Abstract
For decades, autologous ex vivo gene therapy has been postulated as a potential alternative to parenteral administration of recombinant proteins. However, achieving effective cellular engraftment of previously retrieved patient cells is challenging. Recently, our ability to engineer vasculature in vivo has allowed for the introduction of instructions into tissues by genetically modifying the vascular cells that build these blood vessels. In the present study, we genetically engineered human blood–derived endothelial colony-forming cells (ECFCs) to express erythropoietin (EPO) under the control of a tetracycline-regulated system, and generated subcutaneous vascular networks capable of systemic EPO release in immunodeficient mice. These ECFC-lined vascular networks formed functional anastomoses with the mouse vasculature, allowing direct delivery of recombinant human EPO into the bloodstream. After activation of EPO expression, erythropoiesis was induced in both normal and anemic mice, a process that was completely reversible. This approach could relieve patients from frequent EPO injections, reducing the medical costs associated with the management of anemia. We propose this ECFC-based gene-delivery strategy as a viable alternative technology when routine administration of recombinant proteins is needed.
Introduction
Parenteral administration of recombinant proteins to treat pathologic conditions is a standard practice in medicine.1,2 However, these therapies are usually expensive and require sustained patient compliance. For example, anemia in patients with advanced-stage chronic kidney disease is managed with routine injections of recombinant erythropoietin (EPO),3,4 a treatment that cost thousands of dollars annually.5,6 Moreover, there is a severe shortage of donor organs that is exacerbated by an aging population, and this forces patients to remain with dysfunctional kidneys for even longer periods of time.
For decades, gene therapy has been postulated as an alternative means for the administration of recombinant proteins,7,8 and although the validity of the approach is well-documented using animal models,9-12 clinical translation remains a challenge.13 Direct in vivo gene therapy offers the advantage of simplicity, but still raises questions regarding immunogenicity, cell target specificity, and safety associated with the use of viruses in vivo.14 Ex vivo gene therapy using autologous cells would substantially ameliorate most of these constraints because it can be carried out in vitro under controlled and reproducible conditions.14 However, engraftment of transfected cells back into the patient is not trivial.
In recent years, we and others have developed a means to engraft human cells through in vivo neovascular structures.15-18 Using an immunodeficient mouse model of human cell transplantation, we showed that subcutaneous injection of human BM-derived mesenchymal stem cells (MSCs) combined with blood-derived endothelial colony-forming cells (ECFCs) resulted in implants containing extensive human vascular networks that formed functional connections with the mouse vasculature.19 In these networks, ECFCs specifically line lumens of the newly formed blood vessels. This proximity is particularly advantageous for cell-based delivery vehicles, in which any protein secreted by the implanted ECFCs would theoretically be released into the bloodstream and distributed throughout the body.
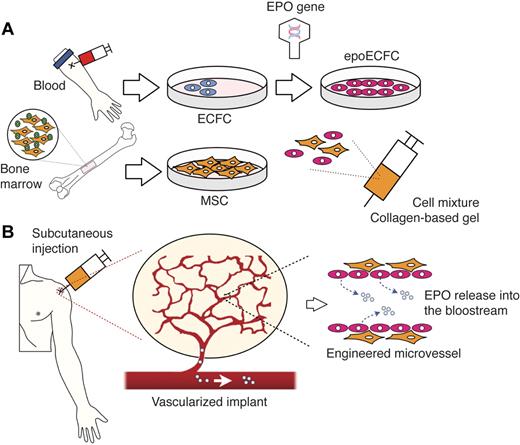
In the present study, we propose an autologous cell-based approach that combines principles of ex vivo gene therapy and vascular network bioengineering with the goal of generating subcutaneous implants that allow delivery of therapeutic EPO in vivo (Figure 1). The implementation of this approach involves several sequential steps: (1) cells must be obtained from the patient's own blood (ECFCs) and BM (MSCs) samples; (2) a gene encoding for human EPO (hEPO) is then inserted into culture-expanded ECFCs; and (3) genetically modified ECFCs and unmodified MSCs are combined in a biocompatible gel and subcutaneously injected back into the patient. We carried out preclinical studies to test the feasibility of this approach.
Combining ex vivo gene therapy and vascular network bioengineering: schematic diagram. (A) ECFCs and MSCs are obtained from the patient's own blood and BM, respectively. A gene encoding for hEPO is inserted into culture-expanded ECFCs, and the transfected cells (epoECFCs) are then selected out from the nontransfected ones. Culture-expanded epoECFCs and MSCs are combined in a collagen/fibrin–based gel and prepared for subcutaneous injection into the patient. (B) After injection, the transplanted cells engraft, forming a network of functional blood vessels that are connected to the patient's vasculature. The EPO-producing cells, epoECFCs, line the lumens of these bioengineered vessels. Consequently, EPO is readily secreted into the bloodstream of the patient and distributed systemically.
