Abstract
Localization of plasmin on macrophages and activation of pro–MMP-9 play key roles in macrophage recruitment in the inflammatory response. These functions are promoted by plasminogen receptors exposing C-terminal basic residues on the macrophage surface. Recently, we identified a novel transmembrane plasminogen receptor, Plg-RKT, which exposes a C-terminal lysine on the cell surface. In the present study, we investigated the role of Plg-RKT in macrophage invasion, chemotactic migration, and recruitment. Plg-RKT was prominently expressed in membranes of human peripheral blood monocytes and monocytoid cells. Plasminogen activation by urokinase-type plasminogen activator (uPA) was markedly inhibited (by 39%) by treatment with anti–Plg-RKT mAb. Treatment of monocytes with anti–Plg-RKT mAb substantially inhibited invasion through the representative matrix, Matrigel, in response to MCP-1 (by 54% compared with isotype control). Furthermore, chemotactic migration was also inhibited by treatment with anti–Plg-RKT mAb (by 64%). In a mouse model of thioglycollate-induced peritonitis, anti–Plg-RKT mAb markedly inhibited macrophage recruitment (by 58%), concomitant with a reduction in pro–MMP-9 activation in the inflamed peritoneum. Treatment with anti–Plg-RKT mAb did not further reduce the low level of macrophage recruitment in plasminogen-null mice. We conclude that Plg-RKT plays a key role in the plasminogen-dependent regulation of macrophage invasion, chemotactic migration, and recruitment in the inflammatory response.
Introduction
Activation of plasminogen, the zymogen of the primary thrombolytic enzyme plasmin, is markedly promoted when plasminogen is bound to cell surfaces (for review, see Miles et al1 ) and cell-associated plasmin is protected from inactivation.2,3 Therefore, cells become armed with the broad-spectrum proteolytic activity of plasmin.4 This provides a mechanism to facilitate both physiologic and pathologic processes requiring cell migration. Plasminogen-dependent cell migration is involved in macrophage recruitment during the inflammatory response,4-10 tissue remodeling,11 wound healing,12,13 tumor cell invasion and metastasis,14,15 skeletal myogenesis,16 neuroendocrine prohormone processing,17,18 and neurite outgrowth.19,20 Studies in plasminogen-deficient mice have demonstrated that plasminogen plays a key role in cell migration in a diverse array of physiologic and pathophysiologic settings, notably, macrophage recruitment in response to inflammatory stimuli in the thioglycollate-induced model of peritonitis. Plasmin-dependent cell migration is accomplished by direct degradation of extracellular matrix components by plasmin and also by activation of matrix metalloproteinases for further degradation of extracellular matrices.4-7
Among the plasminogen-binding proteins, those exposing C-terminal basic residues on cell surfaces are predominantly responsible for the ability of eukaryotic cells to enhance plasminogen activation, because carboxypeptidase B (CpB) treatment abrogates cell surface–dependent plasminogen activation.21 Furthermore, plasminogen-dependent macrophage recruitment is mediated by CpB-sensitive plasminogen-binding sites.22 Recently, we used specific proteolysis with CpB combined with a proteomics technique (multidimensional protein identification technology) to identify a novel, structurally unique plasminogen receptor, Plg-RKT, from murine monocyte progenitor cells stimulated to undergo differentiation. Plg-RKT is an integral membrane protein that exposes a C-terminal lysine on the cell surface in an orientation to bind plasminogen, interacts with tissue plasminogen activator (t-PA), and markedly stimulates t-PA–dependent plasminogen activation.23 Furthermore, Plg-RKT is highly colocalized with the urokinase-type plasminogen activator (uPA) receptor (uPAR).23 In the present study, we evaluated human monocytes for the presence of Plg-RKT and tested the role of Plg-RKT in uPA-dependent plasminogen activation. We evaluated the role of Plg-RKT in monocyte migration and invasion and in a murine model of peritonitis induced by thioglycollate. Our results show that Plg-RKT plays a major functional role in plasminogen-dependent monocyte/macrophage migration, invasion, and recruitment in the inflammatory response.
Methods
Proteins
Human Glu-plasminogen was purified from fresh human blood as described previously.24,25 Single-chain t-PA was from Calbiochem/EMD.
MAb 7H1 was raised in mice against the synthetic peptide CEQSKFFSDK (corresponding to the 9 C-terminal amino acids of rat Plg-RKT with an aminoterminal cysteine added for coupling) coupled to keyhole limpet hemocyanin. Abs were selected for direct binding to immobilized CEQSKFFSDK coupled to BSA. MAb 7H1 was pan-specific, reacting with the C-terminal nonapeptides of mouse, rat, and human Plg-RKT with equivalent affinity. Endotoxin levels in experiments with mAb 7H1 were < 0.05 endotoxin units/mL as determined using the lymphocyte amebocyte lysate assay (Lonza). Fab fragments of mAb 7H1 were prepared using Fab preparation kit number 44885 (Pierce Biotechnology) according to the manufacturer's instructions. Anti–α-enolase mAb 9C1226 was prepared in our laboratory. Polyclonal Abs against MMP-9 (AB19016) and MMP-2 (AB19167) were from Millipore. IgG2a isotype control mAb and Fab fragments (low endotoxin and azide free) were from SouthernBiotech (0103-14).
Cells
Human monocytoid U937 cells and THP-1 cells were cultured as described previously.27 Hoxa9-ER4 cells were a gift from Dr Mark Kamps (University of California San Diego), and were cultured and differentiated with M-CSF as described previously.28 To isolate human peripheral blood monocytes (PBMs), freshly donated human blood was centrifuged over Ficoll-Hypaque (Pharmacia). PBMs at the Ficoll-Hypaque interface were separated into monocyte and lymphocyte populations. PBMs were isolated by adherence for 18 hours on Petri dishes (Corning).29 Human granulocytes, lymphocytes, and RBCs were isolated as described previously.29 Mouse peritoneal exudate cells were collected by peritoneal lavage, centrifuged, and resuspended in complete medium. Macrophages were further purified by adherence on 100- × 20-mm sterile culture dishes (Corning) for 2-3 hours at 37°C. Adherent cells were detached using 0.25% Trypsin-EDTA (Invitrogen) and counted using a hemocytometer.
