Abstract
Abstract 3106
Allogeneic stem cell transplantation (SCT) is potentially curative for patients with CML who fail tyrosine kinase inhibitor (TKIs) treatment but remain in chronic phase (CP). In addition, TKI may ‘downstage’ advanced disease, creating a window of opportunity for SCT in second CP (CP2). Sequential therapy, as proposed by Champlin et al (ASH 2009), using a reduced intensity preparative regimen, followed by low dose donor lymphocyte infusion (DLI) is well tolerated and associated with prolonged disease control, especially for patients in CP1. There is preclinical and clinical evidence that hypomethylating agents have immunomodulatory effects when given after SCT (de Lima, Cancer 2010). We then hypothesized that low dose 5-azacitidine (AZA) given early after SCT will induce a more rapid and durable molecular complete remission (molCR).
To increase the frequency of achievement of molCR following reduced intenstity allogeneic SCT. Eligibility: CML in CP who had failed to respond to treatment with one or more TKI. Patients received a preparative regimen of fludarabine 40 mg/m2 × 4 days, busulfan 130 mg/m2 × 2 days, and thymoglobulin 2.5 mg/kg daily × 3 days followed by allogeneic SCT from an HLA matched related or unrelated donor. Graft-versus-host disease (GVHD) prophylaxis was tacrolimus and mini-methotrexate (5 mg/m2 on SCT days 1, 3 and 6 and 11). Those not in molCR 30 days after SCT receive AZA 32 mg/m2 every 28 days for four cycles starting on day +35. Patients were required to have engraftment and initial hematologic recovery, stable organ function and no active GVHD to start AZA treatment. Patients were treated between 2/09 - 9/10, and those with at least 6 months of follow-up are presented here.
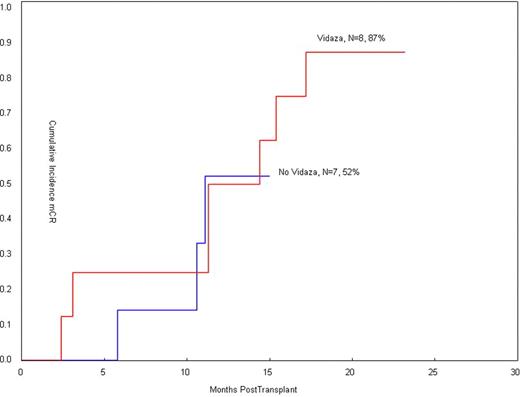
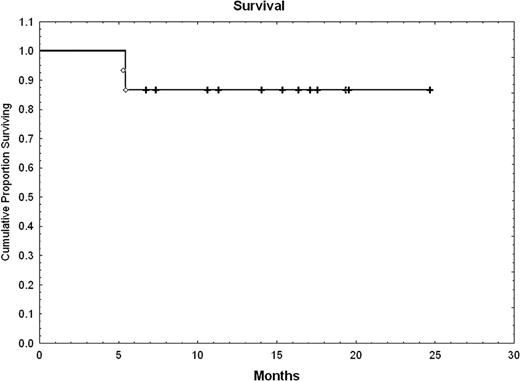
15 patients were entered on study. Eight patients received AZA for a median of 4 cycles. Seven patients did not receive AZA: two were in molCR post transplant, 2 had hematologically progressive disease, 2 were cytopenic, which precluded AZA and 1 patient declined. AZA was administered outpatient with minimal, reversible toxicity: grade I/II/III thrombocytopenia (37.5%), grade I nausea (25%) and grade I fatigue (25%). Grade II-IV acute GVHD occurred in 10 pts. Four AZA patients subsquently received DLI (1 × 107 CD3 cells/Kg) and 75% (3 of 4) converted to MolCR and cytogenetic CR. 7 of the 8 patients that received AZA achieved a MolCR with median follow-up of 12.6 months (range, 3.4–24), compared with MolCR of 57% for those that didn't receive AZA. (Figure 1: cumulative incidence of MolCR) (Table 1). Thirteen patients are alive with a median of 25 months of follow-up (Figure 2) and two died of CML. One patient with overt relapse of chronic phase CML received a second transplant and is alive in MolCR.
