Abstract
Abstract 2498
Diffuse large B-cell lymphomas (DLBCL) account for approximately 40% of lymphomas in adults, with the activated B-cell lymphoma (ABC) subtype being the least curable. ABC lymphoma cells display the phenotype of activated B-cells, which is induced by constitutive activation of the transcription factor Nuclear Factor-kappa B (NF-κB). NF-κB activation in ABC lymphoma can result from several different mutations or abnormal expression of proteins upstream of NF-κB nuclear translocation. No matter the mechanism of NF-κB constitutive activation, the resulting gene expression pattern confers an anti-apoptotic and pro-survival advantage to B-cells, hence driving the oncogenesis and supporting the survival of ABC lymphomas. Therefore, inhibiting NF-κB activity is an attractive strategy for the treatment of ABC lymphomas.
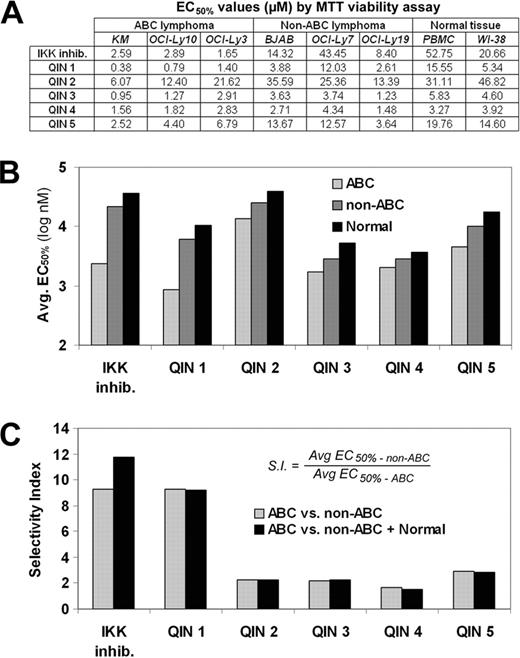
Using a cell line with a reporter for NF-κB transcriptional activity in a high throughput screen of 180,000 compounds we discovered 12 compounds that inhibited NF-κB activation, suggesting these compounds negatively regulate NF-κB. Since ABC lines are generally more dependent on NF-κB signalling for cell survival than are the other types of B-cell lymphoma, the hits from the primary screen were tested in a secondary screen for the ability to selectively inhibit the growth of ABC lymphoma cells. Of the 12 hits, two compounds belonging to the same quinolone chemotype were found to selectively inhibit the growth of an ABC line compared to a non-ABC line and normal human peripheral blood mononuclear cells (PBMCs). Three more structurally related quinolones were obtained to investigate a possible limited structure activity relationship for this class of molecules, designated here as Quinolone Inhibitors of NF-κB (QINs). QIN 1 was significantly more potent (Figure A and B) and selective (Figure B and C) relative to other QINs. The limited structure activity relationship suggests that two structural regions of the chemotype may be important for potency and for ABC selectivity, hence providing impetus to further investigate QINs as possible ABC lymphoma drugs.
QIN1 is more potent in inhibiting the growth of ABC lines than a known inhibitor of NF-κB activity, a commercially available selective IKK inhibitor (CAS 507475-17-4). Active IKK causes degradation of IκBα, the natural inhibitor of NF-κB, hence IKK inhibitors prevent NF-κB activation by preventing degradation of IκBα. In addition to being more potent, QIN1 retains similar ABC selectivity as the IKK inhibitor (Figure C). QIN1 and the IKK inhibitor both cause apoptosis at a similar rate and both cause G1 arrest in ABC lines at equi-toxic concentrations, initially suggesting similar mechanisms of action. Subsequently, QIN1was found to inhibit NF-κB in several different assays using the IKK inhibitor as a positive control. First, QIN1 inhibited the activation of a transcription based NF-κB reporter cell line in response to LPS, an NF-κB activator. Supporting these results, QIN1 inhibited cytokine release from human PBMCs stimulated with LPS. In addition, QIN1 prevented degradation of IκBα in response to NF-κB activating stimuli, further demonstrating the ability of QIN1 to inhibit NF-κB activity. Finally, QIN1 inhibited nuclear translocation of active NF-κB, thus preventing pro-survival gene expression.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.