Abstract
Adoptive transfer of regulatory T cells (Tregs) prevents GVHD in experimental animals. Because antigen activation drives Treg function, we measured the frequency, growth requirements, and function of alloantigen-specific (allospecific) Tregs from human blood. When alloantigen was presented directly, the precursor frequency of allo-specific Tregs in normal individuals was 1.02% (95% confidence interval [95% CI]: 0.65-1.59) and non-Tregs 1.56% (95% CI: 0.94-2.55). When alloantigen was presented indirectly, the frequency of specific Tregs was approximately 100-fold less. Purified Tregs were expanded with APCs, rapamycin, IL-2, and IL-15. In 12 days, allo-specific Tregs expanded 793-fold (95% CI: 480-1107), with duplication approximately every 24 hours. Purified allo-specific Tregs suppressed responses to specific alloantigen selectively and were approximately 100-fold more potent than polyspecific Tregs and nonexpanded Tregs. Allo-specific Tregs maintained high expression of Foxp3, Bcl-2, lymphoid homing receptor CD62L, and chemokine receptor CCR7, predicting sustained function and migration to lymphoid tissues in vivo. Allo-specific Tregs produced TGF-β and IL-10 and expressed more cytoplasmic CTLA-4 compared with non-Tregs. These data provide a platform for the selective expansion of Tregs against major and possibly minor histocompatibility antigens and predict the feasibility of adoptive immunotherapy trials using Tregs with indirect allo-recognition for preventing GVHD while sparing GVL effects.
Introduction
Regulatory T cells (Tregs) play a dominant role in transplantation tolerance, as shown by adoptive cell transfer for the prevention of organ graft rejection and GVHD in experimental models.1-6 Translating Treg therapies to humans requires cell isolation and purification, because transfer of the accompanying effector T cells would exacerbate rather than ameliorate human disease. Naturally occurring Treg populations comprise 1%-3% of the human T-cell repertoire.7 Tregs are characterized by the constitutive expression of CD4, the IL-2 receptor α chain (CD25), and high levels of a nuclear transcription factor, forkhead box p3 (Foxp3), which is critical for their development and suppressive function.8-10 Tregs express little or no IL-7 receptor α chain (CD127).11 Therefore, the constitutive expression of CD25 and low expression of CD127 have been used for identifying and purifying Tregs.12,13 Immunomagnetic cell-separation technology has been adapted for this application. Adoptive transfer of freshly isolated human Tregs has prevented GVHD in patients treated with allogeneic hematopoietic stem cell transplantations partially depleted of conventional T cells.14 However, T cell–replete grafts are more commonly used, and prevention of GVHD after T cell–replete transplantations require the transfer of a higher number of Tregs that can only be obtained through ex vivo expansion.
Tregs can be expanded by more than 1000-fold by anti-CD3 and anti-CD28 Ab-coated beads, but loss of regulatory function and acquisition of effector functions have been a concern with this technology. The addition of rapamycin to artificial APCs has apparently solved this problems, because Tregs maintained Foxp3 expression and in vivo suppressive function in immunodeficient mice.15 Because in vivo Treg protection from GVHD requires alloantigen-specific (allo-specific) engagement of the TCR,16 adoptive immunotherapy with allo-specific Tregs offers more advantages than polyspecific Tregs: selective rather than broad immunosuppression, ability to control the conditions for antigen presentation, and economy of scale because they are a fraction of the entire Treg population. Antigen-specific Tregs expanded in response to dendritic cells (DCs) presenting a single self-peptide17 and suppressed experimental autoimmune diabetes more efficiently than polyclonal Tregs.18 Human allo-specific Tregs could also expand ex vivo,19 but only by 10- to 20-fold.18 Because Tregs constitute 1%-3% of human blood T cells, and effective suppression requires a Treg:effector T cell ratio of 1:1, we surmise that therapeutic applications will require expanding allo-specific Tregs by over 100-fold.20 There is a need to improve on expansion protocols before allo-specific Tregs can be brought to the clinic. Sagoo et al21 have recently adopted a 2-step process for the large-scale expansion of allo-specific Tregs: primary Treg activation by DCs presenting the specific alloantigen directly, followed by sorting Tregs for expression of the activation markers CD69 and CD71, and secondary expansion by artificial APCs plus IL-2. In the present study, we have measured the frequency of human blood Tregs specific against alloantigen presented directly by allogeneic DCs or indirectly by self-DCs, and established a single-step method for the expansion of allo-specific Tregs by approximately 800-fold by DC stimulation in the presence of rapamycin, IL-2, and IL-15.
Methods
Isolation of cells
Fresh buffy coats from healthy donors was obtained from the local blood bank (Florida Blood Services, St Petersburg, FL), and PBMCs were isolated by density gradient centrifugation. Where indicated, class-II HLA-DRB3, HLA-DRB4, HLA-DQA, and HLA-DQB antigens were typed by Luminex using reverse sequence-specific oligonucleotide technology. DRB1 was typed using a high-definition kit (One Lambda). HLA-DPB1 was typed with a high-resolution single stranded binding-protein kit (Invitrogen). CD4+ T cells were purified from PBMCs by negative selection using a cocktail of CD8, CD19, CD123, and CD127 biotin-conjugated Abs and antibiotin magnetic MicroBeads (Miltenyi Biotec). This resulted in a CD4+ T-cell enrichment of > 97%. Tregs were isolated using the CD4+CD25+CD127− Treg isolation kit II (Miltenyi Biotec). Percentage yield was calculated using the formula: (number of Tregs after sort/number of Tregs at start) × 100. For frequency measurement and suppression assays, Tregs were instead isolated on a BD FACSAria II high-speed cell sorter (BD Biosciences). CD4+CD25+CD127− Tregs were sorted under sterile conditions and collected into human heat-inactivated pooled AB serum (SeraCare). Purified Treg populations obtained from magnetic or flow sort methods were 95%-99% pure. Culture medium was RPMI 1640 plus glutamine supplemented with 0.02mM pyruvate, 100 U/mL of penicillin, 100 μg/mL of streptomycin, (Cellgro), and 10% heat inactivated human pooled AB serum (SeraCare). Cells were cultured at 37°C, 95% humidity, and 5% CO2 in 96-well, round-bottom or flat-bottom plates or in 48-well plates (Nunc).