Blood cell differentials
Blood cell differentials were determined using a Vet ABC hematology analyzer (SCIL Animal Company). Mice were killed by CO2 overdose and blood samples (200 μL) were collected by cardiac puncture within 1-2 minutes after death. EDTA (6 mg/mL) was used as anticoagulant. To recover thioglycollate-elicited cells, PBS (7 mL) was injected into the peritoneum and 5 mL was recovered, centrifuged, and resuspended in 50 μL of PBS.
FACS analysis
FACS analyses were performed as described previously.14 Briefly, indirect immunofluorescence staining and dual-color FACS analyses were performed. Cells (5 × 105) were incubated with mAb 7H1 IgG or isotype control IgG for 20 minutes in binding buffer (HBSS containing 0.1% BSA and 2mM EDTA) at 4°C. The cells were washed once with 200 μL of binding buffer and incubated with FITC-labeled secondary IgG for 30 minutes at 4°C in the dark. The cells were washed again, resuspended in 500 μL of binding buffer containing the nonvital dye propidium iodide (PI) at 5 μg/mL, and the cells were immediately analyzed by dual-color FACS as described previously.30 In other experiments, cells were incubated with FITC-plasminogen instead of Abs and populations of cells were gated according to the fluorescence intensity of PI staining. The population of cells with low cell-associated PI fluorescence intensity (cells that excluded PI) were defined as viable.
Western blotting
Cytoplasmic and membrane fractions were prepared as described previously,31 subjected to SDS-PAGE on 4%-20% Tris-glycine gels, and transferred to nitrocellulose membranes (Invitrogen). The membranes were incubated with mAb 7H1 or IgG2a isotype control, washed, incubated with goat anti–mouse HRP-conjugated secondary Ab (Biosource), and developed with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) on Kodak Biomax MR Film.
Plasminogen-binding assay
A peptide corresponding to the C-terminal residues of human Plg-RKT (CEQSKLFSDK, with a C added for coupling) was coated onto wells of microtiter plates at 10 μg/mL and the wells were blocked with 5% BSA. Either mAb 7H1 or isotype control was added. Biotinylated plasminogen was then added, followed by detection with streptavidin-HRP goat anti–mouse IgG (Invitrogen) as described previously.23
Plasminogen activation assay
U937 cells were preincubated with either mAb 7H1 or mouse IgG2a isotype control for 30 minutes at 37°C. Glu-plasminogen (2.7μM) was then added, the mixture was incubated for an additional 30 minutes at 37°C, and then 20nM high molecular weight recombinant uPA (Calbiochem/EMD) was added. Plasmin activity was measured after 12 minutes by diluting the reaction mixture 1:50 into S-2251 (DiaPharma Group) to a final concentration of 1mM and monitoring absorbance at 405 nm as described previously.23
Invasion and chemotactic migration
U937 cells in RPMI 1640 medium (without additions) were pretreated with 7H1 Fab or IgG2a isotype control Fab, and then plasminogen (200nM) was added. The cells (4 × 105) were then placed in the upper chambers of 24-well Transwell plates with polycarbonate membrane filters (Corning). Transwells were either uncoated (5-μm pore size) or coated with Matrigel (BD Biosciences) diluted 1:50 (8-μm pore size). uPA (50nM) was present in the Matrigel invasion assays. As a chemoattractant, MCP-1 (100 ng/mL; R&D Systems) was present in the lower wells. After incubation for 18 hours at 37°C in 5% CO2, cells in the lower chambers were quantified using the CellTiter-Glo Luminescent Cell Viability Assay (Promega).
mAb injection into mice
Plg+/− mice32 were a kind gift from Dr Victoria Ploplis (University of Notre Dame, Notre Dame, IN) and were bred to obtain Plg−/− mice and Plg+/+ littermate controls. C57Bl/6J female mice at 8-9 weeks of age were obtained from the in-house rodent breeding colony. All animal procedures and protocols were approved by the institutional animal care and use committee of The Scripps Research Institute. Either mAb 7H1 (500 μg) or IgG2a isotype control (500 μg) in 200 μL of PBS were injected intravenously into mice (8-9 weeks old). Thirty minutes later, the mice were injected intraperitoneally with 3% thioglycollate (Sigma-Aldrich). A second injection of Ab was administered intravenously 24 hours after thioglycollate. After 72 hours, mice were killed and thioglycollate-recruited cells were collected by peritoneal lavage.
Zymography
Gelatin zymography was carried out as described previously.33 Briefly, samples were electrophoresed on 10% SDS-PAGE gels containing 0.1% gelatin under nonreducing conditions. The gels were washed with 2.5% Triton X-100 and then incubated in 40mM Tris buffer, pH 7.6, supplemented with 200mM NaCl and 10mM CaCl2 for 18 hours at 37°C. The gels were stained with Coomassie Blue and destained in distilled water to visualize bands. Gels were scanned and band intensity was quantified using the UN-SCAN-IT gel Version 6.1 program (Silk Scientific).
Reagents
Aprotinin was from Roche, amiloride was from Sigma-Aldrich, and GM6001was from Millipore.
Statistics
Data are presented as means ± SEM. The Student t test was used to evaluate the significance of differences between groups.
