK2 inhibition also shifted the ratio of CD4+ Tregs to alloreactive effector CD8+CD25+ T cells substantially in favor of Tregs (Figure 4C). These experiments verified that phenotypic Tregs persisted in TG101348-treated allogeneic MLRs.
Given the importance of IL-2 in Treg development, we confirmed that IL-2 signaling remained intact through its common γ chain receptor in the presence of JAK2 inhibition.19-21 This was demonstrated by a significant increase over baseline in IL-2–induced STAT5 phosphorylation via JAK3 in the TG101348-treated group (Figure 4D-E). Whereas the total percentage of pSTAT5 was slightly lower in the TG101348 treated T cells, the STAT5 phosphorylation index was similar in both groups (Figure 4F).
JAK2 inhibition induces durable allotolerance but preserves antiviral immunity
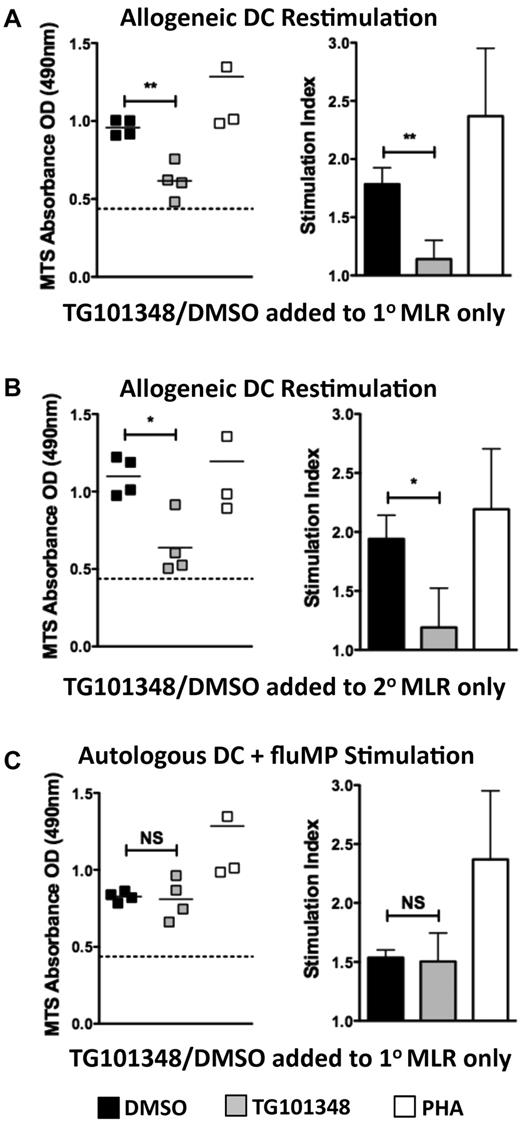
We directly assessed whether JAK2 inhibition achieved consistent suppression of alloreactive T-cell proliferation. T cells exposed to TG101348 at the onset of stimulation by allogeneic DCs remained tolerant to that alloantigen when rechallenged by the same first-party moDCs, even in the absence of additional JAK2 inhibition (Figure 5A). Conversely, T cells primed to allogeneic moDCs in the absence of JAK2 inhibition were unable to mount a secondary response if exposed to TG101348 at the onset of the secondary MLR (Figure 5B). T cells exposed to TG101348 in a primary allogeneic MLR, however, remained capable of responding to a nominal antigen-like fluMP when presented by autologous moDCs (Figure 5C).
JAK2 inhibition induces durable tolerance to alloantigen but preserves antiviral immunity. Graphs show responder T-cell proliferation measured by colorimetric assay after: (A) primary allogeneic MLR to which TG101348 or DMSO diluent control was added (5 days), followed by restimulation of T cells by first-party allogeneic moDCs (3 days); (B) unadulterated primary MLR (5 days), after which T cells were restimulated by first-party allogeneic moDCs in the presence of TG101348 or DMSO diluent control; or (C) T-cell responders from a primary allogeneic MLR to which TG101348 or DMSO diluent control was added as in panel A, stimulated de novo against autologous HLA-A*0201–expressing moDCs loaded with fluMP (5 days). Scatter plot (left) and bar graph (right) show replicate means ± SD from 4 independent experiments; *P < .05; **P = .001-.01; NS, not significant; the permutation-based paired t test was used in panels A and C, and the paired t test was used in panel B. Stimulation indices were calculated as in Figure 2C and F.