Combining ex vivo gene therapy and vascular network bioengineering: schematic diagram. (A) ECFCs and MSCs are obtained from the patient's own blood and BM, respectively. A gene encoding for hEPO is inserted into culture-expanded ECFCs, and the transfected cells (epoECFCs) are then selected out from the nontransfected ones. Culture-expanded epoECFCs and MSCs are combined in a collagen/fibrin–based gel and prepared for subcutaneous injection into the patient. (B) After injection, the transplanted cells engraft, forming a network of functional blood vessels that are connected to the patient's vasculature. The EPO-producing cells, epoECFCs, line the lumens of these bioengineered vessels. Consequently, EPO is readily secreted into the bloodstream of the patient and distributed systemically.
Methods
Cell culture
ECFCs and MSCs were isolated from human CB and BM and expanded in culture as described previously.19 Conditioned medium (CM) was generated in EBM-2 and 5% FBS for 24 hours. The sprouting assay was performed with ECFC-coated Cytodex-3 microcarriers embedded in fibrin gel (2 mg/mL of fibrinogen, 0.15 U/mL of aprotinin, and 0.625 U/mL of thrombin) for 4 days. The tube-formation assay was carried out on Matrigel-coated plates for 24 hours. TNF-α stimulation of ECFCs and the leukocyte adhesion assay were carried out as described previously.20 The proliferation assay was carried out as described previously18 with 10 ng/mL of VEGF-A, 1 ng/mL of bFGF, 10 ng/mL of EGF, and 10 ng/mL of IGF. For apoptosis assay, ECFCs were challenged with or without 0.5mM H2O2 for 14 hours and analyzed with the FITC–annexin-V apoptosis detection kit II (BD Pharmingen). The hematopoietic colony-forming assay was carried out with CB mononuclear cells (1.25 × 104 cells/35-mm dish) for 2 weeks in EPO-free methylcellulose medium (R&D Systems) containing either 100 μL of CM or 3 U/mL of recombinant hEPO (rhEPO; ProSpec).
Plasmid constructs and lentiviral transductions
Transfections were carried out using the Gateway cloning system (Invitrogen); entry vector containing hEPO (Ultimate ORF clone number IOH7334) was transferred to lentiviral pLenti6.3/V5-DEST vector. lacZ vector (pLenti6.3/V5-GW/lacZ) was used as control. For the Tet-regulated system, entry vector was transferred to lentiviral pLenti4/TO/V5-DEST vector and used in combination with a Tet-Repressor vector pLenti6/TR. Virus was generated in HEK 293T with Virapower Lentiviral packaging mix and Lipofectamine 2000 (Invitrogen). Transfected epoECFCs and laczECFCs were selected with Blasticidin and Zeocin. Doxycycline (Dox; 1 μg/mL in medium or 2 mg/mL in drinking water, replaced every 2 days) was used to induce EPO expression in tet-epoECFCs.
Flow cytometry, immunofluorescence, and immunoblotting
Cytometry and indirect immunofluorescence were carried out as described previously.19 Rabbit anti–hEPO (1:100; R&D Systems) was used. Western blots were performed with mouse anti–hEPO (1:500; R&D Systems) and mouse anti–human β-actin (1:10 000; Sigma-Aldrich).
RT-PCR and multigene transcriptional profiling
Total RNA was isolated with a RNeasy kit (QIAGEN), and cDNA was prepared using reverse transcriptase III (Invitrogen). Multigene transcriptional profiling, a form of quantitative RT-PCR, was used to determine the number of mRNA copies per cell normalized to 18S rRNA abundance (∼ 106 18S-rRNA copies/cell).21 Real-time PCR primer sequences were as follows: endogenous EPO (mEPO) (F: GGGACAGATGACCAGGTGTGT, R: GGTGTGGCACAAGCAATGTT), hEPO (F: AGCCCAGAAGGAAGCCATCT, R: GCGGAAAGTGTCAGCAGTGAT), human CD90 (hCD90; Thy1) (F: GCCTAACGGCCTGCCTAGT, R: GGGTGAACTGCTGGTATTCTCAT). In addition, RT-PCR was performed for 35 cycles with primers for hEPO (F: TCACTGTCCCAGACACCAAA, R: CACTGACGGATTTATCCACA) and GAPDH (F: ACCACAGTCCATGCCATCAC, R: TTCACCACCCTGTTGCTGTA).
Mice
Six-week-old nu/nu mice were purchased from Massachusetts General Hospital (Boston, MA). Nu/J mice for renal failure studies were purchased from The Jackson Laboratory. 5/6-Nephrectomy was performed by the vendor 7-14 days before shipping. Mice were housed in compliance with Children's Hospital Boston guidelines, and all animal-related protocols were approved by the institutional animal care and use committee.
In vivo experiments
The formation of vascular networks in vivo was evaluated using our xenograft model19 : 0.8 × 106 ECFCs and 1.2 × 106 MSCs in 200 μL of collagen/fibrin–based solution (3 mg/mL of bovine collagen, 30 μg/mL of human fibronectin, 25mM HEPES, 10% 10× DMEM, 10% FBS, and 3 mg/mL of fibrinogen, pH neutral). Before cell injection, 50 μL of 10 U/mL thrombin was subcutaneously injected. Unless otherwise stated, all experiments were carried out in 4 mice. rhEPO administration (60 IU/200 μL of saline given IP) was carried out every 2-3 days. For radiation-induced anemia, mice were subjected to 4Gy of γ irradiation 1 week after cell implantation. Microvessel density quantification, histology, and immunohistochemistry were performed as described previously.19 Rabbit anti–hEPO (1:100) and rabbit anti–hKi67 (1:200; Thermo Fisher Scientific) Abs were used. Rhodamine-labeled Ulex europaeus agglutinin I (UEA; 1:100; Vector Laboratories) was used to detect human microvessels.