Proliferative responses of CD4+ Tregs to alloantigens
Briefly, 2.5 × 104 freshly isolated Tregs were cultured with irradiated (3000 cGy), HLA-mismatched APCs in the presence or absence of IL-2 (10 U/mL; R&D Systems), IL-15 (10 ng/mL; R&D Systems), and rapamycin (100 ng/mL; EMD Biosciences) using 96-well, round-bottom plates. Monocyte-derived DCs were produced by culture in X-VIVO 15 serum-free medium (Lonza) for 6 days in 1000 U/mL of GM-CSF (R&D Systems) and 1000 U/mL of IL-4 (R&D Systems). APCs were γ-irradiated, either CD4-depleted PBMCs seeded at a Treg:APC ratio of 1:4 or DCs seeded at a ratio of 10:1. For indirect allo-recognition experiments, allogeneic cell lysate was loaded to self-DCs. Briefly, allogeneic or autologous PBMCs were resuspended in 500 μL of PBS and lysed by 3 cycles of freezing to −80°C and thawing at 37°C. The supernatant was collected by centrifugation at 5939g for 5 minutes and loaded overnight onto an equal number of DCs in X-VIVO 15 serum-free medium. The next day, DCs were washed twice with PBS, resuspended in RPMI complete medium/10% AB+ serum, and γ-irradiated. Proliferation was analyzed by [3H] thymidine incorporation using a gas scintillation counter (Matrix 96 β counter; Canberra Packard). Cells were pulsed with 1 μCi/well 3H-thymidine for the last 18 hours in culture and harvested on day 6 to measure proliferation. Results are expressed in counts per minute (CPM) of at least triplicate measurements.
LDA
The frequencies of antigen specific Tregs and non-Tregs were determined by limiting-dilution analysis (LDA) as described previously.22 Briefly, purified Tregs (CD4+CD25+CD127−) and non-Tregs (CD4+CD25−CD127+) were cultured with a fixed concentration of DCs (2 × 103). T cells were plated at various concentrations (1500, 750, 375, 188, 94, 47, 23, and 12 T cells per well) with a minimum of 10-12 replicate wells in a 96-well U-bottom plates. Treg cultures were supplemented with or without IL-2 (10 U/mL), IL-15 (10 ng/mL), and with rapamycin (100 ng/mL). Non-Treg cultures were supplemented with or without IL-2. For indirect allo-recognition experiments, Tregs or non-Tregs were seeded concentrations of 5000, 2500, 1250, 625, 313, 156, 78, and 39 per well with self-DCs loaded with allogeneic cell lysate in the presence of IL-2, IL-15, and rapamycin. Cultures were maintained in a humidified atmosphere at 37°C and 5% CO2 for 6 days and pulsed with 3H-thymidine. Wells were considered positive if the CPM was greater than the mean plus 3 SD over the sum of negative controls, responder and stimulator cells, with each cultured alone. Online extreme LDA (ELDA) (http://bioinf.wehi.edu.au/software/elda/index.html) was used to calculate the frequency and 95% confidence interval (95% CI) of replicating allo-specific Tregs.23
In vitro expansion of allo-specific Tregs
Where indicated, Tregs or non-Tregs were labeled with 1μM carboxyfluorescein diacetate succinimidyl ester (CFDA-SE; Molecular Probes) just before stimulation. A total of 2.5 × 104 labeled T cells was cultured with 1 × 105 irradiated, HLA-mismatched, CD4 cell–depleted PBMCs or 2.5 × 103 DCs in 96-well, round-bottom plates. On day 6, the cultures were transferred to 48-well plates and monitored for cell volume and cell density and split as necessary. Treg cultures were fed every other day with fresh medium, and on day 12, were washed; stained for CD4, CD25, and D127; and analyzed by flow cytometry. Unless otherwise indicated, Treg medium was supplemented with IL-2 (10 U/mL), IL-15 (10 ng/mL), and rapamycin (100 ng/mL), and non-Treg medium was supplemented with IL-2. For indirect expansion, 1 × 106 labeled T cells were cultured with irradiated 1 × 105 cell lysate loaded self-DCs in 48 well, flat-bottom plates. As indicated in selected experiments, cells were counted and restimulated with γ-irradiated allogeneic cell lysate–loaded self-DCs. Polyspecific Tregs were expanded as described by Putnam et al12 and used for the suppression assay. Briefly, CD4+CD25+CD127− Tregs were FACS-sorted, stained for CFSE, and expanded with anti-CD3/anti-CD28–coated MicroBeads (Invitrogen) at a 1:1 bead:cell ratio in the presence of IL-2 (10 U/mL) for 12 days. Cell division was determined by timed acquisition flow cytometric analysis for CFSE− or Ki-67–positive T cells.
In vitro suppression assays
The suppressive capacity of Tregs was assayed in a mixed leukocyte culture suppression assay. Freshly purified or cultured Tregs were sorted with a FACSAria (BD Biosciences), titrated, and mixed with 5 × 104 responder CD4+CD25− T cells from the Treg donor and 5 × 103 irradiated DCs from an HLA-mismatched allogeneic donor. Alloantigen specificity of suppression was examined in cocultures with the specific stimulator or a third-party stimulator. HLA typing of the cell combinations are shown in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Proliferation was analyzed by [3H] incorporation and is expressed as the mean ± SD CPM of 3 measurements.
Phenotypic analysis of expanded population
Phenotypes of expanded Tregs were analyzed by 8-color flow cytometry (LSR II; BD Biosciences). To stain dead cells, DAPI (Sigma-Aldrich) was added to cells before acquisition. Cell surfaces were stained with the following conjugated mAbs: CD4 Alex Fluor 700 (CD4Alex700; BD Biosciences), CD25APCH7 (BD Biosciences), CD127Alex647 (BD Biosciences), CD45PercpCy5.5 (eBioscience), CD62LPE-Cy7 (BioLegend), and CCR7-PE (R&D Systems). Data were analyzed with FlowJo software Version 9.1 (TreeStar). For intracellular staining, cells were labeled first with Live/Dead Yellow (Invitrogen), and then surface stained with CD4Alex700, CD25APCH7, and CD127Alex647. Cells were fixed and permeabilized using the Fix and Fix/Perm buffer (eBioscience) according to the manufacturer's instructions, and then stained with Foxp3-PE (BD Biosciences) or Bcl-2-PE (BD Biosciences). Isotype controls were used for markers or gate settings. To assess the fraction of proliferating Tregs in culture, expanded, CFSE-labeled, alloactivated Tregs were fixed and permeabilized using the Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (BD Biosciences), and stained intracellularly with Foxp3 and Ki-67.
Cytokine analysis and CTLA-4
The intracellular cytokines IL-2, IL-4, IL-10, IFN-γ, TNF-α, and CTLA-4 were analyzed by flow cytometry after cell stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin in the presence of GolgiPlug (brefeldin-A) or GolgiStop (monensin; both from BD Biosciences) for 5 hours.24 Intracellular staining was performed with the Cytofix/Cytoperm Fixation/Permeabilization kit (BD Biosciences) according to the manufacturer's instructions. The following conjugated mAbs (BD Biosciences) were used: CD4Alex700, CD25APCH7, CD127Alexa647, IL-2PercpCy5.5, IFN-γPE, TNF-α PE, IL-4PE-Cy7, IL-10PE, and CTLA-4PE. Corresponding isotype controls were used for gate or marker settings. After 14 days of Treg expansion, cells were washed twice with PBS and cultured in X-VIVO 15 serum-free medium with PMA/ionomycin for 5 hours. Supernatants were tested in triplicate by multiplex ELISA for IL-2, IL-4, IL-10, IL-17, IL-23, IL-15, IL-6, IFN-γ, and TNF-α (Quansys Biosciences). TGF-β was assayed by a cytokine bead array kit (BD Biosciences) on an LSR II flow cytometer (BD Biosciences). Data were analyzed using FCAP Array 1.0.1 software (BD Biosciences).