JAK2 inhibition induces durable tolerance to alloantigen but preserves antiviral immunity. Graphs show responder T-cell proliferation measured by colorimetric assay after: (A) primary allogeneic MLR to which TG101348 or DMSO diluent control was added (5 days), followed by restimulation of T cells by first-party allogeneic moDCs (3 days); (B) unadulterated primary MLR (5 days), after which T cells were restimulated by first-party allogeneic moDCs in the presence of TG101348 or DMSO diluent control; or (C) T-cell responders from a primary allogeneic MLR to which TG101348 or DMSO diluent control was added as in panel A, stimulated de novo against autologous HLA-A*0201–expressing moDCs loaded with fluMP (5 days). Scatter plot (left) and bar graph (right) show replicate means ± SD from 4 independent experiments; *P < .05; **P = .001-.01; NS, not significant; the permutation-based paired t test was used in panels A and C, and the paired t test was used in panel B. Stimulation indices were calculated as in Figure 2C and F.
JAK2 inhibition suppresses IL-6, TNF-α, and the Th1/Th17 response in human allogeneic MLRs
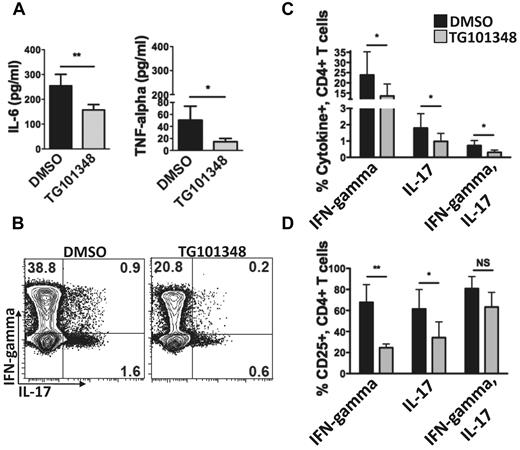
Supernatants from 5-day allogeneic MLRs, to which TG101348 or DMSO control had been added on day 0, were collected and assayed for IL-6 and TNF-α. These cytokines are implicated and therapeutically targeted in GVHD.1,10,11,31 TG101348 significantly decreased the concentrations of both IL-6 and TNF-α in the allogeneic MLR supernatants (Figure 6A).
JAK2 inhibition suppresses IL-6, TNF-α, and the Th1/Th17 response in human allogeneic MLRs. (A) The effect of JAK2 inhibition (TG101348, 1μM) on IL-6 and TNF-α concentrations in the supernatants of allogeneic MLRs compared with DMSO (0.01%). Bar graphs show means ± SD from 5 (IL-6) and 4 (TNF-α) independent experiments; *P < .05; **P = .001-.01 by paired t test. (B) Representative contour plots show the effect of TG101348 (1μM) on allogeneic Th1 and Th17 populations (gating on CD3+CD4+ and then assessing for IFN-γ and IL-17) compared with DMSO (0.01%) after a 6-day allogeneic MLR of sorted CD4+ T cells and cytokine-matured moDCs. (C) The effect of JAK2 inhibition on allogeneic Th1, Th17, and CD4+IFN-γ+IL-17+ populations compared with DMSO. (D) The influence of JAK2 inhibition on CD25 expression, a marker of lymphocyte activation, on allogeneic Th1, Th17, and CD4+IFN-γ+IL-17+ T cells compared with DMSO. Bar graphs show means ± SD from 4 independent experiments; *P < .05; **P = .001-.01; NS, not significant by paired t test.
JAK2 inhibition suppresses IL-6, TNF-α, and the Th1/Th17 response in human allogeneic MLRs. (A) The effect of JAK2 inhibition (TG101348, 1μM) on IL-6 and TNF-α concentrations in the supernatants of allogeneic MLRs compared with DMSO (0.01%). Bar graphs show means ± SD from 5 (IL-6) and 4 (TNF-α) independent experiments; *P < .05; **P = .001-.01 by paired t test. (B) Representative contour plots show the effect of TG101348 (1μM) on allogeneic Th1 and Th17 populations (gating on CD3+CD4+ and then assessing for IFN-γ and IL-17) compared with DMSO (0.01%) after a 6-day allogeneic MLR of sorted CD4+ T cells and cytokine-matured moDCs. (C) The effect of JAK2 inhibition on allogeneic Th1, Th17, and CD4+IFN-γ+IL-17+ populations compared with DMSO. (D) The influence of JAK2 inhibition on CD25 expression, a marker of lymphocyte activation, on allogeneic Th1, Th17, and CD4+IFN-γ+IL-17+ T cells compared with DMSO. Bar graphs show means ± SD from 4 independent experiments; *P < .05; **P = .001-.01; NS, not significant by paired t test.