Blood analysis
Blood was collected by submandibular bleeding in EDTA-coated tubes. Hematocrit, RBCs, WBCs, and hemoglobin were obtained from whole blood using an automated complete blood count test (Department of Laboratory Medicine, Children's Hospital Boston). Blood urea nitrogen concentration in the plasma was measured with a urea assay kit (Abcam). hEPO was measured in plasma with the hEPO Quantikine IVD ELISA Kit (R&D Systems).
Microscopy
Phase microscopy images were taken with a Nikon Eclipse TE300 inverted microscope using Spot Advance Version 3.5.9 software (Diagnostic Instruments) and a 10×/0.3 objective lens. All fluorescent images were taken with a Leica TCS SP2 Acousto-Optical Beam Splitter confocal system equipped with a DMIRE2 inverted microscope (diode 405 nm, argon 488 nm, HeNe 594 nm; Leica Microsystems) using a 63×/1.4 oil objective lens. Nonfluorescent images were taken with a Primo Star microscope (Zeiss) equipped with an AxioCam MRc5 camera (Zeiss) using a 40×/0.6 objective lens.
Statistical analyses
Data are expressed as means ± SD. Single comparisons were performed using 2-tailed Student unpaired t tests with Prism Version 4 software (GraphPad). In addition, multiple comparisons were performed where appropriate by 1- and 2-way ANOVA followed by Bonferroni posttest. P < .05 was considered statistically significant.
Results
Genetic engineering of ECFCs
We evaluated the receptivity of ECFCs to viral transduction and their ability to overexpress and secrete functional EPO (Figure 2). EPO-transfected ECFCs (epoECFCs) successfully overexpressed EPO, as confirmed by RT-PCR (Figure 2A). At the protein level, overexpression was confirmed by immunoblotting analysis of cell lysates (Figure 2B) and immunofluorescent staining of cells in monolayer cultures (Figure 2D and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). lacz-transfected ECFCs (laczECFCs), nontransfected ECFCs, and MSCs served as negative controls. EPO was also detected in CM obtained from epoECFCs (epoECFC-CM), which confirmed extracellular secretion of the protein (Figure 2C and supplemental Figure 2). Secreted EPO was confirmed to be functional in hematopoietic colony-forming assays, because epoECFC-CM induced the formation of EPO-dependent erythrocyte-containing colonies (Figure 2E and supplemental Figure 3). Overexpression of EPO did not alter the endothelial cell (EC) phenotype of ECFCs. Both laczECFCs and epoECFCs displayed typical cobblestone-like morphology and expressed the EC markers CD31 and VE-cadherin in cell-cell borders and vWF in a punctuate pattern in the cytoplasm (supplemental Figure 1). Flow cytometry showed uniform expression of CD31 and vWF and absence of CD90 and CD45 (supplemental Figure 1). Cells retained their ability to respond to EC mitogens, to launch angiogenic sprouts, and to assemble into capillary-like structures in 3D cultures (supplemental Figure 4). Viral transduction did not impair or augment physiologic response to pro-inflammatory cytokines (supplemental Figure 4). Overexpression of EPO did confer superior antiapoptotic capability to epoECFCs (supplemental Figure 5), which is consistent with previous studies revealing a protective, anti-apoptotic role of EPO in vascular ECs.22,23 These observations were clear indications of a preserved EC phenotype after viral infection.
Transduction of ECFCs with rhEPO. Human CB-derived ECFCs were transfected using a lentivirus vector encoding for hEPO (epoECFCs) under control of a CMV promoter. laczECFCs, nontransfected ECFCs, and MSCs served as negative controls for EPO expression. (A) Expression of EPO at the mRNA level was only observed in epoECFCs by RT-PCR. (B) At the protein level, expression of EPO was confirmed in cell lysates by immunoblotting analysis. (C) EPO was also detected by immunoblotting analysis in epoECFC-CM, but not in CM from laczECFCs, ECFCs, or MSCs. (D) Immunofluorescent staining of cells in monolayer cultures using Abs against human CD31 (green) and hEPO (red) showed EPO expression only present in epoECFCs, not in nontransfected ECFCs. Cell nuclei were stained with DAPI. Scale bars indicate 50 μm. (E) Secreted EPO was confirmed to be functional in hematopoietic colony-forming assays. CB-derived mononuclear cells cultured in EPO-free methylcellulose medium containing epoECFC-CM developed EPO-dependent erythrocyte-containing colonies such as BFU-E and CFU-GEMM. Cultures containing control ECFC-CM did not generate BFU-E or CFU-GEMM colonies. Scale bars indicate 200 μm.
