Abstract
Abstract 1908
Chimeric antigen receptors (CARs) are employed to genetically modify T cells to redirect their specificity to target antigens on tumor cells. Typically a second generation CAR is derived by fusing an extracellular domain derived from the scFv of monoclonal antibody (CAR) specific to targeted antigen with CD3 zeta, and CD28 endodomains. CD123 (IL3RA) is expressed on 45% to 95%of acute myelogenous leukemia (AML) and B-cell lineage acute lymphoblastic leukemia (B-ALL). Expression of CD123 is high in the leukemic stem cell (LSC) population, but not in normal hematopoietic stem cells. Thus, CD123 appears to be potential target for immunotherapy in leukemias through chimeric antigen receptor (CAR). We hypothesized that the generation of CD123 specific CAR can redirect the specificity of T cells to CD123 and this was tested by cloning the scFv of CD123 mAb in our CAR construct. The sleeping beauty system was used to express the CAR and DNA plasmids were electroporated into peripheral blood mononuclear cells and cells were numerically expanded on artificial antigen presenting cells genetically modified to express co stimulatory molecules CD86, 4-1BBL, membrane-bound IL-15, and CD123 antigen in presence of IL-21 and 1L–2. CAR+ T numerically expanded to clinically relevant numbers and showed antigen specific cytotoxicity in leukemic celllines. CAR+ T cells expressed both effector and memory markers showing the potential for in vivo persistence after T cell infusion. The bonemarrow homing receptor CXCR4 was expressed by CAR T cells shows the potential to target LSC that reside in BM niches. The preliminary data suggests that mirroring an approach we are using to manufacture clinical grade CD19 specific CAR+ T cells.Close modal
Figure 1:
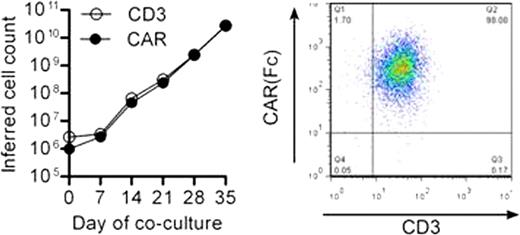
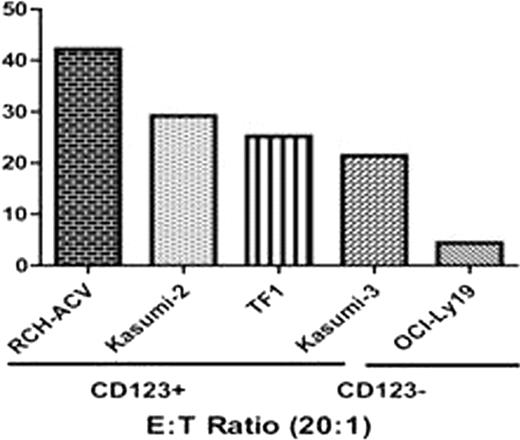
(A) CAR expression on day 35. (B) Cytotoxicity of CD123CAR in leukemic cell lines.
Figure 1:
(A) CAR expression on day 35. (B) Cytotoxicity of CD123CAR in leukemic cell lines.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011