Results
Plg-RKT is expressed by human monocytes
Plg-RKT was first identified in the murine Hoxa9-ER4 cell line.23 Therefore, we examined the expression and subcellular localization of Plg-RKT in human peripheral blood monocytes and human monocytoid cells using an anti–Plg-RKT mAb (mAb7H1) raised in mice against the synthetic peptide CEQSKFFSDK (corresponding to the 9 C-terminal amino acids of rat Plg-RKT with an aminoterminal cysteine added for coupling). This mAb was selected in our hybridoma screening strategy because it reacted with the C-terminal peptides of human, mouse, cow, dog, and rat Plg-RKT with equivalent affinity (data not shown). Furthermore, using confocal analysis, we have shown previously that mAb 7H1 (identified as mouse anti–Plg-RKT mAb in our publication) and plasminogen compete for binding sites on monocytoid cells.23 Membrane and cytoplasmic fractions of human peripheral blood monocytes, human U937 cells, and human THP-1 cells were electrophoresed and Western blotted with anti–Plg-RKT mAb 7H1. A major band corresponding to the molecular mass of Plg-RKT (17 261 Da) was observed in the membrane fractions of human peripheral blood monocytes, U937 cells, and THP-1 cells, but was not detected in the cytoplasmic fractions of these cells (Figure 1A). In positive controls, an immunoreactive band was also detected in the membrane, but not in the cytoplasm, of M-CSF–differentiated murine progenitor Hoxa9-ER4 cells34 from which the Plg-RKT was initially isolated.23 In further specificity controls, U937 cell lysates were immunoprecipitated with mAb 7H1. After Western blotting with mAb 7H1, a specific band was observed at a Mrapp of 17 261; no additional bands were observed compared with Western blotting with isotype control or immunoprecipitation with isotype control followed by Western blotting with mAb 7H1 or with isotype control (data not shown). In addition, when the specific immunoprecipitated band migrating at 17 261 was cut out and subjected to MALDI analysis, peptides corresponding to Plg-RKT were detected in the immunoprecipitate, but no peptides corresponding to other plasminogen-binding proteins were detected (data not shown). Therefore, Plg-RKT is markedly expressed in membranes of normal human peripheral blood cells and human monocytoid cell lines.
Plg-RKT expression by human monocytes. Membrane and cytoplasmic fractions of human peripheral blood monocytes (PBMs), U937 cells, THP-1 cells, and M-CSF–differentiated Hoxa9-ER4 cells (30 μg/lane; A) and lysates of human granulocytes, lymphocytes, RBCs, and M-CSF–differentiated Hoxa9-ER4 cells (40 μg/lane; B) were electrophoresed on 4%-20% SDS gels under reducing conditions and Western blotted with anti–Plg-RKT mAb 7H1. The blots were stripped and reprobed with anti–α-enolase mAb 9C12 (A,B) as a loading control. No immunoreactive bands were detected with isotype control (data not shown).
Plg-RKT expression by human monocytes. Membrane and cytoplasmic fractions of human peripheral blood monocytes (PBMs), U937 cells, THP-1 cells, and M-CSF–differentiated Hoxa9-ER4 cells (30 μg/lane; A) and lysates of human granulocytes, lymphocytes, RBCs, and M-CSF–differentiated Hoxa9-ER4 cells (40 μg/lane; B) were electrophoresed on 4%-20% SDS gels under reducing conditions and Western blotted with anti–Plg-RKT mAb 7H1. The blots were stripped and reprobed with anti–α-enolase mAb 9C12 (A,B) as a loading control. No immunoreactive bands were detected with isotype control (data not shown).
Plg-RKT was also detected in other human peripheral blood cells: highly expressed in lymphocytes, less strongly expressed by granulocytes, and not detected in RBCs (Figure 1B).
Plg-RKT regulates cell-surface plasminogen activation by uPA
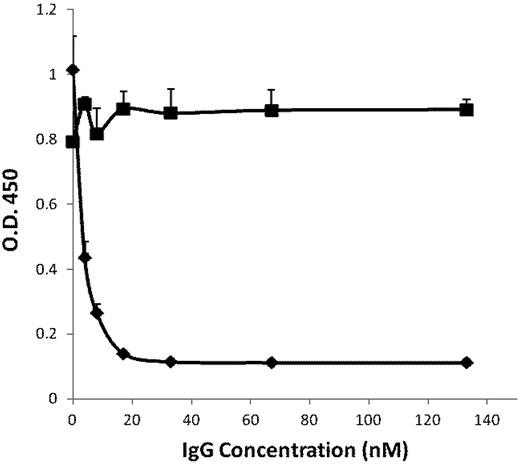
Using confocal analyses, we found previously that Plg-RKT is highly colocalized with uPAR.23 The kinetically favored substrate for uPAR-bound uPA is cell-associated, rather than solution-phase plasminogen.35 Therefore, we investigated whether Plg-RKT regulates uPA-dependent cell surface plasminogen activation using an Ab-inhibition approach. First, we examined whether mAb 7H1 could block plasminogen binding directly to the C-terminal peptide of human Plg-RKT. The peptide was coated onto wells of microtiter plates and the capacity of mAb 7H1 to inhibit plasminogen binding was tested. MAb 7H1 inhibited plasminogen binding in a dose-dependent fashion with an IC50 of 5nM (Figure 2). Maximal inhibition of plasminogen binding was achieved at a concentration of 30nM (Figure 2).
Inhibition of plasminogen binding by anti–Plg-RKT mAb 7H1. A peptide corresponding to the C-terminus of human Plg-RKT with a C-terminal lysine added at the N-terminus, CEQSRFFIDK, was coated onto wells of microtiter plates. Either anti–Plg-RKT mAb 7H1 (♦) or isotype control (■) was added. Biotinylated plasminogen was then added, followed by detection with streptavidin-HRP goat anti–mouse IgG. Total biotinylated plasminogen binding is shown.
Inhibition of plasminogen binding by anti–Plg-RKT mAb 7H1. A peptide corresponding to the C-terminus of human Plg-RKT with a C-terminal lysine added at the N-terminus, CEQSRFFIDK, was coated onto wells of microtiter plates. Either anti–Plg-RKT mAb 7H1 (♦) or isotype control (■) was added. Biotinylated plasminogen was then added, followed by detection with streptavidin-HRP goat anti–mouse IgG. Total biotinylated plasminogen binding is shown.
