Abstract
Significant comorbidites and lethality complicate GVHD and its treatment. Targeting the cytokine milieu may improve GVHD control; and IL6 is an attractive candidate, given its role in dendritic cell activation and T-cell differentiation. Tocilizumab is a humanized mAb to IL6-receptor-α (IL6R-α), which is Food and Drug Administration–approved for treatment of rheumatoid arthritis. Mouse transplant models have demonstrated that IL6 blockade also improves GVHD scores and survival. Definitive immunologic effects of IL6 inhibition have not emerged given inconsistent alterations in regulatory T cells (Tregs) and suppression of T-cell proliferation. Despite on-target suppression of IL6R-α signaling in human monocyte-derived dendritic cells (moDCs) and T cells, our data show no effect on moDC maturation/activation, alloreactive T-cell proliferation, Treg expansion, or allogeneic Th1/Th17 responses in vitro. These findings merit attention in any clinical trials of tocilizumab for GVHD prevention or treatment and provide a rationale for evaluating more specific inhibitors of downstream JAK2/STAT3 signaling as well.
Introduction
Tocilizumab is a humanized mAb to IL6R-α, inhibiting the JAK2/STAT3 signaling pathway.1 It is Food and Drug Administration–approved for treatment of rheumatoid arthritis, with potential efficacy in other autoimmune diseases.2-4 Tocilizumab can cause significant adverse effects, including cytopenias, infections, and gastrointestinal perforation.2-4
IL6 is a proinflammatory cytokine secreted by mature DCs and lymphocytes.5 IL6 is a constituent of monocyte-conditioned medium, and it enhances DC maturation and stimulatory potency.6 Indeed, combinations of inflammatory cytokines that mature DCs include rhu-IL6.6 IL6 enhances the generation of CD8+ cytolytic T cells, supports the development of Th17 lymphocytes that are active in autoimmunity, and impairs Treg differentiation.7-13 IL6 neutralization eliminates this suppressive influence over Tregs.12
Two groups have investigated the efficacy of IL6 inhibition in treating GVHD in mice.14,15 Their data have shown that IL6 inhibition results in decreased GVHD scores and improved survival.14,15 The data are inconsistent, regarding Treg expansion or direct effects on alloreactive T-cell proliferation.14,15 Given the continued interest in IL6 inhibition in the management of GVHD and the paucity of human data, we investigated the immune mechanisms underlying tocilizumab's effects on human DC-stimulated alloreactivity in vitro.
Methods
Cells, media, and reagents
PBMCs were isolated over Ficoll-Paque Plus (GE Healthcare Biosciences) from leukocyte concentrates from healthy, consenting, volunteer donors (Memorial Sloan-Kettering Cancer Center [MSKCC] Donor Room and Blood Bank; NY Blood Center, American Red Cross), in agreement with the Declaration of Helsinki and existing tissue procurement protocols approved by the Institutional Review and Privacy Board of Memorial Hospital, MSKCC. T cells and moDCs were obtained as published,16 with the exception of moDC maturation by exposure to LPS (10ng/mL; Sigma-Aldrich) whenever necessary to avoid IL6. Complete RPMI and IMDM (MSKCC Media Prep Core Facility) with heat-inactivated, pooled, human serum (PHS; Gemini Bioproducts) were supplemented as published.16 Tocilizumab (Actemra; Genen-tech) was purchased from MSKCC Pharmacy and used at 5 ug/mL final. Human immunoglobulin (Grifols) served as a negative control at 5ug/mL final.
Fluorochrome-conjugated anti–human mAbs and flow cytometry
MoDCs: FITC-, PE-, Alexa Fluor647 (AF647)–, APC-, and PE–cyanine-7 (PE-Cy7)–conjugated mouse anti–human mAbs included anti-CD83, anti-CD86, anti–HLA-DR, and anti-pSTAT3 (pY705; BD Biosciences); and FITC-conjugated anti-CCR7 (R&D Systems).