Statistical analysis
Differences in frequency or proliferation of allo-reactive T cells were analyzed by the 2-tailed Student t test, and P < .05 was considered significant.
Results
Treg isolation and allo-specific proliferative response
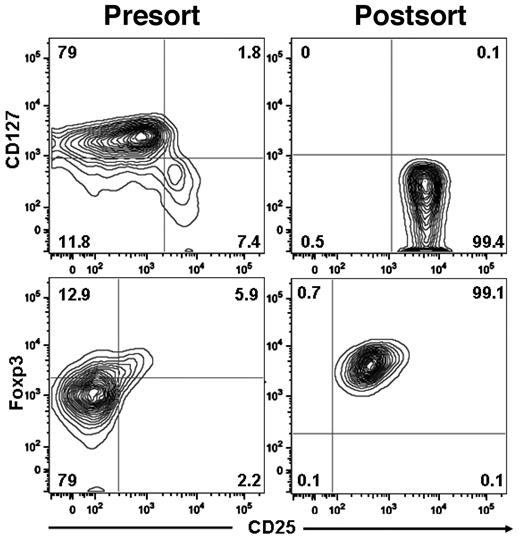
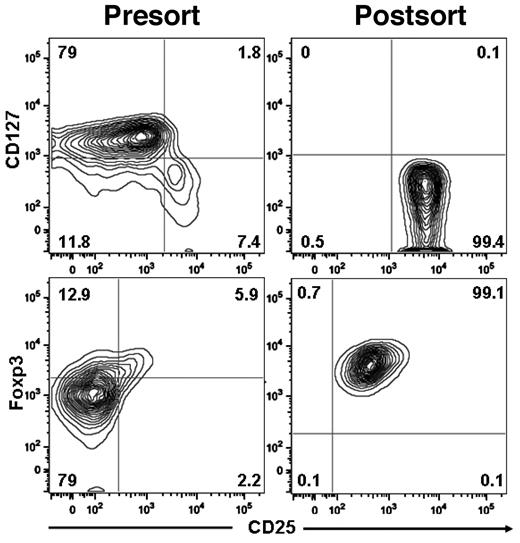
We isolated Tregs from normal human blood buffy coats using a 3-step procedure: (1) PBMCs were obtained by density gradient centrifugation, (2) CD4 cells were enriched by negative immunomagnetic selection, and (3) CD25-bright cells were purified by positive immunomagnetic selection. The yield ranged from 25%-34% (median, 27%; Table 1), and the purity of CD4+CD25+CD127− Tregs ranged from 95%-99% (Figure 1).
Purification of Tregs from human blood. Tregs were isolated using the CD4+CD25+CD127− Treg isolation kit II magnetic MicroBead method (Miltenyi Biotec). The purity of human Tregs and phenotype expression was analyzed as follows: CD4+ cells were gated and analyzed for surface expression IL-7 receptorα chain (CD127) and IL-2 receptorα chain (CD25) and for intracellular expression of Foxp3.
Purification of Tregs from human blood. Tregs were isolated using the CD4+CD25+CD127− Treg isolation kit II magnetic MicroBead method (Miltenyi Biotec). The purity of human Tregs and phenotype expression was analyzed as follows: CD4+ cells were gated and analyzed for surface expression IL-7 receptorα chain (CD127) and IL-2 receptorα chain (CD25) and for intracellular expression of Foxp3.
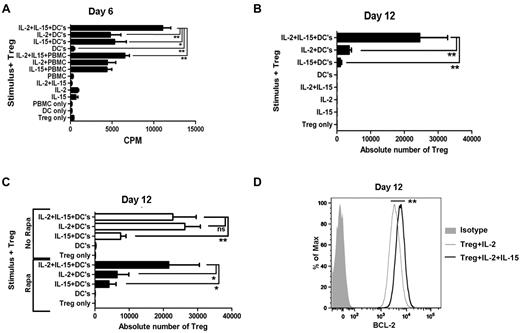
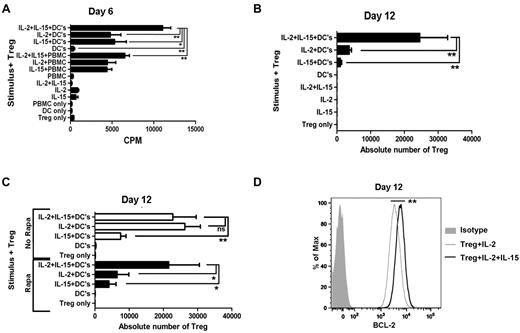
To define optimal conditions for expansion of allo-specific Tregs, we cultured freshly purified Tregs with allogeneic monocyte–derived DCs or CD4-depleted PBMCs to present alloantigen in the presence or absence of IL-2 and IL-15 cytokines to stimulate Treg proliferation and rapamycin to block proliferation of non-Tregs while permitting Treg activation. We found that 6-day proliferation (Figure 2A) and 12-day expansion (Figure 2B) in the presence of rapamycin require IL-2 or IL-15, and the cytokine combination is additive. Stimulation with allogeneic DCs is more effective than PBMCs in inducing proliferation. APCs alone or cytokines alone do not stimulate Treg proliferation. Rapamycin inhibits allo-reactive Tregs cultured with IL-2 alone (Figure 2C). The additive role of IL-15 is critical for the maximal expansion of Tregs in the presence of rapamycin, but is dispensable in the absence of rapamycin. The addition of IL-15 enhances Treg expression of Bcl-2 by 2-fold over IL-2 alone (P < .01; Figure 2D).
Allo-specific Treg-proliferative response and expansion. (A) Tregs were freshly purified and stimulated with γ-irradiated HLA-mismatched APCs plus rapamycin (100 ng/mL) in the presence or absence of IL-2 (10 U/mL) and IL-15 (10 ng/mL). Each bar represents mean ± SD of 3H-thymidine uptake (CPM) on day 6. Results are one representative of 5 individual experiments. (B) Expansion of allo-specific Tregs (2 × 104/well in triplicate) stimulated with allogeneic DCs (10:1) plus rapamycin (100 ng/mL) in the presence or absence of IL-2 (10 U/mL) or IL-15 (10 ng/mL) for 12 days. Each bar represents mean ± SD of the absolute number of Tregs obtained by timed acquisition after 12 days of expansion. A representative of 5 experiments with similar results is shown. (C) Absolute number of Tregs 12 days after stimulation with allogeneic DCs in the presence or absence of rapamycin, IL-2 (10 U/mL), or IL-15 (10 ng/mL). (D) Bcl-2 expression by expanded Tregs in the presence of IL-2 and IL-15 (bold line) or IL-2 alone (thin line) gated on CD4+CD25+CD127− Tregs. Data shown are representative of 3 experiments. *P < .05; **P < .01.