To determine the effect of mAb 7H1 on plasminogen activation, U937 cells were preincubated with either mAb 7H1 or IgG2a isotype control, followed by incubation with plasminogen for 30 minutes. Plasmin generation was measured after the addition of uPA and a chromogenic substrate for plasmin. Treatment with mAb 7H1 markedly suppressed cell-dependent plasminogen activation by uPA, and the extent of suppression was dependent on cell concentration. At a concentration of 3 × 105 cells/mL, cell surface–dependent plasminogen activation by uPA was suppressed by 39% (Figure 3). Therefore, Plg-RKT plays a major role in uPA-dependent plasminogen activation on monocytoid cells.
Plg-RKT regulates cell-surface plasminogen activation by uPA. Plasminogen activation was determined as described in “Plasminogen activation assay” in the presence of different concentrations of U937 cells as indicated and in the presence of 170nM of either anti–Plg-RKT mAb 7H1 (■) or mouse IgG2a isotype control (□). Cell-dependent plasminogen activation is shown after subtracting plasminogen activation in the absence of cells. **P < .001 compared with the corresponding isotype control.
Plg-RKT regulates cell-surface plasminogen activation by uPA. Plasminogen activation was determined as described in “Plasminogen activation assay” in the presence of different concentrations of U937 cells as indicated and in the presence of 170nM of either anti–Plg-RKT mAb 7H1 (■) or mouse IgG2a isotype control (□). Cell-dependent plasminogen activation is shown after subtracting plasminogen activation in the absence of cells. **P < .001 compared with the corresponding isotype control.
Plg-RKT regulates invasion of Matrigel by monocytes
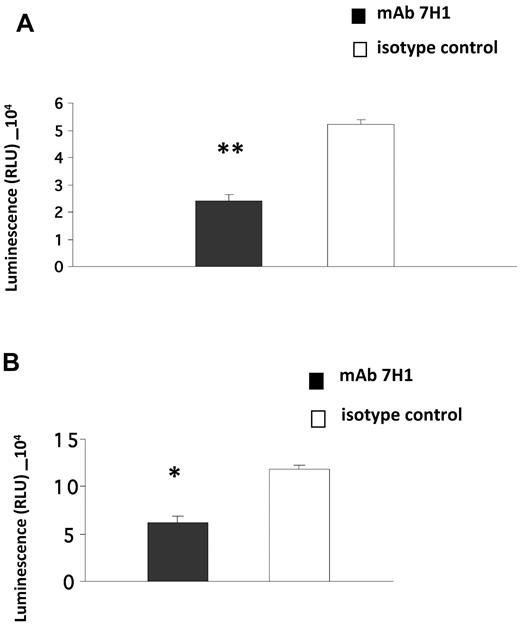
Invasion of Matrigel by monocytoid cells in response to the chemotactic stimulus MCP-1 is enhanced in the presence of plasminogen and also requires active plasmin8,10 and uPA.8 Furthermore, the effect of plasminogen is markedly suppressed in the presence of ϵ-aminocaproic acid (EACA), suggesting a key role of plasminogen receptors in this function.8-10 Therefore, we investigated the role of Plg-RKT in this process. U937 cells were preincubated with anti–Plg-RKT 7H1 Fab or isotype control Fab, plasminogen was added and incubated with the cells, uPA was added, and the cells were placed in the upper chambers of Transwells coated with Matrigel. The chemoattractant MCP-1 was present in the lower chamber. Treatment of the cells with antiPlg-RKT 7H1 Fab (140nM) markedly decreased migration of the cells through Matrigel (by 54%) compared with isotype control Fab (Figure 4A). This Fab concentration provided the maximal effect, because invasion was not further suppressed at a concentration of 280nM 7H1 Fab (data not shown). We also examined the effect of 7H1 on invasion by human peripheral blood monocytes. Matrigel invasion by human peripheral blood monocytes was also markedly inhibited (by 48%) when the cells were pretreated with 7H1 (Figure 4B). These results demonstrate that Plg-RKT regulates plasminogen-dependent Matrigel invasion by human monocytoid cells.
Plg-RKT regulates Matrigel invasion by monocytes. U937 cells (A) or human peripheral blood monocytes (B; 4 × 105) were placed in the upper chambers of Transwells coated with Matrigel in the presence of plasminogen (200nM) and uPA (50nM) and either anti–Plg-RKT Fab 7H1 (140nM) or isotype control Fab (140nM). Matrigel invasion in response to MCP-1 was quantified as described in “Invasion and chemotactic migration.” Data represent the mean of triplicates ± SEM of total invasion. **P < .001 and *P < .05 compared with isotype control.
Plg-RKT regulates Matrigel invasion by monocytes. U937 cells (A) or human peripheral blood monocytes (B; 4 × 105) were placed in the upper chambers of Transwells coated with Matrigel in the presence of plasminogen (200nM) and uPA (50nM) and either anti–Plg-RKT Fab 7H1 (140nM) or isotype control Fab (140nM). Matrigel invasion in response to MCP-1 was quantified as described in “Invasion and chemotactic migration.” Data represent the mean of triplicates ± SEM of total invasion. **P < .001 and *P < .05 compared with isotype control.
Plg-RKT regulates monocyte chemotactic migration
Plasmin promotes chemotactic cell migration across polycarbonate membranes in the absence of extracellular matrix.36 Therefore, we examined the role of Plg-RKT in chemotactic cell migration. First, we characterized the plasminogen dependence of cell migration and U937 cell migration was enhanced 1.5-fold in the presence of plasminogen (Figure 5A). Migration was inhibited by EACA, which is consistent with a plasminogen-receptor dependence of this function (Figure 5B). Cell migration was also inhibited by amiloride and aprotinin, which is consistent with a requirement for active plasmin and uPA (Figure 5B). In another experiment, U937 cells were pretreated with mAb 7H1 or isotype control at different concentrations and migration in the presence of plasminogen was assessed. Preincubation of cells with 7H1 at 70nM reduced cell migration by 64%, and the addition of a higher concentration of 7H1 (280nM) did not cause further inhibition of migration (Figure 5C). The extent of inhibition by 7H1 was similar to the extent of inhibition by EACA. Migration of human peripheral blood monocytes was also markedly inhibited (39% inhibition) by 7H1 compared with isotype control (Figure 5D).