T cells: FITC-, PE-, AF647-, APC-, and PE-Cy7–conjugated mouse anti–human mAbs included anti-CD3, anti-CD8, anti-CD25, anti-pSTAT3 (pY705), and anti–IFN-γ (BD Biosciences); FITC-, AF647-, and APC-conjugated anti-CD3, anti-CD127, anti–human Foxp3, and anti-IL17a (eBioscience); and PE-Texas Red–conjugated anti-CD4 (Invitrogen).
Corresponding fluorochrome-conjugated mouse immunoglobulins were used as isotype controls. Live events were acquired with a FC 500 (Beckman Coulter) flow cytometer and analyzed using FlowJo Version 8.8.7 software (TreeStar).
STAT3 phosphorylation
Resting T cells or immature moDCs were starved in complete RPMI, with either tocilizumab or control Ig at 37°C for 3 hours. The cells were pulsed or not with rhu-IL6 (105 IU/mL; CellGenix) for 10 minutes. The cells were then fixed (Cytofix; BD Biosciences); permeabilized (cold methanol, 90% vol/vol); and stained with anti-CD3 (T cells) or anti–HLA-DR (moDCs), together with anti-pSTAT3.
Allogeneic mixed leukocyte reactions (alloMLR)
AlloMLRs comprised 105 T cells stimulated by moDCs at DC:T ratios of 1:30 to 1:1000. Tocilizumab or control Ig was added once on d0 of the 5-6 days alloMLR. T-cell proliferation was determined by a colorimetric assay (Promega).
Tregs and Th1/Th17 staining
Cytokine matured moDCs were cultured with allogeneic T cells at a DC:T ratio of 1:30, to which tocilizumab or control Ig was added on d0. After 5 days, Tregs were identified by gating on the live CD3+, CD4+, CD25bright cells, then assessing for Foxp3 expression and absence of CD127.17,18
To assess Th1 and Th17 responses, CD4+ T cells were negatively selected from PBMCs (EasySep; Stemcell Technologies) and stimulated by cytokine-matured moDCs at a DC:T ratio of 1:30 with tocilizumab or control Ig. CD4+ T cells were harvested after 6 days and stimulated with PMA/ionomycin in IMDM-10% PHS for 6 hours. Monensin (eBioscience) was added after the first hour of stimulation. CD4+ T cells were surface-stained for CD3, CD4, and CD25, followed by fixation and permeabilization (BD Biosciences) for intracellular staining of IFN-γ and IL17.
Statistical analysis
Statistical comparisons used the paired, 2-tailed, Student t test (GraphPad/Prism Version 5 software). Statistical significance required P value < .05.
Results and discussion
IL6R-α blockade does not impair moDC maturation or suppress alloreactive T-cell proliferation
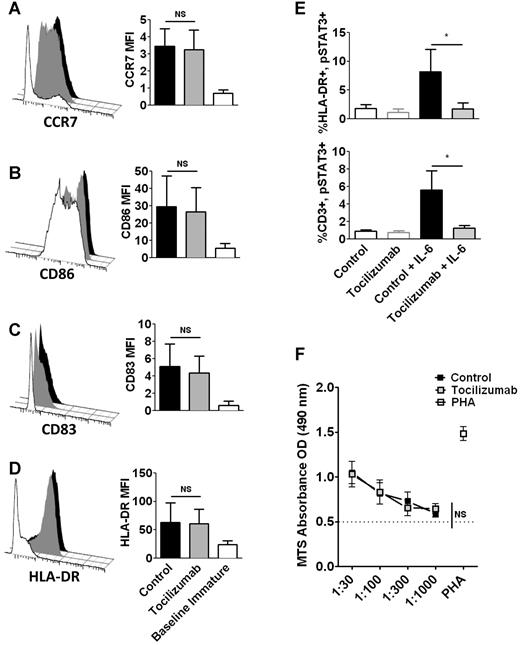