Allo-specific Treg-proliferative response and expansion. (A) Tregs were freshly purified and stimulated with γ-irradiated HLA-mismatched APCs plus rapamycin (100 ng/mL) in the presence or absence of IL-2 (10 U/mL) and IL-15 (10 ng/mL). Each bar represents mean ± SD of 3H-thymidine uptake (CPM) on day 6. Results are one representative of 5 individual experiments. (B) Expansion of allo-specific Tregs (2 × 104/well in triplicate) stimulated with allogeneic DCs (10:1) plus rapamycin (100 ng/mL) in the presence or absence of IL-2 (10 U/mL) or IL-15 (10 ng/mL) for 12 days. Each bar represents mean ± SD of the absolute number of Tregs obtained by timed acquisition after 12 days of expansion. A representative of 5 experiments with similar results is shown. (C) Absolute number of Tregs 12 days after stimulation with allogeneic DCs in the presence or absence of rapamycin, IL-2 (10 U/mL), or IL-15 (10 ng/mL). (D) Bcl-2 expression by expanded Tregs in the presence of IL-2 and IL-15 (bold line) or IL-2 alone (thin line) gated on CD4+CD25+CD127− Tregs. Data shown are representative of 3 experiments. *P < .05; **P < .01.
Frequency of allo-specific Tregs
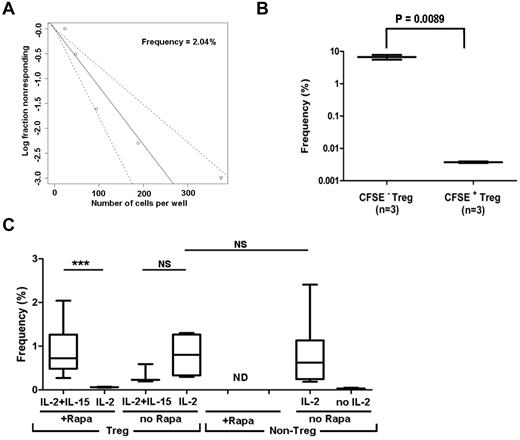
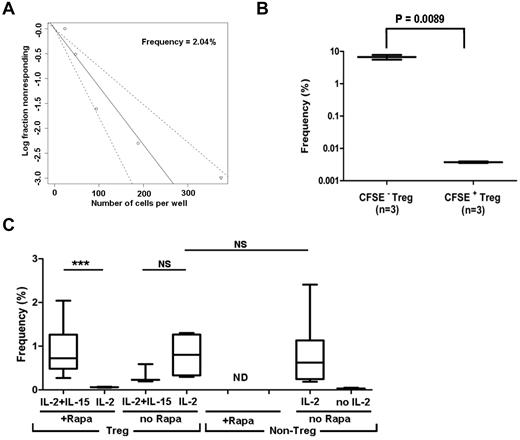
We used LDA to estimate the frequency of Tregs specific for allogeneic HLA-incompatible stimulator (direct allo-recognition) (Figure 3A). We validated the assay by testing flow-sorted, CFSE-labeled Tregs 8 days after alloantigen stimulation. CFSE− (replicated) Tregs showed 1800-fold higher frequency than CFSE+ (resting) Tregs (Figure 3B). Rapamycin decreased the frequency of allo-reactive Tregs detectable in the presence of IL-2, but did not affect the frequency of Tregs detectable in the presence of both IL-2 and IL-15 (Figure 3C), which is consistent with the Treg-expansion data presented in Figure 2C. Under optimal conditions, the precursor frequency of the allo-specific Tregs was between 0.7% and 2.0%, similar to the frequencies of non-Tregs (Figure 3C). Data showing that precursor frequencies of allo-reactive Tregs and non-Tregs are similar supported the idea that therapeutic application would require a more than 100-fold ex vivo expansion of allo-specific Tregs.20
LDA showing frequency of allo-reactive Treg (CD4+CD25+CD127−) and non-Treg (CD4+CD25−CD127+) populations in normal human blood. Serially diluted, purified Tregs or non-Tregs were stimulated with allogeneic DCs in the presence or absence of exogenous cytokines. Each LDA was performed with a minimum of 10-12 replicates per cell concentration. (A) Log-fraction plot of the LDA of allo-specific Tregs. The slope represents log-active cell fraction, bold lines represent frequency estimates, and non-bold lines show 95% CIs of Tregs calculated based on the likelihood ratio test of single-hit model. (B) LDA validation by testing CFSE− (replicated) or CFSE+ (resting) Tregs. Box plot represents the frequency estimate and the error bar shows 95% CIs calculated by extreme LDA. (C) Box plots show the frequencies of Tregs in the presence or absence of IL-2, IL-2 plus IL-15, and non-Tregs in the presence or absence of IL-2 with 95% CIs. *P < .05; **P < .01; ***P < .001.
LDA showing frequency of allo-reactive Treg (CD4+CD25+CD127−) and non-Treg (CD4+CD25−CD127+) populations in normal human blood. Serially diluted, purified Tregs or non-Tregs were stimulated with allogeneic DCs in the presence or absence of exogenous cytokines. Each LDA was performed with a minimum of 10-12 replicates per cell concentration. (A) Log-fraction plot of the LDA of allo-specific Tregs. The slope represents log-active cell fraction, bold lines represent frequency estimates, and non-bold lines show 95% CIs of Tregs calculated based on the likelihood ratio test of single-hit model. (B) LDA validation by testing CFSE− (replicated) or CFSE+ (resting) Tregs. Box plot represents the frequency estimate and the error bar shows 95% CIs calculated by extreme LDA. (C) Box plots show the frequencies of Tregs in the presence or absence of IL-2, IL-2 plus IL-15, and non-Tregs in the presence or absence of IL-2 with 95% CIs. *P < .05; **P < .01; ***P < .001.
