Abstract
Abstract 1628
Historically, relapsed DLBCL and HL has been associated with a high cure rate with salvage regimens followed by high dose chemotherapy and stem cell transplant (ASCT). However, patients (pts) who relapse early following upfront chemotherapy and pts who fail to respond to salvage have a poor overall response rate (ORR) with additional salvage regimens and a poor prognosis even when consolidated with ASCT (von Tresckow & Engert, 2011; Gisselbrecht et al., 2010). At present there is no standard therapy in the third-line setting for pts with DLBCL and HL. We designed a regimen: vinorelbine (30mg/m2) & paclitaxel (175mg/m2) given on day 1; etoposide (100mg/m2) & cisplatin (20mg/m2) given on days 2–5; and cytarabine (2000mg/m2) on days 4 and 5 (VTEPA) for treatment of lymphoma pts with 1° refractory disease or relapse following salvage. In phase 1, VTEPA was safe with a 33% ORR following 1 cycle (Lonial et al, 2006).
To examine the effectiveness of VTEPA, we conducted an IRB approved retrospective review of consecutive cases of relapsed/refractory DLBCL and HL identified from our database from 1999–2011. All pts had evidence of primary refractory disease or stable or progressive disease following first line salvage therapy. Responses following salvage VTEPA were retrospectively assessed using International Working Group Criteria (JCO 1999) for all pts. Responding pts proceeded to ASCT. Survival curves were constructed using the Kaplan-Meier method and compared with the log-rank test.
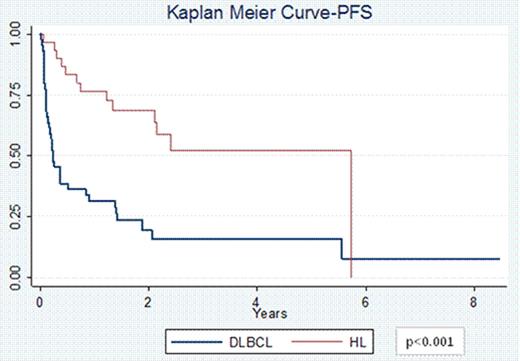
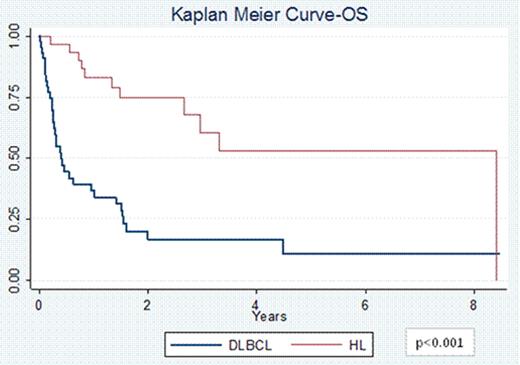
74 pts (44 DLBCL and 30 HL) with a median age at diagnosis of 30 years (range 18–63) for HL and 49 years (range 20–68) for DLBCL were included. 67% of HL pts had primary refractory disease and 33% of pts had relapsed disease; 60% were stage III/IV at diagnosis. 75% of DLBCL pts had primary refractory disease and 25% of pts had relapsed disease; 73% stage III/IV. Pts received a median of 2 prior therapies (range 1–4). 63% pts with HL received prior salvage therapy with ifosfamide, carboplatin, and etoposide (ICE) and 13% with other regimens. 16 pts with HL received 1 cycle of VTEPA, 13 received 2 cycles and 1 pt received 3 cycles of VTEPA. 70% pts with DLBCL had received prior salvage therapy with rituximab (R) + ICE and 16% received other salvage regimens. 32 pts with DLBCL received 1 cycle of R-VTEPA and 12 pts received 2 cycles. The most common reported grade 3/4 toxicities were pancytopenia (97%), nausea/vomiting (58%), fatigue (30%), infectious complications (26%), diarrhea (24%), electrolyte imbalance (19%), and mucositis (16%). 70 pts (43 DLBCL and 27 HL) were evaluable for response. The ORR for DLBCL pts was 44% (9% CR and 35% PR) while that for HL pts was 70% (26% CR and 44% PR, p=0.04). 4 DLBCL pts had treatment related mortality. 34 pts went on to collect ≥2 × 106 CD34+ cells/kg; 3 pts had inadequate stem cell collection. In 23 pts collection was not attempted, and 14 pts collected stem cells prior to R+/−VTEPA. 37 pts (47%) went onto planned ASCT, and 4 pts underwent allogeneic transplantation. The PFS at 2 years for pts with HL was 68% vs. 49% for pts with DLBCL (p<0.001, Figure 1). Similarly the OS at 2 years for pts with HL was 79% vs. 41% for pts with DLBCL (p<0.001, Figure 2).
Treatment with VTEPA for heavily pretreated relapsed/refractory HL and DLBCL pts is feasible with manageable side effects, a high ORR, and permits transplantation for nearly ½ of pts. Nevertheless, outcomes for pts with refractory DLBCL is exceedingly poor compared to refractory pts with HL treated with the same approach. While there remains a great need for novel agents to aid DLBCL pts who are chemotherapy and R refractory, the favorable PFS and OS for relapsed/refractory HL pts suggest VTEPA is a promising salvage regimen for this disease.
Sinha:Celgene: Research Funding. Kaufman:Millenium; Onxy; Novartis; Keryx: Research Funding; Merk, Celgene: Research Funding. Flowers:Spectrum: Consultancy, Research Funding; Millennium/Takeda: Research Funding; Celgene: Consultancy; Genentech/Roche (unpaid): Consultancy; Novartis: Research Funding; Seattle Genetics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.