The addition of tocilizumab to LPS-matured moDCs did not diminish expression of CCR7,19 CD83,20 CD86,21 or HLA-DR21 (Figure 1A-D). We verified that tocilizumab (5ug/mL) blocked IL6 signaling by confirming the absence of IL6 induced pSTAT3 in tocilizumab-treated moDCs and T cells (Figure 1E).1 While IL6 enhances moDC potency, the addition of tocilizumab at the outset of the alloMLR had no effect on T-cell proliferation at any DC:T ratio (Figure 1F). This finding is similar to mouse data, where blocking the IL6R could not inhibit donor T-cell proliferation in alloMLRs.15
IL6R-α neutralization with tocilizumab does not impair the phenotypic maturation of moDCs or alloreactive T-cell proliferation. (A-D) DCs were matured for 48 hours with LPS, in the presence of tocilizumab or control Ig, both at 5ug/mL final. Representative histograms depict selected markers of DC maturation. Bar graphs show the averaged MFI (mean fluorescent intensity) from 4 independent experiments, ± SD; NS = not significant, paired t test. (E) Bar graphs show means from 4 independent experiments, ± SD, evaluating inhibition of IL6-induced pSTAT3 by tocilizumab in immature moDCs (HLA-DR+, top) and resting T cells (CD3+, bottom); *P < .05, paired t test. (F) A fixed number of T cells was cultured with variable doses of allogeneic DCs at DC:T ratios from 1:30 to 1:1000. Tocilizumab or control Ig (5ug/mL) was added once on d0. AlloMLRs were cultured for 5 days at 37°C. T-cell proliferation was assessed using a colorimetric assay. Graph shows the average of triplicate means from 4 independent experiments, ± SEM; NS = not significant, paired t test of area under the curve (AUC).
IL6R-α neutralization with tocilizumab does not impair the phenotypic maturation of moDCs or alloreactive T-cell proliferation. (A-D) DCs were matured for 48 hours with LPS, in the presence of tocilizumab or control Ig, both at 5ug/mL final. Representative histograms depict selected markers of DC maturation. Bar graphs show the averaged MFI (mean fluorescent intensity) from 4 independent experiments, ± SD; NS = not significant, paired t test. (E) Bar graphs show means from 4 independent experiments, ± SD, evaluating inhibition of IL6-induced pSTAT3 by tocilizumab in immature moDCs (HLA-DR+, top) and resting T cells (CD3+, bottom); *P < .05, paired t test. (F) A fixed number of T cells was cultured with variable doses of allogeneic DCs at DC:T ratios from 1:30 to 1:1000. Tocilizumab or control Ig (5ug/mL) was added once on d0. AlloMLRs were cultured for 5 days at 37°C. T-cell proliferation was assessed using a colorimetric assay. Graph shows the average of triplicate means from 4 independent experiments, ± SEM; NS = not significant, paired t test of area under the curve (AUC).