Expansion of allo-specific Tregs
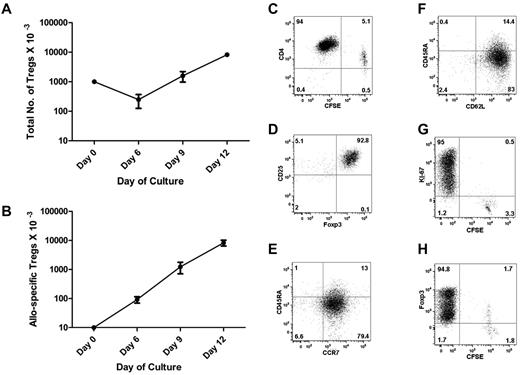
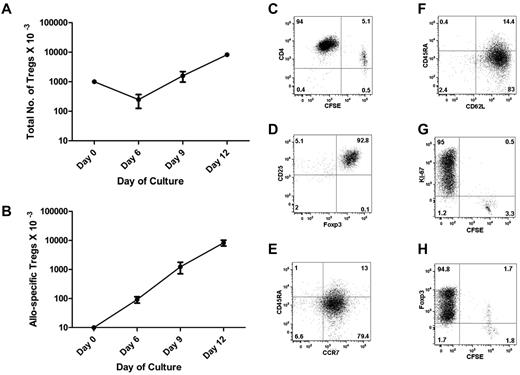
To expand allo-specific Tregs, we used monocyte-derived DCs as a stimulus, labeled purified Tregs with CFSE, and added IL-2, IL-15, and rapamycin to the culture. We calculated the frequency of allo-reactive Tregs by LDA at the start of culture (Figure 3A) and by CFSE dilution after culture (Figure 4C). By day 6, we observed an approximately 10-fold expansion, consistent with just over 3 cell divisions. The cell numbers continued to increase up to approximately 800-fold expansion on day 12, consistent with just over 9 cell divisions (Figure 4B). Assuming that the initial cell duplication started within 72 hours, the Treg duplication time in culture was approximately 24 hours. Because only approximately 1% of the starting population expanded, the total number of cells in culture only increased 8-fold (Figure 4A).
Expansion and characterization of allo-specific Tregs. Tregs were seeded at 1 × 105/well in a 48-well plate with irradiated allogeneic DCs (1 × 104), rapamycin (100 ng/mL), IL-2 (10 U/mL), and IL-15 (10 ng/mL). Cells were cultured for 12 days. (A) The total Treg numbers at each time point were calculated by flow cytometry of timed events. The numbers of starting Tregs were normalized to 1 million at time 0. (B) The diagram outlines the numbers of allo-specific Tregs before and after in vitro expansion, normalized to 10 000 Tregs at time 0. The frequency of allo-specific Tregs at time 0 was calculated by LDA. The frequencies of allo-specific Tregs at subsequent times were calculated by flow microfluorometry of CFSE-labeled Tregs (C). (D-F) Flow cytometry plots showed intracellular expression of Foxp3 and cell-surface expression of CD45RA, CCR7, and CD62L on gated CD4+, CD25+, and CD127− Tregs at the end of culture. Dot plot shows the analysis of CFSE dilution versus Ki-67 nuclear protein (G) or Foxp3 (H) staining in expanded Tregs at the end of culture. We observed a dual level of Foxp3 expression with the BD Biosciences kit (panel H), but not with the eBioscience kit (panel D).
Expansion and characterization of allo-specific Tregs. Tregs were seeded at 1 × 105/well in a 48-well plate with irradiated allogeneic DCs (1 × 104), rapamycin (100 ng/mL), IL-2 (10 U/mL), and IL-15 (10 ng/mL). Cells were cultured for 12 days. (A) The total Treg numbers at each time point were calculated by flow cytometry of timed events. The numbers of starting Tregs were normalized to 1 million at time 0. (B) The diagram outlines the numbers of allo-specific Tregs before and after in vitro expansion, normalized to 10 000 Tregs at time 0. The frequency of allo-specific Tregs at time 0 was calculated by LDA. The frequencies of allo-specific Tregs at subsequent times were calculated by flow microfluorometry of CFSE-labeled Tregs (C). (D-F) Flow cytometry plots showed intracellular expression of Foxp3 and cell-surface expression of CD45RA, CCR7, and CD62L on gated CD4+, CD25+, and CD127− Tregs at the end of culture. Dot plot shows the analysis of CFSE dilution versus Ki-67 nuclear protein (G) or Foxp3 (H) staining in expanded Tregs at the end of culture. We observed a dual level of Foxp3 expression with the BD Biosciences kit (panel H), but not with the eBioscience kit (panel D).
Phenotypic characterization of allo-specific Tregs
Expanded Tregs were analyzed for the expression of molecules critical for their function. After 12 days of expansion, the majority of expanded Tregs were CFSE− (Figure 4C), indicating that they had undergone multiple cell divisions. Virtually all of the CFSE− CD4+ cells retained the expression of CD25 and Foxp3 (Figure 4D), key for the regulatory function of Tregs, and were negative for CD127, the IL-7 receptor α chain that is not used by Tregs (not shown). The expanded Tregs expressed the chemokine receptor CCR7 (Figure 4E) and the lymphoid homing receptor CD62L (Figure 4F), predicting that they can migrate and home to lymphoid tissue in vivo. CD4+CFSE+ cells had lost expression of Foxp3 and were Ki-67−, whereas CFSE− CD4+ cells were Ki-67+ and Foxp3+, indicating active replication at the end of culture (Figure 4G-H).
Suppressive function of expanded allo-specific Tregs
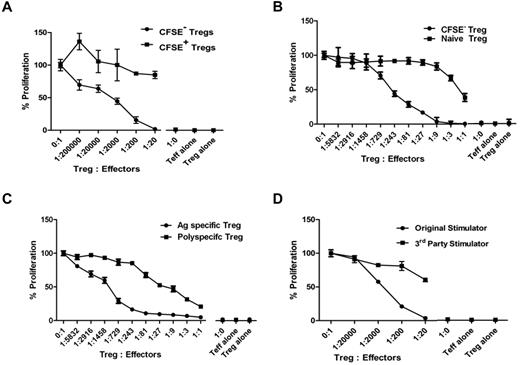
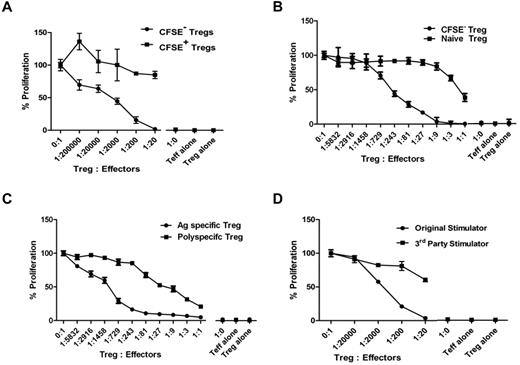
To determine whether the expanded CFSE−CD4+CD25+CD127− Treg population was enriched for cells with allo-specific suppression, we sorted CFSE+ and CFSE− Tregs and cocultured them at various ratios with naive autologous CD4+CD25− responder T cells and allogeneic DCs from the original stimulator. Proliferation was maximally reduced by CFSE− Tregs at a regulator-to-responder cell ratio of 1:20, and 50% inhibition was still achieved at ratios of 1:2000. No suppressive effect was found with the CFSE+ Tregs that had not undergone cell division in response to alloantigen (Figure 5A). This experiment demonstrates that virtually all allospecific Tregs replicate in culture after stimulation with allogeneic DC, IL-2, and IL-15.
