Abstract
Abstract 1007
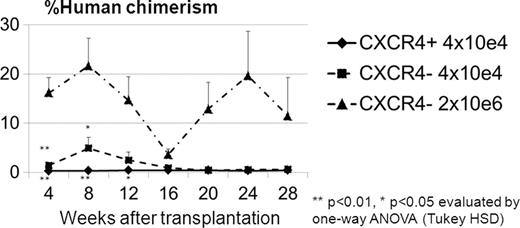
CXCR4 is an α-chemokine receptor specific for the ligand stromal-derived-factor-1 (SDF-1). CXCR4 is expressed on several cell types, including CD34+ cells. In this study, we sought to evaluate the difference between subpopulations of human and rhesus CD34+ cells that express CXCR4. Rhesus CD34+ cells were mobilized using a five day course of G-CSF (10mcg/kg SQ) and a single dose (1mg/kg SQ) of the CXCR4 antagonist, AMD3100, on the 5th day. Leukapheresis was performed two hours following the last dose of G-CSF and AMD3100 and CD34+ cells were isolated by immunoselection from the product. Human CD34+ cells were collected by leukapheresis and immunoselection following a 5 day course of 480 mcg G-CSF SQ. CXCR4+ and CXCR4- fractions in the CD34+ cells were separated by cell sorting. Rhesus and human CD34+CXCR4+ and CD34+CXCR4- cells were used for microarray analysis as well as cultured and transduced with an EGFP-expressing lentiviral vector. On average (SD) 21.6 ± 4.4% of AMD3100+G-CSF mobilized rhesus (n=6) and 39.3 ± 4.3% of G-CSF mobilized human (n=3) CD34+ cells express CXCR4. Two days following in vitro culture, rhesus CD34+CXCR4+ cells showed greater numbers of adherent spindle-shaped cells (56.4 ± 7.4%) when compared to CXCR4- cells (1.3 ± 0.4%, p<0.01) and bulk CD34+ cells (21.0 ± 2.0%, p<0.01). Following transduction, the rhesus CD34+CXCR4+ derived cells also had significantly lower EGFP expression (7.0 ± 0.3%) when compared to their CD34+CXCR4- counterpart (25.4 ± 1.2%, p<0.01) and bulk CD34+ cells (22.6 ± 2.2%, p<0.01). In CFU assay, human CD34+CXCR4+ cells showed fewer colony numbers (Erythroid (E): 6.2 ± 1.7/1000 cells, Myeloid (M): 7.0 ± 0.0/1000 cells) when compared to CD34+CXCR4- derived cells (E: 21.5 ± 7.9/1000 cells, ns, M: 24.7 ± 4.8/1000 cells, p<0.05) and bulk CD34+ cells without lentiviral transduction (E: 37.3 ± 1.2/1000 cells, p<0.05, M: 24.7 ± 4.8/1000 cells, p<0.01). To determine whether the CD34+CXCR4+ or CD34+CXCR4- subpopulation contained hematopoietic repopulating cells, human CD34+CXCR4+ cells (4×10e4 cells/mouse) and CD34+CXCR4- cells (4×10e4 cells/mouse or 2×10e6 cells/mouse) with lentiviral gene-marking were transplanted into NOD/SCID/IL2Rγ null mice. Human cell chimerism and %EGFP in the peripheral blood cells were analyzed every 4 weeks for over 6 months (Figure). CD34+CXCR4+ cell-transplanted mice showed no engraftment of human cells (less than 1% of human CD45+ cells) at all time points, while human cells were detected in the peripheral blood of the CD34+CXCR4- cell-transplanted mice (4×10e4 cells/mouse) until 12 weeks after transplantation. The CD34+CXCR4- cell-transplanted mice (2×10e6 cells/mouse) showed human cell engraftment for over 6 months. These data suggest that the CD34+CXCR4- cell population contain hematopoietic repopulating cells. To evaluate the nature of the CD34+CXCR4+ cell population, global microarray gene expression analysis was performed both on human and non-human primate CD34+CXCR4+ cells. Microarray signatures showed different gene profiles between CD34+CXCR4+ and CD34+CXCR4- populations in both human and rhesus CD34+ cells. Both human and rhesus CD34+CXCR4+ populations demonstrated strong monocyte- and neutrophil-mediated inflammatory response gene expression profiles, suggesting that the CD34+CXCR4+ subpopulation in both human and non-human primates contain progenitors instrumental in the innate immune response. These results indicate that while the addition of AMD3100 to G-CSF mobilization protocols may increase the number of CD34+ cells mobilized, some of these cells may not be as potent for gene therapy and transplantation applications and support the design of immunoselection protocols which target specific subpopulations of CD34+ cells for therapeutic applications in the future.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011