Abstract
Abstract 1400
Activating mutations of the FLT3 gene are common in AML, with approximately 25–30% of cases containing an internal tandem duplication (ITD) in the juxtamembrane domain. The presence of a FLT3-ITD mutation is associated with a poor prognosis which can be modulated by the combination of co-operating mutations. In animal models, expression of FLT3-ITD by transgenesis, bone marrow transplantation or gene knock-in does not lead to an acute leukemia but a myeloproliferative disease, resembling CMML, suggesting a requirement for additional co-operating mutations (Lee et al, Cancer Cell. 12: 367). This is supported by in vivo models which demonstrate that the combination of the FLT3-ITD mutation with other genetic lesions leads to an acute leukemia in mice.
To identify novel genes co-operating with FLT3-ITD to induce acute leukemia by using an N-ethyl-N-Nitroso-urea (ENU) mutagenesis strategy in mice with a FLT3-ITD background.
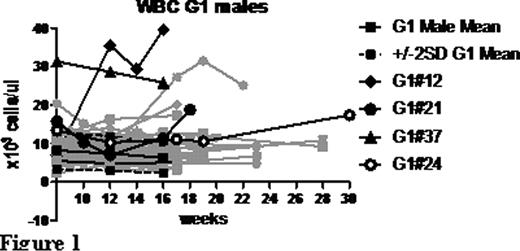
An autosomal dominant screen for FLT3-ENU co-operating mutations was carried out at the Australian Phenomics Facility by mating ENU mutagenised male mice homozygous for the FLT3-ITD knock-in allele to females homozygous for the FLT3-ITD knock-in allele. G1 mice were screened for changes in blood cell parameters indicative of an altered disease state. Peripheral blood differential analysis was performed at 8, 12 and 16 weeks of age as well as immunophenotyping for the myeloid markers Mac1 and Gr1 and the progenitor marker c-kit. Analysis of a non-mutagenised cohort of mice indicated a sex-specific differential effect of FLT3-ITD on multiple blood cell parameters, so male and female data were analysed separately. Mice with blood cell parameters outside two standard deviations of the relevant G1 mean were identified as potential mutation carriers (Figure 1) and bred to FLT3-ITD homozygous knock-in mice to test for heritability of the phenotype (Figure 2).
To date 150 G1 mice have been screened, leading to the identification of four heritable phenotypes (Designated pedigrees 12, 21, 24 and 37, Figure 1). All pedigrees were characterised by increased WBC counts compared to ‘unaffected’ mice (FLT3-ITD homozygous background) and by further expansion of the myeloid compartment. Preliminary analysis of the bone marrow from affected mice identified an increase in colony replating ability in response to GM-CSF, suggestive of a block in differentiation. Three out of the four pedigrees showed disease penetrance only in males, with several mice from pedigree 24 succumbing to disease (Figure 2). Whole exome sequencing is being undertaken to identify the sequence variants that segregate with the phenotype in each of the pedigrees.
It is possible through mutagenesis to identify co-operating mutations with FLT3-ITD. Further characterisation of these pedigrees, the associated phenotype and identification of the associated mutation in each case, will provide important information regarding pathways that co-operate with FLT3-ITD in leukemogenesis. Translation of these findings to human AML will have potential implications for predicting the course of disease in AML patients and may indicate pathways that will be targets for new and complementary treatments in AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.