Plg-RKT regulates monocyte chemotactic migration. (A) U937 cells (4 × 105) were placed in the upper chambers of Transwells in the absence (open bars) or presence of MCP-1 alone (hatched bars) or MCP-1 and plasminogen (200nM; closed bars), and cell migration was quantified as described in “Invasion and chemotactic migration.” *P < .05 compared with cells treated with MCP-1 but without the addition of plasminogen. (B) U937 cells were pretreated with EACA (200mM), aprotinin (2.5μM), or amiloride (200μM), and cell migration in the presence of plasminogen was quantified. **P < .001 and *P < .05 compared with cells with plasminogen alone. (C) Cells were preincubated with either mAb 7H1 (■) or isotype control (□) at the indicated concentrations for 30 minutes, and cell migration in the presence of plasminogen was quantified. *P < .05 compared with isotype control mAb. (D) Human peripheral blood monocytes were preincubated with either 7H1 mAb (140nM; ■) or isotype control mAb (140nM; □) for 30 minutes, and cell migration in the presence of plasminogen was quantified. **P < .01 compared with isotype control mAb. Data represent the mean of triplicates ± SEM of total migration.
Plg-RKT regulates monocyte chemotactic migration. (A) U937 cells (4 × 105) were placed in the upper chambers of Transwells in the absence (open bars) or presence of MCP-1 alone (hatched bars) or MCP-1 and plasminogen (200nM; closed bars), and cell migration was quantified as described in “Invasion and chemotactic migration.” *P < .05 compared with cells treated with MCP-1 but without the addition of plasminogen. (B) U937 cells were pretreated with EACA (200mM), aprotinin (2.5μM), or amiloride (200μM), and cell migration in the presence of plasminogen was quantified. **P < .001 and *P < .05 compared with cells with plasminogen alone. (C) Cells were preincubated with either mAb 7H1 (■) or isotype control (□) at the indicated concentrations for 30 minutes, and cell migration in the presence of plasminogen was quantified. *P < .05 compared with isotype control mAb. (D) Human peripheral blood monocytes were preincubated with either 7H1 mAb (140nM; ■) or isotype control mAb (140nM; □) for 30 minutes, and cell migration in the presence of plasminogen was quantified. **P < .01 compared with isotype control mAb. Data represent the mean of triplicates ± SEM of total migration.
We also investigated whether another anti–Plg-RKT mAb (13B6) raised against the C-terminal peptide of human Plg-RKT with an aminoterminal cysteine added for coupling (CEQSRFFIDK) would affect invasion and chemotaxis. MAb 13B6 recapitulated the ability of the pan-specific mAb 7H1 (raised against rat Plg-RKT) to inhibit invasion and migration (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
We also investigated whether, in addition to chemotaxis, Plg-RKT was also involved in chemokinesis (ie, nondirectional cell motility in response to MCP-1). Checkerboard analysis (Table 1) demonstrated that, in the presence of plasminogen, a constant MCP-1 concentration and a positive concentration gradient induced U937 motility. Both processes were inhibited by anti–Plg-RKT Ab 7H1, with chemokinesis being suppressed almost to the background level.
Regulation of the inflammatory response by Plg-RKT
After establishing the presence of Plg-RKT on human monocytoid cells and its role in invasion and chemotactic migration in vitro, we examined the role of Plg-RKT in monocyte migration in vivo. Plg+/+ mice were injected IV with either mAb 7H1 (500 μg) or IgG2a isotype control (500 μg). After 30 minutes, the mice were injected IP with thioglycollate to induce a sterile inflammatory response, and a second dose of Ab was given intravenously 24 hours after thioglycollate injection. After 72 hours (when most recruited leukocytes are macrophages5,37,38 ), cells were collected by peritoneal lavage and macrophages were purified by adherence. The adherent cells were detached and counted using a hemocytometer. Macrophage recruitment was significantly (49%) impaired in the animals that were injected with mAb 7H1 compared with those injected with the isotype control (2.46 × 105 ± 0.28 × 104 for mice injected with 7H1 vs 4.82 × 105 ± 0.33 × 104 for mice injected with isotype control, n = 5, P = .00048; Figure 6). In 3 other experiments, C57Bl/6J mice injected with 7H1 had a 53% ± 4% reduction in peritoneal macrophages compared with mice injected with the isotype control. The effect of the 500-μg concentration of mAb injected appeared to approach saturation, because when mice were injected with 250 μg of 7H1, the percent reduction in recruited macrophages compared with the isotype control was 44%. We verified in controls that mAb 7H1 had no effect on the adherence method we used to recover peritoneal macrophages. C57Bl/6J female mice were injected with thioglycollate and peritoneal cells collected after 72 hours. Macrophage adherence was performed in the presence of increasing concentrations of mAb 7H1 or isotype control. Adherent macrophages were detached and counted. There was no statistically significant effect of mAb 7H1 on macrophage recovery compared with isotype control (supplemental Figure 2).
Effect of Plg-RKT on thioglycollate-induced monocyte recruitment. Both plasminogen-deficient (Plg−/−) and wild-type littermate (Plg+/+) mice were injected IV with either mAb 7H1 (■) or isotype control (□; 500μg). After 30 minutes, thioglycollate was injected intraperitoneally. A second injection of Ab was given 24 hours later. After 72 hours, thioglycollate-recruited cells were collected by peritoneal lavage and macrophages were purified by adherence. The adherent cells were detached and counted using a hemocytometer. Data represent mean ± SEM; n = 5 in each group.
Effect of Plg-RKT on thioglycollate-induced monocyte recruitment. Both plasminogen-deficient (Plg−/−) and wild-type littermate (Plg+/+) mice were injected IV with either mAb 7H1 (■) or isotype control (□; 500μg). After 30 minutes, thioglycollate was injected intraperitoneally. A second injection of Ab was given 24 hours later. After 72 hours, thioglycollate-recruited cells were collected by peritoneal lavage and macrophages were purified by adherence. The adherent cells were detached and counted using a hemocytometer. Data represent mean ± SEM; n = 5 in each group.