Suppressive activity of expanded allo-specific Tregs. (A-C) The suppressive activity of cultured CFSE− versus CFSE + (A), CFSE− (replicated) versus naive Tregs (B), or allo-specific versus polyspecific Tregs (C) were tested at different ratios to self-CD4+CD25− T-cell responders in the presence of original allogeneic DCs. (D) Allo-specific suppressive function of cultured CFSE− Tregs was tested against the original stimulator or a HLA class II–mismatched third party stimulator. Results are shown as average CPM of triplicates measured by the incorporation of 3H-thymidine in cocultures at day 6 after subtracting the CPM of background wells without Tregs. Results shown are representative of 3 individual experiments for each of the panels.
Suppressive activity of expanded allo-specific Tregs. (A-C) The suppressive activity of cultured CFSE− versus CFSE + (A), CFSE− (replicated) versus naive Tregs (B), or allo-specific versus polyspecific Tregs (C) were tested at different ratios to self-CD4+CD25− T-cell responders in the presence of original allogeneic DCs. (D) Allo-specific suppressive function of cultured CFSE− Tregs was tested against the original stimulator or a HLA class II–mismatched third party stimulator. Results are shown as average CPM of triplicates measured by the incorporation of 3H-thymidine in cocultures at day 6 after subtracting the CPM of background wells without Tregs. Results shown are representative of 3 individual experiments for each of the panels.
To quantify the enrichment in suppressive activity after ex vivo expansion, we compared the potency of expanded and nonexpanded Tregs from the same individual. The 50% inhibition of response was achieved at a regulator-to-responder ratio of approximately 1:243 for expanded Tregs and 1:1 for freshly isolated Tregs, denoting at least a 100-fold enrichment of allo-specific suppression (Figure 5B).
To compare the suppressive potency of expanded allo-specific Tregs versus anti-CD3/anti-CD28-stimulated, polyspecific Tregs from the same individual, CFSE− allo-specific or polyspecific Tregs were flow-sorted on day 12 and assayed. Allo-specific Tregs were more potent regulators, with 50% inhibition of day-6 proliferation at an approximately 1:1275 regulator-to-responder ratio compared with polyspecific Tregs, which achieved 50% inhibition at an approximately 1:18 ratio (Figure 5C). Similar potency differences between allo-specific and polyspecific Tregs were observed on day 5 (1:1822 vs 1:9) and day 7 (1:1200 vs 1:12) suppression assays.
To assess whether the suppressive function of the expanded, CFSE− Tregs was specific for the original alloantigen, they were tested in cocultures with the original or a HLA class II mismatched third-party stimulator. Responses to the original stimulator were suppressed by > 95% at a 1:20 ratio, but responses to the third party were minimally suppressed (Figure 5D). This experiment demonstrates that the expanded Tregs were alloantigen specific.
Treg cytokine profile and CTLA-4 expression
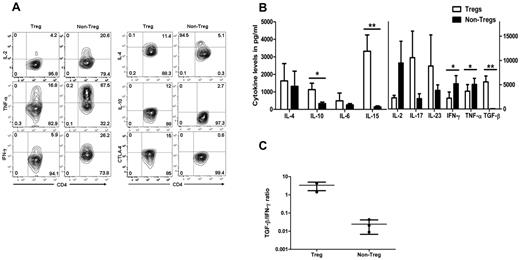
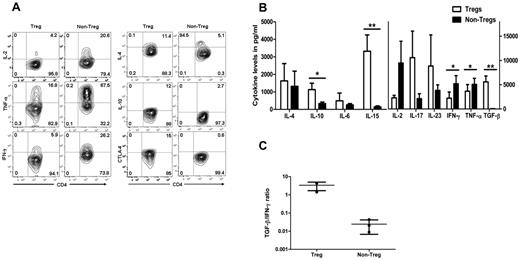
To analyze the cytokine profile, antigen-activated Tregs were stimulated by PMA, ionomycin, and brefeldin-A or monensin, and cytoplasmic cytokines or CTLA-4 were analyzed by flow cytometry. There was no difference in cytokine profile detected between CFSE− and CFSE+ Tregs (not shown). Data from CFSE− Tregs was compared in expanded non-Tregs from the same individual. More Tregs were positive for IL-4, IL-10, and CTLA-4 compared with non-Tregs. In contrast, non-Tregs had higher expression levels of the IFN-γ, TNF-α, and IL-2 cytokines (Figure 6A).
Cytokine release and CTLA-4 expression of expanded allo-specific Tregs. (A) Cytoplasmic staining of cytokines produced by expanded allo-specific CFSE− Tregs and non-Tregs. Flow cytometric contour plots show the relative number of cytokine-producing CD4+ T cells. (B) Cytokine secretion of expanded allo-specific Tregs. Supernatants were removed after incubation of expanded Tregs and non-Tregs with PMA and ionomycin in serum-free medium. Each bar represent mean ± SD of cytokine levels of 4 individual experiments. (C) TGF-β/IFN-γ cytokine ratio of Tregs and non-Tregs. *P < .05; **P < .01.
Cytokine release and CTLA-4 expression of expanded allo-specific Tregs. (A) Cytoplasmic staining of cytokines produced by expanded allo-specific CFSE− Tregs and non-Tregs. Flow cytometric contour plots show the relative number of cytokine-producing CD4+ T cells. (B) Cytokine secretion of expanded allo-specific Tregs. Supernatants were removed after incubation of expanded Tregs and non-Tregs with PMA and ionomycin in serum-free medium. Each bar represent mean ± SD of cytokine levels of 4 individual experiments. (C) TGF-β/IFN-γ cytokine ratio of Tregs and non-Tregs. *P < .05; **P < .01.
Cytokine secretion was also measured in the culture supernatant of expanded Tregs and non-Tregs. Approximately 100-fold higher levels of TGF-β were found in the culture supernatant of expanded Tregs compared with non-Tregs. IL-2, IFN-γ, and TNF-α levels were significantly lower in the culture supernatant of expanded Tregs compared with those of non-Tregs (Figure 6B). Secretion of IL-15 was also increased in the culture supernatant of expanded Tregs. The ratio of TGF-β to IFN-γ displayed in Figure 6C shows the greater than 100-fold difference between expanded Tregs and non-Tregs.