Transduction of ECFCs with rhEPO. Human CB-derived ECFCs were transfected using a lentivirus vector encoding for hEPO (epoECFCs) under control of a CMV promoter. laczECFCs, nontransfected ECFCs, and MSCs served as negative controls for EPO expression. (A) Expression of EPO at the mRNA level was only observed in epoECFCs by RT-PCR. (B) At the protein level, expression of EPO was confirmed in cell lysates by immunoblotting analysis. (C) EPO was also detected by immunoblotting analysis in epoECFC-CM, but not in CM from laczECFCs, ECFCs, or MSCs. (D) Immunofluorescent staining of cells in monolayer cultures using Abs against human CD31 (green) and hEPO (red) showed EPO expression only present in epoECFCs, not in nontransfected ECFCs. Cell nuclei were stained with DAPI. Scale bars indicate 50 μm. (E) Secreted EPO was confirmed to be functional in hematopoietic colony-forming assays. CB-derived mononuclear cells cultured in EPO-free methylcellulose medium containing epoECFC-CM developed EPO-dependent erythrocyte-containing colonies such as BFU-E and CFU-GEMM. Cultures containing control ECFC-CM did not generate BFU-E or CFU-GEMM colonies. Scale bars indicate 200 μm.
Engraftment and systemic release of EPO in vivo
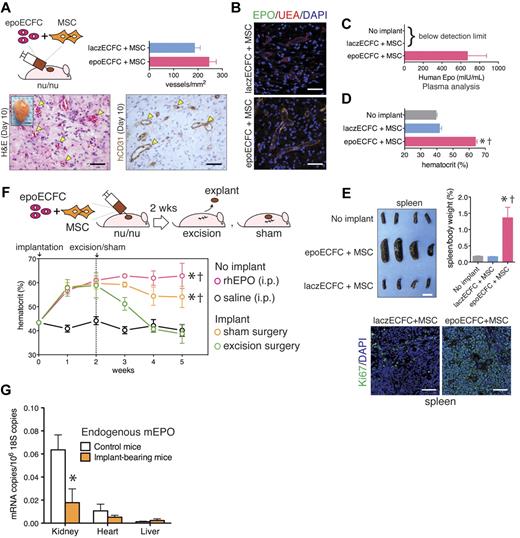
To determine the ability of transduced ECFCs to form vascular networks, we implanted epoECFCs with MSCs subcutaneously into nude mice for 10 days (Figure 3). Histologic examination of explants revealed an extensive network of perfused blood vessels that expressed human CD31 (Figure 3A and supplemental Figure 6). Quantification of microvessel density revealed no differences between implants prepared with epoECFCs and laczECFCs (245 ± 52 and 186 ± 47 vessels/mm2, respectively; P = .1754; Figure 3A). The epoECFC-lined microvessels, identified by binding of UEA, expressed EPO (Figure 3B and supplemental Figure 6). laczECFC-lined lumens showed no expression of hEPO. EPO was also detected in the plasma of mice bearing epoECFC-based implants, but it was undetectable in both naive mice and mice bearing laczECFC-based implants (Figure 3C). These results show that epoECFCs were able to engraft vascular networks and that they retained their ability to express and secrete EPO into the bloodstream.
Vascular network formation and EPO delivery in vivo. (A) epoECFCs and MSCs were subcutaneously injected into nude mice. Macroscopic view of explant at day 10 is shown in the inset (scale bar indicates 2 mm). H&E and hCD31 immunohistochemistry showed formation of numerous blood vessels (yellow arrowheads; scale bar indicates 50 μm). Microvessel density was quantified at day 10 (n = 4). (B) Double immunofluorescent staining of microvessels using UEA binding (red) and an Ab against hEPO (green; scale bars indicate 50 μm). (C) HEPO detected by ELISA in plasma at day 10. (D) Hematocrit at day 10 (epoECFCs vs no implant, †P < .0001; epoECFCs vs laczECFCs, *P < .0001). (E) Macroscopic view of spleens at day 10 (scale bar indicates 10 mm). Spleens/body weight ratio (epoECFCs vs laczECFCs, †P < .005; epoECFCs vs no implant,*P < .005). Immunofluorescent staining using an Ab against Ki67 (green). Cell nuclei were stained with DAPI. Scale bars indicate 50 μm. (F) Hematocrit was monitored for 5 weeks; implants were surgically excised at 2 weeks. Sham-operated mice, and mice treated (IP) with rhEPO and saline served as control (all vs excision, *P < .001; all vs saline, †P < .001). (G) Quantitative RT-PCR analyses of endogenous mEPO expression in kidney, heart, and liver at 5 weeks (*P < .01).
Vascular network formation and EPO delivery in vivo. (A) epoECFCs and MSCs were subcutaneously injected into nude mice. Macroscopic view of explant at day 10 is shown in the inset (scale bar indicates 2 mm). H&E and hCD31 immunohistochemistry showed formation of numerous blood vessels (yellow arrowheads; scale bar indicates 50 μm). Microvessel density was quantified at day 10 (n = 4). (B) Double immunofluorescent staining of microvessels using UEA binding (red) and an Ab against hEPO (green; scale bars indicate 50 μm). (C) HEPO detected by ELISA in plasma at day 10. (D) Hematocrit at day 10 (epoECFCs vs no implant, †P < .0001; epoECFCs vs laczECFCs, *P < .0001). (E) Macroscopic view of spleens at day 10 (scale bar indicates 10 mm). Spleens/body weight ratio (epoECFCs vs laczECFCs, †P < .005; epoECFCs vs no implant,*P < .005). Immunofluorescent staining using an Ab against Ki67 (green). Cell nuclei were stained with DAPI. Scale bars indicate 50 μm. (F) Hematocrit was monitored for 5 weeks; implants were surgically excised at 2 weeks. Sham-operated mice, and mice treated (IP) with rhEPO and saline served as control (all vs excision, *P < .001; all vs saline, †P < .001). (G) Quantitative RT-PCR analyses of endogenous mEPO expression in kidney, heart, and liver at 5 weeks (*P < .01).