Because JAK2 inhibition permitted Treg survival at the expense of CD8+ effectors, we then evaluated whether TG101348 would conversely reduce allostimulated Th1 and/or Th17 expansion. Given the reduction in IL-6, which is critical to the development of Th17 lymphocytes, we expected a corresponding decrease in this T-cell subset.6 The JAK2 inhibitor significantly reduced the Th1 response by 1/2 and the Th17 response by 2/3 (Figure 6B-C). Furthermore, effector T-cell activation measured by CD25 expression was substantially decreased by TG101348 in what remained of both the Th1 and Th17 populations (Figure 6D). A rare population of CD4+ T cells expressing both intracellular IFN-γ and IL-17 also decreased with JAK2 inhibition. Interestingly, this group of T cells retained surface expression of CD25 despite exposure to TG101348.
Discussion
We have demonstrated that JAK2 is a biologically relevant target for restricting alloantigen-specific T-cell reactivity, with implications for both GVHD and solid organ allograft rejection. Whereas several lines of data in mouse models show that JAK2 inhibition ameliorates various autoimmune syndromes,15,16 we have generated preclinical data with primary human cells in vitro, which prove that JAK2 blockade durably suppresses alloreactivity. Such inhibition of JAK2, however, does not impair subsequent development of adaptive immunity by other T cells in the responder population against a nominal pathogenic antigen like fluMP.
We initially investigated the immunosuppressive effects of JAK2 blockade with a nonselective reagent, JAK inhibitor I, which blocked the IL-6/JAK2/pSTAT3 signaling pathway, partially impairing the phenotypic maturation of moDCs and suppressing alloreactive T-cell proliferation. However, unlike specific JAK2 inhibition with TG101348, JAK inhibitor I also suppressed IL-2 signaling through JAK3/pSTAT5 in the treated T cells. Others have demonstrated that selective JAK3 inhibition reduces allograft rejection and murine GVHD.32-34 Disruption of JAK3 function is potentially detrimental to common γ chain cytokine signaling, however, because it affects IL-2, which is critical for Treg development.14,19-21 IL-15 also signals through JAK3/pSTAT5 and, along with IL-2, is important for generation of Ag-specific T-cell effectors.9,35
Whereas there are numerous JAK2 inhibitors, we chose TG101348 because it is the most selective JAK2 inhibitor currently available. TG101348 also has 300-fold higher binding affinity for JAK2 than JAK3,18 so it avoids off-target effects on the common γ chain cytokine pathways. TG101348 additionally blocks other inflammatory cytokine signaling through the JAK2/pSTAT3 pathway, which simple inhibition of IL-6R-α by tocilizumab could not prevent.13
JAK2 inhibitor treatment of moDCs interfered with their complete phenotypic maturation yet did not impair their potency as stimulators in allogeneic MLRs. Irrespective of any pretreatment effects on moDCs, however, achieving negative immunomodulatory effects on alloreactive T-cell responses required the presence of only 1μM TG101348 during the initial interactions between alloantigen-specific T-cell responders and allogeneic moDC stimulators. This resulted in a profound, durable, and antigen-specific tolerance by these T cells on reexposure to the same alloantigen without impairing the capacity of other T cells in the responder population to react de novo against a viral antigen such as fluMP. Moreover, we also observed similarly potent suppression of DC-stimulated T-cell proliferation when TG101348 was added only to the secondary allogeneic MLR after unrestricted T-cell priming against alloantigen in the primary MLR. These conditions mimic in vitro the respective clinical settings for using JAK2 inhibitors for GVHD prophylaxis and treatment. These data cannot distinguish whether the expansion of alloantigen-specific Tregs within the responder population or the induction of anergy in the alloreactive T cells mediates the specific suppression of T-cell responses. The data are clear, however, that JAK2 inhibition impairs alloreactive effector T-cell expansion, preserves the development of Tregs, and induces durable tolerance to alloantigen on reexposure without compromising immunity to a different, nominal antigen. Such selective and specific effects of TG101348 are distinct from current immunosuppressive agents used in HSCT or solid organ transplantation.