Indirect allo-recognition
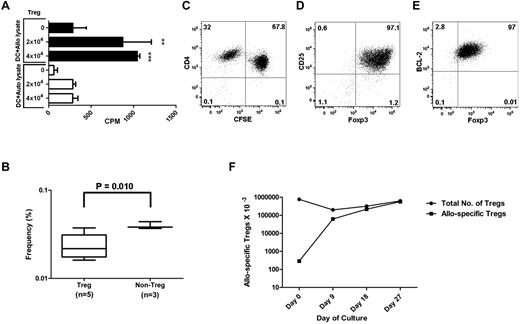
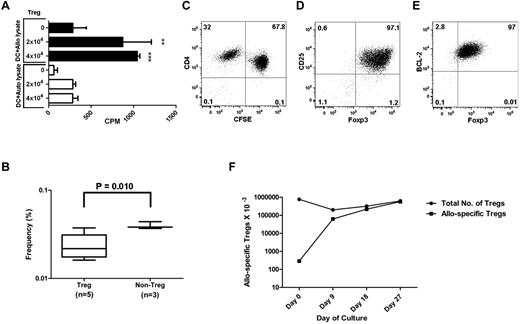
To measure the frequency of Tregs specific for alloantigen presented indirectly by self-HLA, we loaded autologous or allogeneic mononuclear cells or fibroblast lysate on self-DCs to stimulate Tregs. We found significant Treg proliferation when Tregs were stimulated by self-DCs loaded with allogeneic compared with autologous cell lysate (Figure 7A). For proliferation, Tregs required DC loading with allogeneic cell lysate and IL-2 plus IL-15 cytokines. The precursor frequency of Tregs with indirect allo-recognition was 0.02% (95% CI: 0.01-0.04), similar to the precursor frequency of non-Tregs, which was 0.04% (95% CI: 0.03-0.06; Figure 7B). CFSE dilution (Figure 7C) and cytoplasmic (data not shown) and intracellular (Figure D-E) phenotypes of expanded Tregs with indirect allo-specificity were similar to those of Tregs with direct allo-specificity. Expansion kinetics were determined by calculating the frequency of allo-reactive Tregs by LDA at the start of culture and CD4 Ki67 positive for the proliferative cells. After restimulation on day 14 with allogeneic cell lysate loaded self-APCs, allo-specific Treg continue to expand to over 2000-fold by day 27 (Figure 7F).
Indirect allo-recognition by Tregs. (A) Tregs were cultured with γ-irradiated autologous monocyte-derived DCs (10:1) pulsed overnight with allogeneic or autologous whole-cell lysate in the presence of cytokines and rapamycin. Results shown are average CPM of triplicates measured by the incorporation of 3H-thymidine in cocultures on day 6. (B) LDA showing frequency of allo-reactive purified CD4+CD25+CD127− Tregs or CD4+CD25−CD127+ non-Tregs stimulated with self-DCs and pulsed with allogeneic whole-cell PBMC lysate in the presence of rapamycin, IL-2, and IL-15. Box plot represents the frequency estimate and the error bar shows 95% CI calculated by extreme LDA. Dot plot shows the proliferation of CFSE-labeled Tregs with indirect allospecificity after 14 days in culture (C) and the expression of CD25, Foxp3, and BCL-2 after 27 days in culture (D-E). (F) Expansion kinetics of allo-specific Tregs when antigen was presented indirectly. Tregs were seeded at 7.7 × 105/well in a 48-well plate with irradiated allogeneic cell lysate–loaded self-DCs (7.7 × 104), rapamycin (100 ng/mL), IL-2 (10 U/mL), and IL-15 (10 ng/mL). Cells were cultured for 27 days with APC restimulation on day 14. The total Treg numbers at each time point were calculated by flow cytometry using timed event acquisition. The frequency of allo-specific Tregs at time 0 was calculated by LDA. The frequencies of allo-specific Tregs at subsequent times were calculated by flow microfluorometry of Ki-67+ Tregs. Results shown are from 1 representative experiment of 3. **P < .01; ***P < .001.
Indirect allo-recognition by Tregs. (A) Tregs were cultured with γ-irradiated autologous monocyte-derived DCs (10:1) pulsed overnight with allogeneic or autologous whole-cell lysate in the presence of cytokines and rapamycin. Results shown are average CPM of triplicates measured by the incorporation of 3H-thymidine in cocultures on day 6. (B) LDA showing frequency of allo-reactive purified CD4+CD25+CD127− Tregs or CD4+CD25−CD127+ non-Tregs stimulated with self-DCs and pulsed with allogeneic whole-cell PBMC lysate in the presence of rapamycin, IL-2, and IL-15. Box plot represents the frequency estimate and the error bar shows 95% CI calculated by extreme LDA. Dot plot shows the proliferation of CFSE-labeled Tregs with indirect allospecificity after 14 days in culture (C) and the expression of CD25, Foxp3, and BCL-2 after 27 days in culture (D-E). (F) Expansion kinetics of allo-specific Tregs when antigen was presented indirectly. Tregs were seeded at 7.7 × 105/well in a 48-well plate with irradiated allogeneic cell lysate–loaded self-DCs (7.7 × 104), rapamycin (100 ng/mL), IL-2 (10 U/mL), and IL-15 (10 ng/mL). Cells were cultured for 27 days with APC restimulation on day 14. The total Treg numbers at each time point were calculated by flow cytometry using timed event acquisition. The frequency of allo-specific Tregs at time 0 was calculated by LDA. The frequencies of allo-specific Tregs at subsequent times were calculated by flow microfluorometry of Ki-67+ Tregs. Results shown are from 1 representative experiment of 3. **P < .01; ***P < .001.
Discussion
In this study, we purified human Tregs and selectively expanded those Tregs specific for alloantigen presented by DCs directly or indirectly. The average 800-fold allo-specific Treg expansion shown here exceeds the theoretical 300-fold expansion required to reach a 1:1 ratio of Tregs:non-Tregs that is most effective for Treg immunotherapy in experimental animals. There are many novelties in this study.
Purity
In current study, none of the many allo-reactive Treg cultures contained live, detectable non-Tregs. Keys to these results were: (1) the use of a first negative selection step that enriched for CD4 T cells depleted of CD8, CD14, CD19, and the CD4+/CD127+ subset, and (2) the use of rapamycin, which prevented overgrowth of non-Tregs. Whereas we cannot exclude the possibility that Tregs might be reprogrammed to conventional T cells after in vivo transfer, it is unlikely that reprogramming will happen under the cover of rapamycin in vivo. Unpublished data from our laboratory showed that sirolimus immune suppression after hematopoietic cell transplantation favors the expansion of Tregs versus non-Tregs.