IL6R-α neutralization does not alter the Treg/Th17 axis
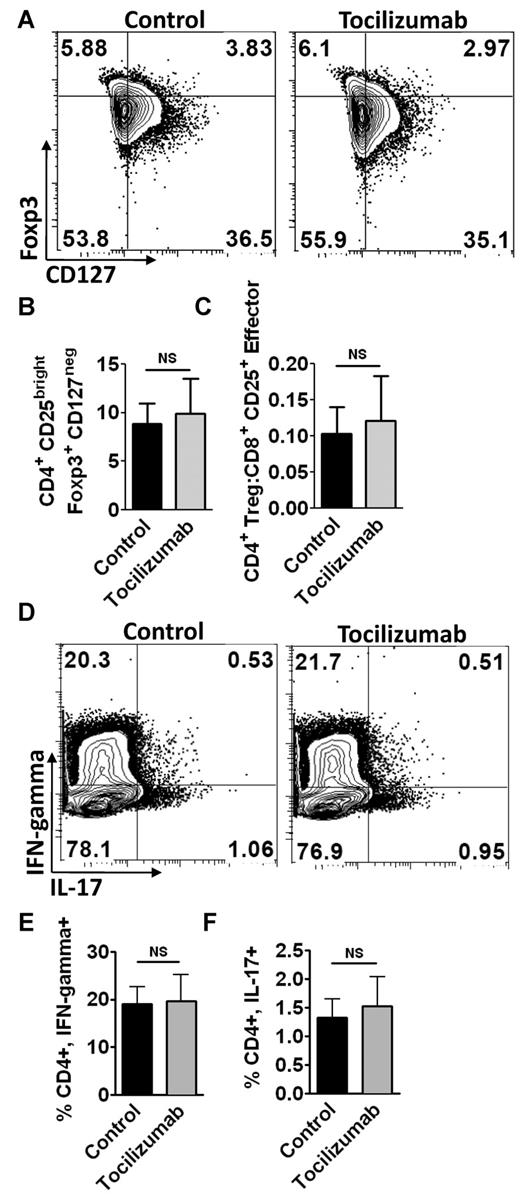
While IL6 antagonizes Treg development, data from mouse GVHD models regarding donor Treg expansion are inconsistent.14,15 We identified Tregs in human alloMLRs by gating on CD3+, CD4+, CD25bright T cells, followed by confirmation of Foxp3 expression and absence of CD127 (Figure 2A).17,18 The addition of tocilizumab neither increased the percentage of alloreactive human Tregs nor the ratio of CD4+ Tregs to effector CD8+ CD25+ T cells (Figure 2B-C).
IL6R-α neutralization with tocilizumab does not alter the Treg/Th17 axis. (A) Representative contour plots of allogeneic Treg populations. Tregs were identified by gating on the moDC-stimulated allogeneic CD3+, CD4+, CD25bright T cells, and then assessing for expression of Foxp3 and lack of CD127. (B) Bar graph depicts the means from 4 independent experiments (paired t test), ± SD, for the percentages of Tregs in alloMLRs treated with tocilizumab or control Ig (5ug/mL). (C) Effect of IL6R α inhibition on the ratio of CD4+ Tregs to CD8+CD25+ effector T cells in alloMLRs, compared with control. Bar graph shows means from 3 independent experiments, ± SD; NS = not significant, paired t test. (D) Representative contour plots of moDC-stimulated allogeneic Th1 and Th17 cells. Th1 and Th17 cells were identified by gating on the CD3+ CD4+ population and then evaluating for intracellular IFN-γ and IL17, respectively. (E) Bar graph shows means from 3 independent experiments, ± SD (paired t test, NS = not significant), representing the percentages of CD3+, CD4+, IFN-γ+ cells in the alloMLRs treated with tocilizumab (5 ug/mL) or control. (F) Bar graph shows means from 3 independent experiments, ± SD (paired t test, NS = not significant) for the percentages of CD3+, CD4+, IL17+ cells in the alloMLRs treated with tocilizumab (5 ug/mL final) or control.
IL6R-α neutralization with tocilizumab does not alter the Treg/Th17 axis. (A) Representative contour plots of allogeneic Treg populations. Tregs were identified by gating on the moDC-stimulated allogeneic CD3+, CD4+, CD25bright T cells, and then assessing for expression of Foxp3 and lack of CD127. (B) Bar graph depicts the means from 4 independent experiments (paired t test), ± SD, for the percentages of Tregs in alloMLRs treated with tocilizumab or control Ig (5ug/mL). (C) Effect of IL6R α inhibition on the ratio of CD4+ Tregs to CD8+CD25+ effector T cells in alloMLRs, compared with control. Bar graph shows means from 3 independent experiments, ± SD; NS = not significant, paired t test. (D) Representative contour plots of moDC-stimulated allogeneic Th1 and Th17 cells. Th1 and Th17 cells were identified by gating on the CD3+ CD4+ population and then evaluating for intracellular IFN-γ and IL17, respectively. (E) Bar graph shows means from 3 independent experiments, ± SD (paired t test, NS = not significant), representing the percentages of CD3+, CD4+, IFN-γ+ cells in the alloMLRs treated with tocilizumab (5 ug/mL) or control. (F) Bar graph shows means from 3 independent experiments, ± SD (paired t test, NS = not significant) for the percentages of CD3+, CD4+, IL17+ cells in the alloMLRs treated with tocilizumab (5 ug/mL final) or control.