Azacitidine is well tolerated post nonmyeloablative SCT and may contribute to achieveent of molCR. Larger number of patients and longer follow-up are necessary to define the efficacy of this intervention.
| . | Azacitidine post transplant (N=8) . | No azacitidine post transplant (N=7) . |
|---|---|---|
| Age at diagnosis | 40 (24–55) | 39.5(23–65) |
| Age at transplant | 47(28–59) | 47(24–66) |
| Disease phase at BMT | Chronic phase(CP) 1 (7) CP2 (1) | CP1 (2) CP2 (5) |
| Median # of TKI failed prior to SCT | 2 (range, 1–5) | 2 (1–3) |
| Tyrosine kinase domain mutation | Yes (5) | Yes (1) |
| No (3) | No (6) | |
| Unknown (1) | ||
| Molecularly BCR-ABL positive post transplant | 8 | 5* |
| Molecular response immediately after AZA | CR – 2 | —- |
| PR – 2 | ||
| PD – 4 | ||
| Molecular response @ 6 mo post BMT | CR – 2 | CR – 2 |
| PR – 5 | PR – 1 | |
| PD – 1 | PD – 3 | |
| NR - 1 | ||
| Molecular response @ 12 mo post BMT | CR – 4 | CR – 3 |
| PD – 4 (received DLI) | PR – 1 | |
| PD – 1 (DLI) | ||
| Death due to disease – 2 | ||
| DLI | 4 (3/4 responses) | 1 (no response) |
| Final Response | molCR – 7 | molCR – 4 |
| PD - 1 | PD – 1 | |
| No response – 2 (death from disease) | ||
| Graft Failure | 1 | 0 |
| Death | 0 | 2 |
| . | Azacitidine post transplant (N=8) . | No azacitidine post transplant (N=7) . |
|---|---|---|
| Age at diagnosis | 40 (24–55) | 39.5(23–65) |
| Age at transplant | 47(28–59) | 47(24–66) |
| Disease phase at BMT | Chronic phase(CP) 1 (7) CP2 (1) | CP1 (2) CP2 (5) |
| Median # of TKI failed prior to SCT | 2 (range, 1–5) | 2 (1–3) |
| Tyrosine kinase domain mutation | Yes (5) | Yes (1) |
| No (3) | No (6) | |
| Unknown (1) | ||
| Molecularly BCR-ABL positive post transplant | 8 | 5* |
| Molecular response immediately after AZA | CR – 2 | —- |
| PR – 2 | ||
| PD – 4 | ||
| Molecular response @ 6 mo post BMT | CR – 2 | CR – 2 |
| PR – 5 | PR – 1 | |
| PD – 1 | PD – 3 | |
| NR - 1 | ||
| Molecular response @ 12 mo post BMT | CR – 4 | CR – 3 |
| PD – 4 (received DLI) | PR – 1 | |
| PD – 1 (DLI) | ||
| Death due to disease – 2 | ||
| DLI | 4 (3/4 responses) | 1 (no response) |
| Final Response | molCR – 7 | molCR – 4 |
| PD - 1 | PD – 1 | |
| No response – 2 (death from disease) | ||
| Graft Failure | 1 | 0 |
| Death | 0 | 2 |
Rationale for not receiving post transplant AZA (2 progressed early to blast crisis, 2 due to cytopenias/GVHD, 1 declined)
PD = Progressive Disease PR = Partial Response CR = Complete Response NR = Nonresponder
DLI = Donor Lymphocyte Infusion
De Lima:Celgene: Research Funding. Off Label Use: azacitidine used post transplant.
Author notes
Asterisk with author names denotes non-ASH members.