Abstract
Abstract 2609
The monosomal karyotype (MK+) has recently been identified as the single most adverse cytogenetic prognosticator of AML outcome (1, 2). We made the recurrent - and seemingly paradoxical - observation that MDS/AML patients (pts) with complex karyotypes (CK+) including monosomy 7 showed encouraging responses to decitabine (DAC, ref.s 3, 4). We now evaluated a large cohort of AML pts >60 years ineligible for induction chemotherapy treated on a single phase II multicenter DAC study (trial 00331) for the effect of the MK+ genotype upon outcome.
Comparisons of response rate (RR), i.e. attainment of complete and partial remissions (CR/PR) and overall survival (OS) were performed in 179 treatment-naive AML pts with available cytogenetics (median age 72 years) treated with DAC as described (ref. 3, given over 72 hours, every 6 weeks), for up to 4 courses, followed by “maintenance” with 3 daily 1-hour infusions of DAC 20 mg/m2 every 4–6-weeks. Median white blood cell counts prior to treatment was 7.500/μl (range 0.5–241,000), 79% had a performance status ECOG >1. Cytogenetic risk groups are shown in Table 1. All karyotypes were centrally reviewed and scored for MK (A.H.). Analyses were adjusted for the parameters which had a strong effect on outcome in multivariate analyses of RR and OS, i.e., performance status (on RR and OS) and platelet counts (on OS).
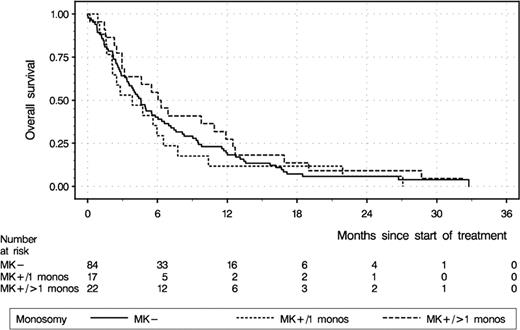
By the established definition of MK+, i.e either 1 autosomal monosomy and at least one structural change (MK1) or 2 or more autosomal monosomies (MK2), 39/179 patients were scored as MK+, with chromosomes 7 (n=17), 17 (n=17), and 5 (n=8) being most frequently lost. 17 pts had a single monosomy, as part of a median of 5 abnormalities (range, 2–21), 22 pts had multiple monosomies (median 3, range 2–11), as part of a median of 9.5 abnormalities (range, 4–17). As shown in Table 1, abnormal cytogenetics (AA) were associated with a lower RR and OS compared to pts with normal karyotype. When analyzing pts with AA (n=123) according to the presence (n=39) or absence of MK+ (n=84), MK+ patients had a higher RR than MK-, irrespective of a CK+ genotype. Since it has been shown by several groups that multiple monosomies may herald an even worse prognosis of AML than single monosomy, it was notable that pts with multiple monosomies (MK2, n=22) had a 45% RR and 25% 1-year OS (Fig. 1) compared with 24% and 12% in those with a single monosomy (MK1, n=17), both comparisons p=0.11.
Patients with normal-karyotype AML had a better outcome with DAC treatment than those with chromosomal abnormalities. Patients with single or even multiple monosomies still appeared to respond to this treatment, supporting our previous, surprising observation that pts with highly complex karyotype including monosomies may benefit from DAC. Because of a lack of control group in our trial, it is difficult to hypothesize if this encouraging result may be related to modes of action of DAC which differ from low-dose chemotherapy, e.g., with cytarabine. The role of MK will be further studied prospectively in a randomized clinical trial in the same patient population (NCT00867672), and retrospectively in a randomized trial of higher-risk MDS patients treated with either DAC or Best Supportive Care (EORTC 06011).
| Characteristic . | . | Response (CR/PR) . | Overall survival (OS) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N . | CR/PR rate (%) . | Odds Ratio . | 95%- Confidence Interval . | p-value . | 1-year OS rate (%) . | Hazard Ratio . | 95%- Confidence Interval . | p-value . | |
| All patients with known cytogenetics | 179 | 28 | 26 | 0.0006 | |||||
| Cytogenetics | 0.13 | ||||||||
| normal | 56 | 38 | 1.00 | − | 43 | 1.00 | − | ||
| abnormal | 123 | 24 | 1.72 | [0.85,3.48] | 19 | 1.86 | [1.30,2.65] | ||
| MK/CK | 0.053 | 0.38 | |||||||
| MK+ | 39 | 36 | 1.00 | − | 21 | 1.00 | − | ||
| MK-/CK+ | 17 | 12 | 4.75 | [0.88,25.6] | 18 | 1.43 | [0.79,2.59] | ||
| MK-/CK- | 67 | 19 | 2.83 | [1.08,7.41] | 18 | 0.96 | [0.63,1.45] | ||
| MK+ | 0.11 | 0.11 | |||||||
| MK1 (1 monosomy) | 17 | 24 | 1.00 | − | 12 | 1.00 | − | ||
| MK2 (>2 monosomies) | 22 | 45 | 0.27 | [0.05,1.35] | 27 | 0.57 | [0.29,1.14] | ||
| Characteristic . | . | Response (CR/PR) . | Overall survival (OS) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N . | CR/PR rate (%) . | Odds Ratio . | 95%- Confidence Interval . | p-value . | 1-year OS rate (%) . | Hazard Ratio . | 95%- Confidence Interval . | p-value . | |
| All patients with known cytogenetics | 179 | 28 | 26 | 0.0006 | |||||
| Cytogenetics | 0.13 | ||||||||
| normal | 56 | 38 | 1.00 | − | 43 | 1.00 | − | ||
| abnormal | 123 | 24 | 1.72 | [0.85,3.48] | 19 | 1.86 | [1.30,2.65] | ||
| MK/CK | 0.053 | 0.38 | |||||||
| MK+ | 39 | 36 | 1.00 | − | 21 | 1.00 | − | ||
| MK-/CK+ | 17 | 12 | 4.75 | [0.88,25.6] | 18 | 1.43 | [0.79,2.59] | ||
| MK-/CK- | 67 | 19 | 2.83 | [1.08,7.41] | 18 | 0.96 | [0.63,1.45] | ||
| MK+ | 0.11 | 0.11 | |||||||
| MK1 (1 monosomy) | 17 | 24 | 1.00 | − | 12 | 1.00 | − | ||
| MK2 (>2 monosomies) | 22 | 45 | 0.27 | [0.05,1.35] | 27 | 0.57 | [0.29,1.14] | ||
Kaplan-Meier overall survival (OS) estimates of patients with abnormal cytogenetics (n=123) and presence of a single monosomy (n=17, dotted line) or multiple monosomies (n=22, broken line)
Kaplan-Meier overall survival (OS) estimates of patients with abnormal cytogenetics (n=123) and presence of a single monosomy (n=17, dotted line) or multiple monosomies (n=22, broken line)
Off Label Use: Decitabine is approved for treatment of different types of MDS, in the present study its activity in AML of older patients was studied. Germing:Celgene: Consultancy, Research Funding.
1.
2.
3.
4.
Author notes
Asterisk with author names denotes non-ASH members.