Automated complete blood count analyses of blood samples drawn from mice 10 days after implantation showed that all erythropoiesis-related parameters—hematocrit level (Figure 3D), RBC concentration (supplemental Figure 7), and hemoglobin concentration (supplemental Figure 7)—were significantly higher in mice bearing epoECFC-based implants than in controls. In particular, the hematocrit increased from a basal 39.5% ± 1.4% to 63.9% ± 2.1% in implant-bearing mice (Figure 3D). Post mortem examination of organs at 10 days revealed splenomegaly in mice with epoECFC-based implants (Figure 3E). In addition, enlarged spleens contained significantly higher numbers of proliferating Ki67-positive cells (Figure 3E and supplemental Figure 8). Increased hematocrit and hypersplenism suggested extramedullary erythropoiesis in mice bearing epoECFC-based implants. To demonstrate reversibility, we excised implants surgically 2 weeks after implantation. After excision, the hematocrit level returned to basal level in the following 2 weeks (38.7% ± 3.9% at 5 weeks; Figure 3F). Sham-operated mice, which retained the implants throughout the study, maintained high hematocrit levels in the subsequent weeks after the operation (54.1% ± 8.8% at 5 weeks; Figure 3F). Furthermore, excised implants continued secreting EPO ex vivo for 24 hours, and the rate of EPO secretion was correlated with the hematocrit level observed in the mice before the excision (supplemental Figure 9). These results confirmed that epoECFC-based implants were solely responsible for the increased hematocrit and illustrate the ease with which this process can be reversed. The same observation was valid for hemoglobin and RBC concentrations, which were significantly higher in sham-operated mice than in excision-operated mice at 5 weeks (supplemental Figure 10). However, both groups presented similar body weight, WBCs, and spleen weight, the latter suggesting that the early splenomegaly may have been transient (supplemental Figure 10). Implants containing epoECFCs and MSCs were able to maintain an elevated hematocrit for up to 4-5 weeks (Figure 3F and supplemental Figure 11); after that, the hematocrit returned to the basal level (supplemental Figure 11), presumably because of replacement of human cells by the host.19 As expected, the presence of MSCs was critical to maintaining EPO expression, because successful ECFC engraftment is dependent on the presence of perivascular cells.15,16,18,19,24 Indeed, in the absence of MSCs, the hematocrit increased only during the first week, but returned rapidly to basal levels 2 weeks after implantation (supplemental Figure 11).
The measurement of mRNA in whole organs revealed a significant down-regulation of mEPO in the kidneys of mice that had borne implants for 5 weeks (74.1% reduction; *P < .01; Figure 3G), an indirect confirmation of exogenous hEPO functionality. Two other organs, heart and liver, showed low-to-undetectable levels of mEPO expression and no differences between control and implant-bearing mice. In addition, 5 weeks after implantation, the presence of both epoECFCs and MSCs in organs and tissues away from the implant was nondetectable based on levels of hEPO and hCD90 mRNA expression (see Table 1 and supplemental Figure 12 for the sensitivity of this evaluation), suggesting confined cellular engraftment.
Regulation of EPO expression with Dox
To achieve control over EPO expression, we used a vector that incorporated a tetracycline operator element upstream of the hEPO gene and a second vector encoding for a tetracycline repressor (tetR) protein (Figure 4A). The capacity to regulate EPO by the transfected ECFCs (tet-epoECFCs) was assessed by administration (on) or absence (off) of Dox (Figure 4). In vitro, tet-epoECFCs cultured in the absence of Dox did not express EPO (day 0); however, once Dox was provided (1 μg/mL), tet-epoECFCs progressively expressed EPO (days 1 and 4; Figure 4B). After day 4, Dox was removed from the medium and cells stopped the production of EPO, which was low and then undetectable at days 5 and 7, respectively (Figure 4B). Similar results were found by analyzing CM from tet-epoECFCs (Figure 4C-D). In vivo, implant-bearing mice with access to Dox (2 mg/mL; water changed every 2 days) had increased hematocrit during the first 2 weeks that was maintained afterward (59.7% ± 3.7% and 56.2% ± 5.9% at 2 and 4 weeks, respectively; Figure 4E). In contrast, without Dox, the hematocrit remained low (43.7% ± 1.1% and 44.2 ± 0.9% at 2 and 4 weeks, respectively; Figure 4E). Finally, when Dox was intermittently added following a weekly on/off regime, the hematocrit fluctuated accordingly, demonstrating the feasibility of modulating EPO expression on demand. The effects of this modular regulation of EPO was extended to RBC and hemoglobin concentrations. In addition, this inducible system allowed terminating or initiating the expression of EPO in vivo at will (supplemental Figure 13).
