Abstract
Abstract 1009
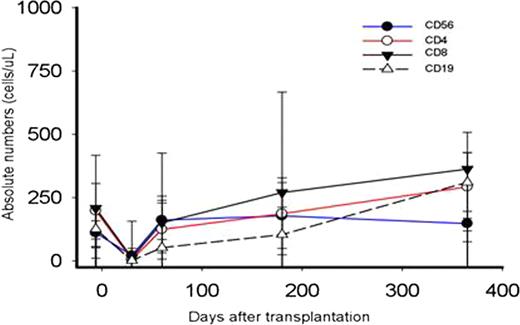
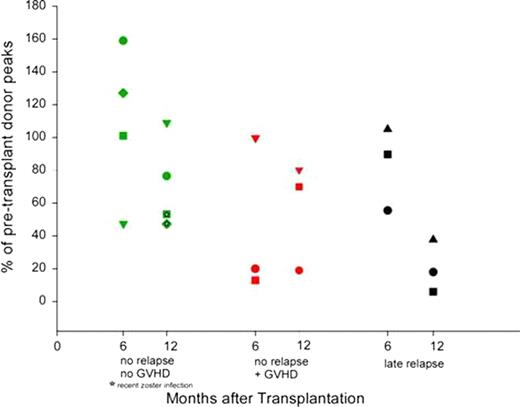
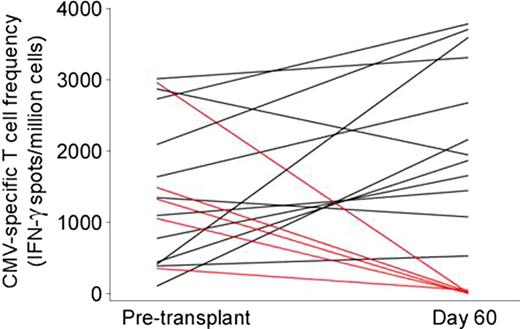
Delayed immune reconstitution with increased risk of opportunistic infection is a major complication of HLA-haploidentical stem cell transplantation, especially in protocols employing extensive T cell depletion of the graft. Previous studies at our institution with high-dose, post-transplantation Cy (PT/Cy) have reported low rates of non-relapse mortality and serious opportunistic infections. Here we characterize immune reconstitution in fifty-three consecutive hematologic malignancies patients receiving nonmyeloablative conditioning, T cell-replete, HLA-haploidentical bone marrow transplantation (BMT), and graft versus host disease prophylaxis including PT/Cy. Patients with advanced hematologic malignancies (median age 51, range 14–71; 5 AML, 2 ALL, 4 MDS, 2 CML, 4 CLL, 1 CMML, 25 NHL, 7 Hodgkins, 3 mantle cell) received Cy 14.5 mg/kg/day IV on days −6 and −5, fludarabine 30 mg/m2/day IV on days −6 to −2, 200 cGy of TBI on day -1 and T cell replete bone marrow from donors with a median age of 44 (range 14–68). GVHD prophylaxis consisted of Cy (50 mg/kg/day) on days 3 and 4, mycophenolate mofetil for 30 days, and tacrolimus for 6 months. Grafts contained an infused median TNC/kg of 4.1 e8 (range 2.6–6.6 e8), CD3+/kg 3.6 e7 (range 1.7–6.7e7) and a CD34+/kg of 3.5e6 (range 1.4–7.0e6). Sustained engraftment of donor cells occurred in 86% of evaluable patients (44/51).The median times to neutrophil (>500/μL) and platelet recovery (>20,000/μL) were 17 days (range, 13–92 days) and 28 days (range, 13–580 days), respectively. Post-transplantation recovery of lymphocyte subsets is shown in Table 1 and Figure 1 and is notable for the following: 1) The median lymphocyte count at day 30 after transplantation is >180/ml and recovers to over 800/ml by day 60; 2) CD4+ T cell counts recover to a median >120/ml by day 60 and >220/ml by day 180 after transplantation; and 3) recovery of CD31+ recent thymic emigrants and CD45RA+ naïve T cells is delayed compared to recovery of memory T cells. T cell receptor spectratyping analysis on a subset of 10 patient/donor pairs chosen specifically for having no relapse/no GVHD (n=4), GVHD and no relapse (n=3), or late relapse (n=3) revealed that patients without relapse, GVHD, or recent viral infection had excellent reconstitution of the T cell repertoire to the level of the pre-transplant donor, as early as 6 months post-transplant (Figure 2). CMV specific T cell response using ELISPOT measured on a subset of 17 patients whose donors were reactive to CMV, revealed that donor-derived immunity to CMV returns by Day 60 in about 70% of patients (12/17) (Figure 3). In conclusion, immune reconstitution after non-myeloablative haploidentical T cell replete BMT with PT/Cy compares favorably with other reduced intensity conditioning alternative donor regimens and suggests that PT/Cy selectively preserves pathogen-specific memory T cells necessary to protect against infection. Further correlations of immune reconstitution with specific infectious and overall outcomes are being analyzed.
Table 1.