TG101348 selectively ablates signaling downstream of JAK2 but spares JAK3 function by way of IL-2– or IL-15–induced phosphorylation of STAT5. JAK2 inhibition favorably shifted the CD4+ Treg to CD8+ effector T-cell ratio because of a relative decrease in the latter population. We also observed a decrease in the proinflammatory Th17-cell population. IL-6 is a key proinflammatory cytokine involved in the expansion of Th17 cells, and its function depends on JAK2 signaling.6 The lack of IL-6/JAK2/pSTAT3 signaling, paired with the availability of uninterrupted IL-2/JAK3/pSTAT5 signaling, may therefore explain the alteration in the Treg/Th17 axis.7 The reductions in Th1 and Th17 responses with maintenance of Tregs after exposure to JAK2 inhibition are compatible with findings in mouse models of autoimmunity.15,16
Whereas we found a significant decrease in the supernatant levels of IL-6 and TNF-α with TG101348 in the allogeneic MLRs, correlative studies in patients receiving TG101348 as part of a phase 1 trial in myelofibrosis did not show a suppressive effect on these proinflammatory cytokines.22 These divergent findings may be because of cytokine variations in an allogeneic system in vitro compared with a noninflammatory state in vivo or to the inherent challenges of measuring accurate circulating cytokine levels in humans.
One might reasonably question the downside of also blocking signaling by the p40 cytokines IL-12p70 and IL-23, which share the downstream JAK2/pSTAT3 pathway with IL-6. Type I IFNs from plasmacytoid DC precursors provide an alternative to IL-12p70 for natural killer cell activation.36 We have also reported substantial evidence favoring human Langerhans-type DCs over moDCs in the generation of antigen-specific cytolytic T lymphocytes; although unlike moDCs, which produce copious amounts of IL-12p70, Langerhans-type DCs secrete little to none (Ratzinger et al9 and E. Romano, J. Cotari, R. Barreira da Silva, B.C.B, F. Avogadri, M. Fink, E.T.S., B. Mehrara, G.H., C. Münz, G. Altan-Bonnet, and J.W.Y., manuscript under revision). Finally, blockade of IL-23 signaling may prove advantageous, given the evidence for its pivotal role in intestinal inflammation where it mediates an inflammatory cytokine cascade involving increased levels of TNF-α, IL-6, IFN-γ, and IL-17.37-39 In fact, given that all conventional human DC subtypes secrete IL-23 (Romano and J.W.Y. unpublished data), this cytokine is a prime candidate to account for the lack of negative immunomodulation achieved by isolated blockade of IL-6R-α with tocilizumab,13 in contrast to specific JAK2 inhibition, which can curtail signaling by both IL-6 and IL-23.
Early-phase clinical trials in patients with myeloproliferative neoplasms have shown that JAK2 inhibition does not pose an increased risk of opportunistic infections requiring antimicrobial prophylaxis.22 JAK2 inhibitors have also not caused serious dose-limiting cytopenias that would portend poorly for use of such drugs in patients undergoing allogeneic HSCT.
Whereas we previously failed to observe any immune-modulatory effect by blocking IL-6R-α with tocilizumab,13 JAK2 inhibition induced significant and stable alloantigen-specific tolerance in our model of human alloreactivity. These experiments using primary human cells were of necessity in vitro, but the data conclusively demonstrated that the induced tolerance requires specific JAK2 blockade during T-cell interactions with allogeneic moDCs, is alloantigen specific, and does not lead to broad nonspecific anergy. Nonalloreactive T cells exposed to TG101348 during allogeneic moDC stimulation remain fully capable of responding to a viral recall antigen represented by fluMP. These preclinical studies support early-phase clinical trials of specific JAK2 inhibition for the treatment of steroid-refractory GVHD or solid organ allograft rejection. A positive therapeutic effect in these high-risk, often heavily pretreated patient populations would also support evaluation of JAK2-specific inhibition as targeted prophylaxis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Mortimer J. Lacher Fellowship from the Lymphoma Foundation (to B.C.B.); by the National Cancer Institute of the National Institutes of Health (T32 CA009207 to B.C.B., R01 CA151949 to R.L.L., R01 CA83070 to J.W.Y., and P01 CA23766 to G.H. and J.W.Y.); by the William H. Goodwin and Alice Goodwin of the Commonwealth Foundation for Cancer Research through The Experimental Therapeutics Center of MSKCC (to J.W.Y.); and by Swim Across America (to J.W.Y.).
National Institutes of Health
Authorship
Contribution: B.C.B. designed the overall study, performed the experiments, analyzed and interpreted the data, and wrote the manuscript; O.A.-W. and S.A.C. assisted with the design, analysis, and interpretation of data for selected experiments; E.T.S. and P.K. assisted with the performance of experiments; G.H. provided statistical design and analysis of the data; J.W.Y. and R.L.L. designed the overall study, supervised performance of the experiments, and managed the analysis and interpretation of the data; and J.W.Y. cowrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for B.C.B is Moffitt Cancer Center, University of South Florida, Tampa, FL.
Correspondence: James W. Young, MD, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: youngjw@mskcc.org.