Regulation of EPO expression with Dox. (A) ECFCs were transduced using lentivirus vectors encoding for a tetracycline operator element (TetO2) upstream of the hEPO gene and TetR under the control of a CMV promoter. The capacity to regulate EPO by the transduced ECFCs (tet-epoECFCs) was assessed by administration (on) or absence (off) of Dox. (B) Double immunofluorescent staining for CD31 (green) and hEPO (red) showed progressive expression of EPO on administration of Dox (day 0-4). Thereafter, in the absence of Dox, EPO expression ceased progressively (day 4-7). Cell nuclei were stained with DAPI. Scale bars indicate 50 um. (C-D) Regulation of EPO with Dox was also demonstrated by analyzing CM from tet-epoECFCs with ELISA (C) and immunoblotting (D). (E) Regulation of the hematocrit in vivo was assessed in mice bearing tet-epoECFC–based implants subjected to 3 different Dox regimes for 4 weeks (n = 4 each group). The hematocrit in mice that did not receive Dox (off) was compared with those subjected to permanent administration of Dox (on vs off; *P < .01; **P < .001) and an intermittent Dox regime (off vs on/off; ‡P < .05; †P < .01).
Regulation of EPO expression with Dox. (A) ECFCs were transduced using lentivirus vectors encoding for a tetracycline operator element (TetO2) upstream of the hEPO gene and TetR under the control of a CMV promoter. The capacity to regulate EPO by the transduced ECFCs (tet-epoECFCs) was assessed by administration (on) or absence (off) of Dox. (B) Double immunofluorescent staining for CD31 (green) and hEPO (red) showed progressive expression of EPO on administration of Dox (day 0-4). Thereafter, in the absence of Dox, EPO expression ceased progressively (day 4-7). Cell nuclei were stained with DAPI. Scale bars indicate 50 um. (C-D) Regulation of EPO with Dox was also demonstrated by analyzing CM from tet-epoECFCs with ELISA (C) and immunoblotting (D). (E) Regulation of the hematocrit in vivo was assessed in mice bearing tet-epoECFC–based implants subjected to 3 different Dox regimes for 4 weeks (n = 4 each group). The hematocrit in mice that did not receive Dox (off) was compared with those subjected to permanent administration of Dox (on vs off; *P < .01; **P < .001) and an intermittent Dox regime (off vs on/off; ‡P < .05; †P < .01).
Alleviation of anemia after sublethal radiation
We tested our implants in a murine model of radiation-induced anemia. Immediately after radiation, control nude mice developed anemia and leukopenia; the recovery from anemia was accelerated by parenteral administration of rhEPO (from 3-4 weeks to 1-2 weeks; supplemental Figure 14). Using this model, we investigated whether mice bearing tet-epoECFC–based implants would have accelerated recovery from anemia after radiation (Figure 5A). One week after implantation, mice were subjected to sublethal radiation (4 Gy); 1 week later, the hematocrit of mice with access to Dox was significantly higher than in those without Dox (36.2% ± 4.9% versus 28.1% ± 1.8%; *P < .001; Figure 5B). Afterward, mice without Dox took 4 weeks to recover from anemia, but mice with Dox took 1-2 weeks to recover basal hematocrit values and exceeded basal levels thereafter. Finally, when mice were kept in the presence of Dox only for 2 weeks, their recovery was accelerated (49.0% ± 3.0% at 2 weeks; Figure 5B), and the hematocrit was maintained thereafter without Dox (42.7% ± 2.8% and 41.5% ± 1.0% at 4 and 5 weeks, respectively; Figure 5B). As expected, leukopenia was unaltered regardless of the Dox regime followed (Figure 5C). These results illustrate the benefit of having a regulatory element. EPO expression was induced for a controlled period of time until we obtained a therapeutic benefit (ie, an increase in hematocrit). Once EPO was no longer needed, we switched its production off, avoiding an excessive increase in hematocrit. In addition, these results also indicate that implants maintained their capacity to produce EPO even after being exposed to sublethal doses of radiation.
Delivery of EPO in a mouse model of radiation-induced anemia. (A) Mice were injected with tet-epoECFC–based implants and kept in the absence of Dox for 1 week. Then, mice received sublethal doses of radiation (4 Gy) and were thereafter subjected to 3 different Dox regimes for 4 weeks (n = 4 each group). (B) The hematocrit in mice that did not received Dox (off) was compared with those subjected to permanent administration of Dox (on vs off; *P < .001) and an intermittent Dox regime (off vs on/off; †P < .001). The hematocrit in mice subjected to permanent administration of Dox was also compared with those on an intermittent regime (on vs on/off; ‡P < .001; §P < .05). (C) WBCs in mice subjected to each Dox regime were monitored and compared for the entire period of the experiment, with no statistically significant differences observed.