Treg frequency
Other studies have expanded allo-specific Tregs, but without a measure of their precursor frequency, there was no chance to assess the efficiency of the expansion process. In the present study, we used an LDA to characterize the precursor frequency of Tregs across full HLA disparity, and found an extraordinarily high frequency: approximately 1%, which is on the same order of magnitude as non-Tregs. The frequency of Tregs specific for alloantigen presented indirectly is approximately 100-fold less. The T-cell precursor frequency data allow us to predict what order of magnitude non-Tregs can be expected in HLA-disparate transplantations (direct presentation). Hopefully, our data on indirect presentation does approximate the Treg precursor frequency with mHa disparity in HLA-identical sibling transplantations, although formal testing for this hypothesis is still ongoing in our laboratory. Some investigators have reported that the potency of expanded allo-specific Tregs is higher than natural Tregs in vitro and in vivo.18,25,26 Our data comparing expanded allo-specific versus polyspecific or versus non-expanded Treg populations support this concept (Figure 5). Our LDA and CFSE data point to the increased frequency of allo-specific Tregs during expansion entirely accounting for the increased potency of the expanded population.
Expansion efficiency and Foxp3 stability
DCs have been identified as having unique capabilities for expanding Tregs,27 and protocols using BM-derived DCs for the large-scale expansion of polyclonal Tregs have been established in a murine model.28 We surmise that both the expansion efficiency and Foxp3 stability observed here may be related to the use of DCs as more physiologic APCs, but cytokines and rapamycin have also contributed to the favorable culture conditions. Tregs stimulated from artificial APCs often experience Foxp3 down-regulation and loss of the suppressive phenotype. Within 12 days in culture after single antigenic stimulation in the presence of IL-2, IL-15, and rapamycin, we have not witnessed either limitation. Resting Tregs express the common β and γ chains of the IL-2/IL-7/IL-15 receptors, but antigen-activated Tregs also express IL-15 receptor-α.29 IL-15 enhances Treg survival through bcl-2 induction and Treg suppressive function through STAT5 activation and expression of Foxp3.24 IL-15 stimulation has also allowed the use of rapamycin and a low concentration of IL-2 that is slightly inhibitory for T cells, thereby avoiding the recruitment of non-Tregs or Tregs that had not been activated by alloantigen (we used IL-2 at 10 U/mL vs 250 U/mL by others).21 Both IL-15 and rapamycin promote stable Foxp3 expression.24,30
Functional phenotype
Ex vivo–expanded allo-specific Tregs retained expression of CD25 and Foxp3, which are required for Treg replication and regulatory functions,31,32 and of the lymphoid homing receptor CD62L and the chemokine receptor CCR7, which are required for migration to lymphoid tissue in vivo,33 but not the IL-7 receptor α chain CD127.11,34,35 We had expected CD45RA down-regulation during cell replication, however, we found that CD45RA was expressed in a substantial fraction of the expanded Treg population, which is consistent with a functional Treg phenotype.34 Several in vitro studies have shown Treg suppression to be dependent on either cell-to-cell contact between Tregs and their target cells9 or membrane-bound TGF-β36 and IL-10.37 Other mechanisms include CTLA-4 on Tregs up-regulating indoleamine 2,3-dioxygenase expression in DCs, which in turn down-regulates immune responses.38 The expanded allo-specific Tregs produced IL-10 and TGF-β and expressed cytoplasmic CTLA-4 to significantly higher degree than non-Tregs, suggesting that those molecules are involved in regulation. The TGFβ:IFNγ ratio was approximately 1000 in Tregs, proposing a rapid and reproducible potency assay with which to qualify a functional Treg populations for clinical use. We need to investigate whether IL-15 detected in the supernatant was secreted by the expanded Tregs or shed exogenous cytokine. As in our expansion experiments, the fraction of nonreplicating Tregs was minute when considering the numbers of Tregs in a peripheral blood stem cell graft; furthermore, the nonreplicating Tregs will not need to be separated off, as suggested previously.39 The efficiency of Treg expansion for in vivo use may be even greater without CFSE labeling. CFSE dilution was precisely correlated with Ki-67 expression in replicating cells. Therefore, we plan to use Ki-67 staining to verify replication of Tregs expanded for in vivo use.
Immune specificity
Most protocols for Treg expansion have used artificial APC plus IL-2 to expand polyspecific Tregs by approximately 300- to 1000-fold.40 In comparison, we expanded allo-specific Tregs by approximately 800-fold in a single culture. Given the similar magnitude of expansion, and that the suppressive potency against immune response to the specific alloantigen is higher for allo-specific than polyspecific Tregs, our data support the idea that allo-specific Tregs have higher therapeutic potential.21 Comparing the efficacy of Tregs for immunotherapy, the need for expanded allo-specific Tregs in one system was found to be 500 times less than that for polyspecific Tregs.41 A clear advantage of using allo-specific Tregs is to avoid broad immune suppression. Another advantage is that the immunomodulatory function of allo-specific Tregs is targeted at the site of alloantigen source and immune activation.42 Whereas crossreactivity is a possibility, especially against MHC incompatibility,43 it is not the rule. In our original murine experiments, Tregs would only suppress GVHD in vivo in the presence of the cognate antigen.26,44 Therefore, the ability to exploit a Treg response to indirectly presented antigens opens wide opportunities for translation in allogeneic transplantation for the prevention of GVHD while sparing immune response against malignancy.
Indirect presentation
In the present study, we report that polyclonal human Tregs can be expanded by self-DC–presenting alloantigen, indirectly confirming and extending findings by Jiang et al, who used a model allopeptide.45 Because the loaded cell lysate included HLA leader and expressed HLA peptides, it is likely that the Treg response was directed at least in part to these antigens. However, it is also possible that many other polymorphic proteins were presented as peptides, including minor histocompatibility (mHA) antigens. This finding opens the opportunity to study a variety of tissues as source of antigen for indirect presentation to activate and expand therapeutic Tregs. The implication is clear in the case of HLA-compatible siblings, in whom Tregs could be expanded against ubiquitous mHA antigens such as H-Y, thereby preventing GVHD while sparing immune responses to hematopoietic-restricted mHA antigens such as HA-1 and HA-2 that can drive GVL responses. If effective, this approach could prevent GVHD while sparing at least in part GVL or graft-versus-tumor responses.
The online version of this article contains a data supplement.
Presented in abstract form at the 52nd Annual Meeting of the American Society of Hematology, Orlando, FL, December 6, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Elizabeth Chang (HLA Laboratory, Department of Pathology Medicine, All Children's Hospital) for HLA class-II antigen typing.
This work was supported in part by the National Institutes of Health (grants CA132197, AI082498, and CA076292 to C.A., and CA118116 to X.Y.) and was facilitated by the flow cytometry core facilities at the Moffitt Cancer Center.
National Institutes of Health
Authorship
Contribution: A.V. performed the experiments; A.V., F.B., and C.A. analyzed the results and produced the figures; and A.V., J.P., X.-Z.Y., and C.A designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudio Anasetti, MD, Department of Blood and Marrow Transplantation, Moffitt Cancer Center, 12902 Magnolia Dr, FOB3-BMT, Tampa, FL 33612; e-mail: claudio.anasetti@moffitt.org.