The decreased response in recruitment to the peritoneum could not be explained by a decreased level of monocytes in the circulation. Differences in the blood levels of monocytes in animals treated with mAb 7H1 compared with isotype control were not statistically significant. At 72 hours, the blood level of monocytes in mice treated with isotype control was 0.68 ± 0.00008 × 103/μL, whereas the level of monocytes in mice treated with mAb 7H1 was 1.22 ± 0.33 × 103/μL (n = 5). Furthermore, the blood levels of granulocytes and lymphocytes also were not statistically different in mice treated with mAb 7H1 compared with isotype control. The level of granulocytes was 4.76 ± 0.48 × 103/μL and 7.6 ± 1.8 × 103/μL (n = 5) in blood from mice treated with isotype control or mAb 7H1, respectively. The level of lymphocytes was 3.26 ± 0.25 × 103/μL and 4.35 ± 0.63 × 103/μL (n = 5) in blood from mice treated with isotype control or mAb 7H1, respectively.
We investigated whether mAb 7H1 affected plasminogen binding to thioglycollate-elicited mouse macrophages using FACS analysis. First, we verified direct binding of mAb 7H1 to the cells. In the presence of 40 μg/mL of isotype control, the mean fluorescence intensity (MFI) was 6.8 ± 0.9, whereas in the presence of mAb 7H1, the MFI was 11.0 ± 0.08. mAb 7H1 blocked FITC-plasminogen binding to the peritoneal macrophages. The MFI for FITC-plasminogen binding in the presence of isotype control was 36 ± 0.27 and for FITC-plasminogen binding in the presence of mAb 7H1, it was 20 ± 2.7.
To assess whether the effect of mAb 7H1 in the peritonitis model was consistent with the plasminogen-binding function of Plg-RKT, we examined the effect of injection of anti–Plg-RKT mAb 7H1 in Plg−/− mice. Thioglycollate recruitment in Plg−/− mice injected with isotype control was significantly decreased (by 73%) in Plg−/− compared with Plg+/+ littermates, as described previously (56%5 to 65%4 ). When Plg−/− mice were treated with mAb 7H1, there was no effect on the remaining macrophage recruitment in Plg−/− mice (Figure 6). Therefore, the effect of the anti–Plg-RKT mAb 7H1 was entirely dependent on plasminogen, which is consistent with Plg-RKT exhibiting plasminogen receptor function in vivo.
We also examined the effect of mAb 7H1 on the recruitment of other leukocytes to the peritoneum in wild-type mice. At 6 hours, when granulocyte levels are maximal,5 neutrophil and eosinophil recruitment to the peritoneum was not statistically different in mice treated with mAb 7H1 compared with isotype control. Total peritoneal neutrophil recruitment in mice treated with isotype control was 1.73 ± 0.58 × 106 and in mice treated with mAb 7H1 was 1.8 ± 0.66 × 106 (n = 5). Total peritoneal eosinophil recruitment in mice treated with isotype control was 1.08 ± 0.4 × 106 and in mice treated with mAb 7H1 was 1.45 ± 0.59 × 106 (n = 5). In contrast, there was an effect of mAb 7H1 injection on lymphocyte recruitment. Total recruited peritoneal lymphocytes after 72 hours in mice treated with isotype control was 5.57 ± 0.63 × 105, and the number of total peritoneal lymphocytes in mice treated with mAb 7H1 was 2.17 ± 0.22 × 105 (n = 4, P = .002).
Role of Plg-RKT in the regulation of MMP activation
The activation of pro–MMP-9 is required for plasminogen-dependent macrophage recruitment in response to thioglycollate administration in mice.4 In addition, pro–MMP-2 activation was shown to be impaired in Plg−/− mice in a model of abdominal aortic aneurysm. Pro–MMP-2 activation has not been quantified in thioglycollate-treated mice.4 Therefore, we examined the role of Plg-RKT in activation of these matrix pro-metalloproteinases in the thioglycollate recruitment model. We examined MMP activity in peritoneal lavage fluid of mice injected with 500 μg of either mAb 7H1 or isotype control before thioglycollate treatment. By gelatin zymography, murine pro–MMP-9 (105 kDa) and active MMP-9 (95 kDa, 88kDa) were detected in the peritoneal lavage fluid collected after 72 hours under both treatment conditions (Figure 7A). The bands were identified based on molecular-weight markers. Because the peritoneal exudate of mice injected with isotype control contained more macrophages, and therefore more protein, we compared the ratios of active MMP-9 to pro–MMP-9 based on densitometric scanning of the gels. Treatment with mAb 7H1 resulted in a 64% decrease in MMP-9 activation in the peritoneal fluid compared with mice injected with isotype control. After Western blotting to verify the results of zymography, active MMP-9 was detected in peritoneal lavage fluid of mice injected with isotype control, but not in that of mice injected with mAb 7H1 (Figure 7C). Gelatin zymography also revealed the presence of pro–MMP-2 and active MMP-2 (72 kDa) in peritoneal lavage fluids. When the ratio of active MMP-2 to pro–MMP-2 was determined in each sample, peritoneal lavage fluid from mice treated with mAb 7H1 showed a 44% reduction in activation of MMP-2 (Figure 7A). Western blotting confirmed the active band as corresponding to active MMP-2 based on electrophoretic mobility (Figure 7B). To assess whether mAb 7H1 had a direct effect on monocytoid cells, we investigated whether mAb 7H1 affected the activation of pro–MMP-9 and pro–MMP-2 by U937 cells in culture. The cells were incubated with MCP-1 and plasminogen in the presence of either mAb 7H1 or isotype control. Both active MMP-9 and active MMP-2 were detected in the conditioned media of cells incubated with isotype control, but were not detected in the conditioned media of cells incubated with mAb 7H1 (Figure 7D-E). These results suggest that mAb 7H1 directly blocks activation of these matrix pro-metalloproteinases synthesized by monocytoid cells.