Delivery of EPO in a mouse model of radiation-induced anemia. (A) Mice were injected with tet-epoECFC–based implants and kept in the absence of Dox for 1 week. Then, mice received sublethal doses of radiation (4 Gy) and were thereafter subjected to 3 different Dox regimes for 4 weeks (n = 4 each group). (B) The hematocrit in mice that did not received Dox (off) was compared with those subjected to permanent administration of Dox (on vs off; *P < .001) and an intermittent Dox regime (off vs on/off; †P < .001). The hematocrit in mice subjected to permanent administration of Dox was also compared with those on an intermittent regime (on vs on/off; ‡P < .001; §P < .05). (C) WBCs in mice subjected to each Dox regime were monitored and compared for the entire period of the experiment, with no statistically significant differences observed.
Correction of anemia in a renal failure model
One of the main clinical indications for EPO is the management of anemia in patients with renal failure.1 Therefore, we adopted a murine model of renal failure created by a 2-step 5/6 nephrectomy.25 Two weeks after the second surgery, nephrectomized nude mice developed marked anemia, with significantly lower hematocrit than control littermates (28.0% ± 4.3% vs 46.0% ± 1.0%; **P < .001; supplemental Figure 15). Using this model, we investigated whether our implants would correct anemia in nephrectomized mice (Figure 6A). One week after implantation, the hematocrit of mice with access to Dox was significantly higher than in mice without Dox (60.0% ± 7.8% vs 27.8% ± 2.0%; *P < .001), and these significant differences were maintained for 3 weeks (Figure 6B). This recovery occurred despite their insufficient renal function, which was confirmed by the development of uremia (Figure 6C). In addition, these results were extended to RBC and hemoglobin concentrations, but not to other physiologic parameters such as body weight and WBCs (supplemental Figure 16). Post mortem examination revealed that the left kidney was larger in the nephrectomized mice than in control littermates, independently of the Dox regime (*P < .001; Figure 6D). Moreover, 5 weeks after nephrectomy, the left kidney was statistically heavier in implant-bearing mice that had no access to Dox than in those in the presence of Dox (366.8 ± 55.2 and 292.5 ± 12.3 mg, respectively; *P < .001; Figure 6D), suggesting an involvement of EPO in compensatory kidney growth. These differences were not observed in spleen, liver, or heart, which maintained their weights in all conditions (supplemental Figure 17). Compensatory kidney growth led to progressive spontaneous correction of hematocrit over a period of 5 weeks in the absence of Dox (Figure 6B). In addition to this endogenous compensatory process,26 implant-bearing mice exhibited correction of anemia in just 1 week and were able to maintain a higher-than-basal level of hematocrit for up to 3 weeks with no more intervention than the administration of Dox (Figure 6B and supplemental Figure 16).
Delivery of EPO in a mouse model of renal failure. (A) Mice were subjected to a 2-step 5/6-nephrectomy and then, 2 weeks after surgery, were injected with tet-epoECFC–based implants and maintained in either the presence or absence of Dox for 5 weeks (n = 4 each group). (B) The hematocrit in mice that did not received Dox (off) was monitored and compared with those subjected to permanent administration of Dox (on vs off; *P < .0001). (C) Blood urea nitrogen content was monitored in 5/6-nephrectomized implant-bearing mice (Nephr.) in either the presence (Dox+) or absence (Dox−) of Dox for 5 weeks and compared with non-nephrectomized control mice (Ctr.; n = 4 each group; Nephr./Dox+ vs Ctr/Dox+, *P < .001; Nephr./Dox- vs Ctr/Dox−, †P < .001). (D) Macroscopic view of excised kidneys 5 weeks after subcutaneous implantation. Scale bar indicates 5 mm. The wet weights of kidneys excised from 5/6-nephrectomized mice were compared with those from non-nephrectomized mice. Independently of the Dox regime followed, left kidneys were significantly larger in nephrectomized than in control implant-bearing mice (*P < .001). In addition, left kidneys in nephrectomized mice in the absence of Dox were larger than in those in which Dox was administered (†P < .001).
Delivery of EPO in a mouse model of renal failure. (A) Mice were subjected to a 2-step 5/6-nephrectomy and then, 2 weeks after surgery, were injected with tet-epoECFC–based implants and maintained in either the presence or absence of Dox for 5 weeks (n = 4 each group). (B) The hematocrit in mice that did not received Dox (off) was monitored and compared with those subjected to permanent administration of Dox (on vs off; *P < .0001). (C) Blood urea nitrogen content was monitored in 5/6-nephrectomized implant-bearing mice (Nephr.) in either the presence (Dox+) or absence (Dox−) of Dox for 5 weeks and compared with non-nephrectomized control mice (Ctr.; n = 4 each group; Nephr./Dox+ vs Ctr/Dox+, *P < .001; Nephr./Dox- vs Ctr/Dox−, †P < .001). (D) Macroscopic view of excised kidneys 5 weeks after subcutaneous implantation. Scale bar indicates 5 mm. The wet weights of kidneys excised from 5/6-nephrectomized mice were compared with those from non-nephrectomized mice. Independently of the Dox regime followed, left kidneys were significantly larger in nephrectomized than in control implant-bearing mice (*P < .001). In addition, left kidneys in nephrectomized mice in the absence of Dox were larger than in those in which Dox was administered (†P < .001).