Post-transplantation Lymphocyte Subset Recovery
| . | Median (cells/μL) (N) . | Interquartile range (cells/μL) . |
|---|---|---|
| ALC | ||
| Donor | 1765 (46) | 1480–2100 |
| Recipient pre-BMT | 840 (45) | 425–1295 |
| Day 30 | 184 (49) | 54–402 |
| Day 60 | 820 (38) | 470–1260 |
| Day 180 | 915 (34) | 670–1560 |
| Day 365 | 3060 (22) | 820–2030 |
| CD3+CD4+CD45RA+ (naïve) | ||
| Donor | 119 (33) | 82–189 |
| Recipient pre-BMT | 22 (34) | 4–38 |
| Day 30 | 0.33 (35) | 0.07–1 |
| Day 60 | 3 (29) | 1–9 |
| Day 180 | 11 (23) | 5–31 |
| Day 365 | 23 (13) | 13–92 |
| CD3+CD4+CD45RA−CCR7+ (central memory) | ||
| Donor | 135 (33) | 95–158 |
| Recipient pre-BMT | 54 (34) | 11–79 |
| Day 30 | 2 (35) | 0.5–11 |
| Day 60 | 34 (29) | 10–79 |
| Day 180 | 61 (23) | 35–117 |
| Day 365 | 89 (13) | 60–122 |
| CD3+CD4+CD45RA−CCR7− (effector memory) | ||
| Donor | 187 (33) | 130–245 |
| Recipient pre-BMT | 87 (34) | 14–134 |
| Day 30 | 3 (35) | 1–18 |
| Day 60 | 59 (29) | 14–122 |
| Day 180 | 102 (23) | 43–179 |
| Day 365 | 142 (12) | 64–204 |
| CD3+CD4+CD45RA+CD31+ (recent thymic emigrants) | ||
| Donor | 61 (33) | 30–97 |
| Recipient pre-BMT | 6 (34) | 1–16 |
| Day 30 | 0.9 (35) | 0.03–0.4 |
| Day 60 | 1 (29) | 0.5–2 |
| Day 180 | 4 (23) | 1–11 |
| Day 365 | 7 (13) | 2–12 |
| CD3+CD4+Foxp3+ | ||
| Donor | 28 (33) | 23–35 |
| Recipient pre-BMT | 13 (34) | 6–24 |
| Day 30 | 1 (35) | 0.1–5 |
| Day 60 | 8 (29) | 4–16 |
| Day 180 | 13 (23) | 7–25 |
| Day 365 | 14 (13) | 7–17 |
| . | Median (cells/μL) (N) . | Interquartile range (cells/μL) . |
|---|---|---|
| ALC | ||
| Donor | 1765 (46) | 1480–2100 |
| Recipient pre-BMT | 840 (45) | 425–1295 |
| Day 30 | 184 (49) | 54–402 |
| Day 60 | 820 (38) | 470–1260 |
| Day 180 | 915 (34) | 670–1560 |
| Day 365 | 3060 (22) | 820–2030 |
| CD3+CD4+CD45RA+ (naïve) | ||
| Donor | 119 (33) | 82–189 |
| Recipient pre-BMT | 22 (34) | 4–38 |
| Day 30 | 0.33 (35) | 0.07–1 |
| Day 60 | 3 (29) | 1–9 |
| Day 180 | 11 (23) | 5–31 |
| Day 365 | 23 (13) | 13–92 |
| CD3+CD4+CD45RA−CCR7+ (central memory) | ||
| Donor | 135 (33) | 95–158 |
| Recipient pre-BMT | 54 (34) | 11–79 |
| Day 30 | 2 (35) | 0.5–11 |
| Day 60 | 34 (29) | 10–79 |
| Day 180 | 61 (23) | 35–117 |
| Day 365 | 89 (13) | 60–122 |
| CD3+CD4+CD45RA−CCR7− (effector memory) | ||
| Donor | 187 (33) | 130–245 |
| Recipient pre-BMT | 87 (34) | 14–134 |
| Day 30 | 3 (35) | 1–18 |
| Day 60 | 59 (29) | 14–122 |
| Day 180 | 102 (23) | 43–179 |
| Day 365 | 142 (12) | 64–204 |
| CD3+CD4+CD45RA+CD31+ (recent thymic emigrants) | ||
| Donor | 61 (33) | 30–97 |
| Recipient pre-BMT | 6 (34) | 1–16 |
| Day 30 | 0.9 (35) | 0.03–0.4 |
| Day 60 | 1 (29) | 0.5–2 |
| Day 180 | 4 (23) | 1–11 |
| Day 365 | 7 (13) | 2–12 |
| CD3+CD4+Foxp3+ | ||
| Donor | 28 (33) | 23–35 |
| Recipient pre-BMT | 13 (34) | 6–24 |
| Day 30 | 1 (35) | 0.1–5 |
| Day 60 | 8 (29) | 4–16 |
| Day 180 | 13 (23) | 7–25 |
| Day 365 | 14 (13) | 7–17 |
ALC, absolute lymphocyte count; WBC, white blood cell count; Treg, regulatory T cell
Disclosures:
Jones:Aldagen: Patents & Royalties.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011