Effect of Plg-RKT on MMP activation. C57BL/6 mice were injected intravenously with 500 μg of either mAb 7H1 or isotype control. After 30 minutes, thioglycollate was injected intraperitoneally. A second injection of Ab was given 24 hours later. After 72 hours, peritoneal lavage fluid from 5 mice/group was collected and pooled. Then, 25 μL of peritoneal lavage fluid from each treatment condition was electrophoresed on 10% SDS gels containing 0.1% gelatin and evaluated as described in “Zymography”: (A) 25 μL was electrophoresed on 4%-20% SDS gels and Western blotted with either anti–MMP-2 (B) or anti–MMP-9 (C). U937 cells (1 × 106 cells/mL) were incubated with plasminogen (200nM) in the presence of either mAb 7H1 (140nM) or isotype control (140nM) overnight at 37°C. Conditioned media (25 μL) was Western blotted with either anti–MMP-2 (D) or anti–MMP-9 (E).
Effect of Plg-RKT on MMP activation. C57BL/6 mice were injected intravenously with 500 μg of either mAb 7H1 or isotype control. After 30 minutes, thioglycollate was injected intraperitoneally. A second injection of Ab was given 24 hours later. After 72 hours, peritoneal lavage fluid from 5 mice/group was collected and pooled. Then, 25 μL of peritoneal lavage fluid from each treatment condition was electrophoresed on 10% SDS gels containing 0.1% gelatin and evaluated as described in “Zymography”: (A) 25 μL was electrophoresed on 4%-20% SDS gels and Western blotted with either anti–MMP-2 (B) or anti–MMP-9 (C). U937 cells (1 × 106 cells/mL) were incubated with plasminogen (200nM) in the presence of either mAb 7H1 (140nM) or isotype control (140nM) overnight at 37°C. Conditioned media (25 μL) was Western blotted with either anti–MMP-2 (D) or anti–MMP-9 (E).
Discussion
Studies in plasminogen-deficient mice have revealed a major role for plasminogen in macrophage recruitment in the inflammatory response.5-7 Optimal macrophage recruitment requires the activation of plasminogen to plasmin,4 as well as the localization of plasmin on the monocyte/macrophage surface7,22 by plasminogen receptors with C-terminal basic residues.22 In the present study, we examined the contribution of the novel plasminogen receptor Plg-RKT (an integral membrane protein that exposes a C-terminal lysine on the cell surface23 ) to monocyte migration in vitro and in vivo. We found that (1) Plg-RKT was highly expressed in the membranes of human peripheral blood monocytes and human monocytoid cells; (2) Plg-RKT regulated cell-surface plasminogen activation by uPA; (3) Plg-RKT regulated Matrigel invasion by human monocytes; (4) Plg-RKT regulated chemotactic/chemokinetic migration in the absence of extracellular matrix; (5) Plg-RKT regulated plasminogen-dependent macrophage recruitment in the thioglycollate model of peritonitis; and (6) Plg-RKT promoted activation of pro–MMP-9 and pro–MMP-2 within the peritoneum in the inflammatory response to thioglycollate.
Plg-RKT was initially isolated from a murine monocyte progenitor cell line.23 In the present study, we provide the first demonstration that Plg-RKT protein is markedly expressed in the membranes of normal human peripheral blood monocytes and human monocytoid cell lines. Our results are consistent with Plg-RKT microarray mRNA expression data documenting the expression of Plg-RKT mRNA by monocytes.39
Plg-RKT was also expressed in other human peripheral blood cells, was highly expressed in lymphocytes and less strongly expressed by granulocytes, and was not detected in RBCs (Figure 1B). The capacity of lymphocytes for plasminogen is 30-fold higher than the capacity of granulocytes for plasminogen.29 Furthermore, RBCs do not appreciably bind plasminogen.29 Therefore, the relative expression of Plg-RKT by these cells corresponds to their plasminogen-binding capacity.
Plg-RKT plays a major functional role in cell surface–dependent plasminogen activation by t-PA.23 In the present study, we found that treatment of cells with anti–Plg-RKT mAb 7H1 also markedly reduced cell-dependent plasminogen activation by uPA. This marked inhibition may be associated with the colocalization of Plg-RKT with the uPAR.23 Our results demonstrate that Plg-RKT regulates plasminogen activation by the 2 major plasminogen activators.
Invasion of the representative extracellular matrix Matrigel by monocytes is dependent on both plasminogen and uPA.8 In the present study, we found that treatment with 7H1 markedly reduced invasion by human peripheral blood monocytes and monocytoid U937 cells, which is consistent with the function of Plg-RKT in promoting plasminogen activation by uPA. Plasmin also promotes chemotactic cell migration in the absence of extracellular matrix.36 We found that Plg-RKT played a major role in migration of unstimulated human peripheral blood monocytes and monocytoid cells through uncoated polycarbonate membranes toward a chemotactic stimulus (MCP-1). Chemotaxis/chemokinesis was inhibited by EACA, aprotinin, and amiloride, which is consistent with the requirement for cell-associated plasmin and uPA in this function. Furthermore, the extent of inhibition by 7H1 was similar to the extent of inhibition by EACA. Therefore, under these conditions, it appears that Plg-RKT could account for the majority of the requirement for plasminogen receptor function in chemotactic/chemokinetic migration.
Plg-RKT regulated plasminogen-dependent macrophage recruitment in vivo. Treatment of wild-type mice with 7H1 markedly decreased macrophage recruitment to the peritoneum in response to thioglycollate (by 49%). Plg−/− mice show markedly decreased macrophage recruitment to the peritoneum in this peritonitis model.4,5 We found no effect of 7H1 treatment on the low level of macrophages recruited to the peritoneum in Plg−/− mice. Therefore, the effect of 7H1 was entirely dependent on plasminogen, which is consistent with Plg-RKT exhibiting plasminogen receptor function in vivo. Injection of mAb 7H1 into mice also produced a marked inhibition of lymphocyte recruitment in the peritonitis model. In contrast, we did not observe an effect on neutrophil or eosinophil recruitment. Monocyte and lymphocyte recruitment are markedly decreased in Plg−/− mice, whereas neutrophil recruitment is unaffected by plasminogen deficiency in this model.4,5 Therefore, these results are also consistent with Plg-RKT functioning as a plasminogen receptor in vivo.