Discussion
The presence of ECFCs in peripheral blood27,28 and their robust blood vessel–forming ability15-18 create an unique opportunity to achieve the specific cellular engraftment needed in ex vivo gene therapies. In the present study, we transduced CB-derived ECFCs with a lentiviral vector encoding for hEPO, and demonstrated the ability to secrete this protein while preserving their EC phenotype. Transduced ECFCs retained their blood vessel–forming ability in vivo; when combined with BM-derived MSCs and injected into immunodeficient mice, epoECFCs were able to form extensive vascular networks that were connected with the murine vasculature over the course of 1 week. Once engrafted, epoECFCs expressed and secreted functional EPO in vivo, as determined by the detection of human-specific EPO on endothelial lumens and in blood plasma. The delivery of EPO unequivocally induced erythropoiesis. Implant-bearing mice consistently showed enhanced hematocrit, RBC count, and hemoglobin concentration; splenomegaly; and down-regulation of endogenous EPO expression in the kidneys. Remarkably, most of these effects were maintained for 5 weeks with no other exogenous EPO supply than that originated by the implants. For example, achieving a similar induction in control mice required multiple injections of rhEPO every week.
To achieve control over EPO expression, we included a tetracycline-regulated system. In vitro, tet-epoECFCs were able to sharply regulate the production and secretion of EPO, depending on Dox exposure, with undetectable levels of leak expression. In vivo, erythropoiesis was rapidly induced by simply adding Dox to the drinking water of implant-bearing mice. We demonstrated that the regulation of EPO expression has multiple advantages: (1) Dox is a small molecule that, unlike recombinant EPO, can be administered orally; (2) EPO treatment can be initiated and terminated at will, independently of when the cells were transplanted; (3) it allows selecting different target hematocrits over time by adjusting the Dox regime; and (4) it allows activating EPO expression independently of the animal's physiologic state. Importantly, the validity of our approach was demonstrated in both normal and anemic mice. For example, the recovery of implant-bearing mice from radiation-induced anemia was significantly accelerated; these experiments also indicated that radiation per se did not compromise the functioning of the transfected ECFCs inside the subcutaneous implants, allowing implantation to be performed ahead of radiation. Our implants were also capable of correcting anemia in 5/6-nephrectomized mice, validating applicability to situations of renal failure.
Collectively, the approach proposed here combines the advantages of ex vivo gene therapy with the benefit of highly specific cellular engraftment. In recent years, pre-clinical studies using a variety of cellular vehicles have been proposed for multiple therapeutic purposes, including the delivery of EPO.29-32 However, the challenge of efficient cell engraftment remain largely unresolved. Many authors have proposed encapsulation technologies in an attempt to improve cell retention9,10,33-35 ; however, this means of engraftment is not ideal for prolonged cell survival and it often leads to graft failure.36 Recently, the advantages of using EC as cellular vehicles are becoming more evident.37 For instance, Compte et al have used human umbilical vein ECs (HUVEC) for the delivery of recombinant Abs to treat human colon carcinoma in a xenograft model.38 However, HUVEC are only available at birth and therefore have less clinical potential than ECFCs, which circulate in peripheral blood even in adults.18,28 We achieved precise cellular engraftment because of the inherent ability of both ECFCs and MSCs to coordinately assemble into functional blood vessels that connect to the host vasculature. We also achieved tight cellular confinement: the presence of either ECFCs or MSCs was undetectable in organs and tissues away from the implant. This confinement reduces potential safety concerns and allows effective reversibility by a simple implant excision.
In conclusion, we propose ECFCs as ideal cellular vehicles for the delivery of EPO because: (1) the cells are readily available in the peripheral blood and can be isolated with ease; (2) after transduction, ECFCs are efficient at producing and secreting functional EPO; and (3) their inherent blood vessel–forming ability can be capitalized to achieve specific cellular engraftment in vivo. Although recombinant EPO has good safety and clinical records, the development of this ECFC-based gene-delivery strategy may lead to an alternative treatment for chronic patients waiting for a kidney transplantation, relieving them from frequent injections and potentially reducing the medical costs associated with the management of anemia. The presence of ECFCs in the peripheral blood of large mammals—for example, sheep39 and swine40 —will facilitate additional preclinical studies that should address the efficacy and longevity of a full autologous approach in immune-competent animals. Beyond the delivery of EPO, another prospective application is to combine gene therapy with an ECFC-based vascular network bioengineering to develop a platform technology for the delivery of a diverse range of therapeutic proteins in clinical and research applications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Histology was supported by the Specialized Research Pathology Core, Longwood facility, of the Dana-Farber/Harvard Cancer Center (P30 CA06516). Automated complete blood counts were supported by the Core Laboratory of the Department of Laboratory Medicine at Children's Hospital Boston. This work was partially supported by a grant from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health (K99EB009096 to J.M.M.-M.).
National Institutes of Health
Authorship
Contribution: R.-Z.L. designed and performed the experiments, interpreted the data, and edited the manuscript; A.D. and K.A. assisted with the cell transfections; D.L. and S.-C.S.J. performed the quantitative RT-PCR; A.C.D. assisted with the radiation experiments and edited the manuscript; and J.M.M.-M. designed and performed the experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Juan M. Melero-Martin, Department of Cardiac Surgery, Children's Hospital Boston, Harvard Medical School, 300 Longwood Ave, Enders 349, Boston, MA 02115; e-mail: juan.meleromartin@childrens.harvard.edu.