IL6 induces naive CD4+ T cells to differentiate into Th17 cells, suggesting that tocilizumab should impair Th17 expansion.9,13 Mouse studies have shown that IL6 blockade does suppress Th1 responses in GVHD.14 We identified moDC-stimulated Th1 and Th17 responder lymphocytes by gating on CD3+ CD4+ cells, followed by assessment of intracellular IFN-γ or IL17, respectively (Figure 2D). The addition of tocilizumab did not diminish the Th1 or Th17 response in human moDC-stimulated alloMLRs (Figure 2E-F).
Apart from the amelioration of mouse GVHD by blockade of IL6 signaling,14,15 there is limited information regarding details of the relevant allogeneic interactions. In fact, while the observed effects of IL6 inhibition have not been in complete accord, the benefit occurs primarily through protection of the host gastrointestinal epithelium.14,15 Because of the additional absence of published information regarding human allogeneic interactions after IL6/IL6R blockade, we have examined the immune mechanisms of tocilizumab on human moDC-stimulated alloreactivity in vitro. We have identified potential limitations of IL6 blockade as a strategy to control clinical GVHD. Tocilizumab failed to suppress moDC maturation, alloreactive T-cell proliferation, or alter the Treg/Th17 axis in human alloMLRs. We have not yet evaluated the effects of tocilizumab on other conventional human DC subsets. Their similar potency to stimulate alloreactive T cells is well-established,22 so any differences with tocilizumab should be negligible.
Clinical case reports and a small clinical trial have ascribed some benefit to tocilizumab therapy in steroid-refractory GVHD,23,24 although prior and ongoing treatment with other immunosuppressants can always have confounding contributions to outcomes. While these reports have been intriguing, the findings presented herein merit careful attention in any clinical trials of tocilizumab for GVHD prevention or treatment. Even if tocilizumab were to prove effective in clinical GVHD, the inconsistent immune effects would be difficult to correlate with disease response and clinical outcome. Finally, as in most biologic systems, there may be sufficient redundancy for other unrecognized factors to signal through shared distal pathways, thus circumventing IL6R-α blockade by tocilizumab. Hence there is a strong rationale to evaluate more specific inhibitors of downstream JAK2/STAT3 signaling to control alloreactivity.25
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Mortimer J. Lacher Fellowship from the Lymphoma Foundation (B.C.B.), T32 CA009207 (B.C.B.); R01 CA83070 (J.W.Y.), P01 CA23766 (J.W.Y.) from the National Cancer Institute, National Institutes of Health; William H. Goodwin and Alice Goodwin of the Commonwealth Foundation for Cancer Research through The Experimental Therapeutics Center of MSKCC (J.W.Y.); and Swim Across America (J.W.Y.).
National Institutes of Health
Authorship
Contribution: B.C.B. designed the overall study, performed experiments, analyzed and interpreted data, and wrote the manuscript; E.T.S.A. and M.K. assisted with performance of experiments; and J.W.Y. designed the overall study, supervised performance of experiments, supervised analysis and interpretation of data, and cowrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for B.C.B. is Moffitt Cancer Center, University of South Florida, Tampa, FL. The current affiliation for M.K. is School of Medicine, Wayne State University, Detroit, MI.
Correspondence: James W. Young, MD, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: youngjw@mskcc.org.