The activation of pro–MMP-9 is required for plasminogen-dependent macrophage recruitment in response to thioglycollate.4 We found that treatment of wild-type mice with anti–Plg-RKT mAb markedly reduced activation of pro–MMP-9 in the peritoneal fluid. In addition, functional blockade of Plg-RKT, also markedly reduced pro–MMP-2 activation in the peritoneal fluid of mice stimulated with thioglycollate. In a previous study, changes in activation of pro–MMP-2 were not quantified in Plg−/− mice in this model.4 However, both pro–MMP-9 activation and pro–MMP-2 activation are markedly reduced in Plg−/− mice in an aortic aneurysm model.4 Based on reconstitution studies, the activation of pro–MMP-9 is necessary for the plasminogen-dependent component of macrophage migration in response to thioglycollate treatment.4 However, reconstitution with active MMP-2 is not able to reverse defective recruitment of macrophages in Plg−/− mice in thioglycollate-induced peritonitis.4 Therefore, activation of MMP-2 is likely to have functions not required for macrophage recruitment in this model.
Functional Ab blockade of Plg-RKT markedly inhibited cell-dependent plasminogen activation by uPA. However, there was an additional component of stimulation of uPA-dependent plasminogen activation that was not affected by 7H1. These results are consistent with the participation of additional plasminogen receptors with surface-exposed C-terminal basic residues in cell-dependent stimulation of plasminogen activation.21 Previously, other members of this class of plasminogen receptors, α-enolase, histone H2B,8 and S100A10,8,10 were shown to contribute substantially to uPA-dependent plasminogen activation on monocytes.
7H1 blocked Matrigel invasion in a dose-dependent manner, with a maximal inhibition of 54%. This result is also consistent with the participation of additional monocytoid plasminogen receptors in this plasminogen-dependent function. Important roles for histone H2B,8 α-enolase,9 S100A10,10 and annexin 240 have also been documented in invasion through extracellular matrix. In Ab blockade experiments, a 70% contribution of histone H2B8 and a 60% contribution of annexin 240 have been demonstrated,8 whereas S100A10-knockout mice exhibited a 45% decrease in Matrigel invasion.10 A role for α-enolase in Matrigel invasion has been demonstrated on lipopolysaccharide (LPS)–stimulated monocytes, but not on untreated monocytes, and accounted for ∼ 50% of Matrigel invasion by the stimulated cells.9 It is likely that the overlap in function of S100A10 and annexin 2 is due to the ability of Abs to annexin 2 to also block S100A10 function, because these proteins comprise the annexin A2 heterotetramer.41 It is possible that the overlap in function of all of these plasminogen receptors and Plg-RKT may be because of different functional contributions to invasion. Specifically, Plg-RKT contributed uniquely to directed chemotaxis/chemokinesis. This result clearly distinguishes a functional role of Plg-RKT that is not exhibited by other plasminogen receptors with C-terminal lysines (eg, histone H2B,8 α-enolase,9 S100A10,10 and Annexin 240 ) on monocytoid cells, and the effect on chemotaxis/chemokinesis may also play a role in invasion. (In contrast, when U937 cells and human peripheral blood monocytes are stimulated to increase cell surface α-enolase expression by treatment with LPS or by transfection, Abs to α-enolase inhibit LPS-dependent chemotaxis of these cells through polycarbonate membranes.9 )
It is apparent that the sum of the effects of functional blockade of specific plasminogen receptors analyzed to date in the thioglycollate-induced peritonitis model is a > 100% reduction in plasminogen-dependent macrophage recruitment. Intravenous injection of specific Abs to histone H2B results in 48% less macrophage recruitment,8 and injection of specific Abs to α-enolase results in 24% less recruitment (compared with injection of nonimmune control).8 In S100A10−/− mice, macrophage recruitment in response to thioglycollate is 53% less in S100A10 −/− mice compared with wild-type mice.10 In the present study, injection of mice with anti–Plg-RKT mAb 7H1 resulted in 49% less macrophage recruitment compared with mice treated with isotype control. Therefore, it is likely that each specific plasminogen receptor may be required at different steps in the inflammatory response; for example, chemotactic migration to the peritoneum or perhaps crossing different layers of peritoneal tissue, where different contributions of direct plasmic cleavage of the extracellular matrix or activation of MMP-9 for collagen degradation4 may predominate. A reduction in pro–MMP-9 activation has been demonstrated in S100A10−/− peritoneal macrophages in culture,10 and there may be overlap in this function as well. The contribution of distinct plasminogen receptors to macrophage recruitment may also be tissue and stimulus specific. For example, in a model of monocyte recruitment to the alveolar compartment, α-enolase appears to play a predominant role.9
In summary, the results presented here establish Plg-RKT as a new player that makes a major contribution to plasminogen-dependent cell migration in the inflammatory response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Victoria A. Ploplis for the Plg+/− mice and Dr Mark Kamps for the Hoxa9-ER4 cells.
This work was supported by the National Institutes of Health (grants HL38272, Hl45934, and HL 081046 to L.A.M.; HL50398 to R.J.P.; and training grant T32 HL007195 to S.L.) and by the Department of Veterans Affairs (to R.J.P.). This is publication number 21172 from the Scripps Research Institute.
National Institutes of Health
Authorship
Contribution: S.L. designed the experiments, performed the research, analyzed the data, and wrote the manuscript; N.B., J.E.D., and S.K. designed the experiments, performed the research, and analyzed the data; R.J.P. analyzed the data and participated in drafting the manuscript; and L.A.M. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lindsey A. Miles, PhD, Department of Cell Biology, The Scripps Research Institute, 10550 N Torrey Pines Rd, SP30-3020, La Jolla, CA 92037; e-mail: lmiles@scripps.edu.