Abstract
GADD45A is a tumor suppressor gene that plays cell-type dependent roles in cellular stress, coordinating DNA repair and de-methylation, cell cycle arrest, and pro-apoptotic or pro-survival responses. GADD45A expression is normally rapidly induced in response to radiation and cytotoxic drugs1 and ectopic expression of GADD45A in the M1 leukemic cell line sensitises cells to stress-induced apoptosis in response to a range of genotoxic agents.2 Silencing of GADD45A by promoter methylation is a hallmark of many tumors,3–5 however to date there have been no investigations to determine whether this is associated with response to therapy. In AML, we have shown GADD45A expression is broadly down-regulated6 and here we investigate the mechanism of GADD45A repression in AML and its clinical significance.
We analysed 131 diagnostic bone marrow mononuclear cell samples from a retrospective cohort of patients with de novo AML. 93 of these patients were treated with induction chemotherapy. Patients 60 years or under were treated with chemotherapy regimens containing idarubicin and high dose cytarabine, and patients older than 60 received idarubicin and standard dose cytarabine. We used matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Sequenom MassARRAY) to analyze methylation of 4 GADD45A promoter CpG dinucleotides previously shown to be associated with silencing of GADD45A in breast and prostate cancer.4,5 We determined association of CpG hyper-methylation with outcome in our treated patient cohort. For AML cells with GADD45A hyper-methylation, and for those with normal levels of GADD45A promoter methylation, we also determined the response to cytotoxic agents in vitro in the presence and absence of hypo-methylating agents.
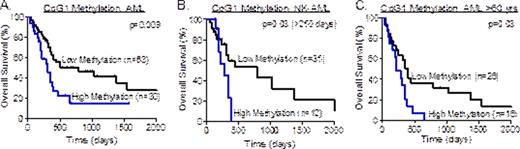
We observed hyper-methylation of the 4 CpG residues in the proximal promoter of GADD45A in 49 of 131 (37%) de novo AML patients and in 6 AML cell lines. Multivariable analysis showed that methylation of a single CpG (CpG1) was an independent predictor of poor survival in AML overall (median survival 281 days versus 794 days, HR 2.25, p=0.009), in normal karyotype AML (NK-AML) (median survival of 281 versus 793 days, HR 5.77, p=0.03, >250 days), and in the elderly patient group (>60 years, median survival of 218 versus 392 days, HR 2.64, p=0.03)(see Figure 1). Additionally, treatment of AML cell lines and patient blasts with decitabine resulted in selective induction of GADD45A mRNA in samples with GADD45A hyper-methylation, and this was associated with increased sensitivity to daunorubicin.
Kaplan-Meier survival curves comparing overall survival of high versus low GADD45A CpG1 methylation in AML overall (A), and in normal karyotype (B) and elderly AML (C).
Kaplan-Meier survival curves comparing overall survival of high versus low GADD45A CpG1 methylation in AML overall (A), and in normal karyotype (B) and elderly AML (C).
DNA methylation of the GADD45A proximal promoter is an independent predictor of poor outcome particularly in AML patients with normal karyotype and in the elderly group. Our biological data shows that induction of GADD45A mRNA expression with decitabine in hyper-methylated samples is associated with increased response to cytotoxic agents. Thus GADD45A promoter CpG methylation represents a new biomarker that may provide prognostic information in the heterogeneous NK-AML group, and in elderly patients. Given that recent trials are combining Azacitidine with chemotherapy and other agents for initial induction treatment of AML9, this may represent a marker to help define patients that will benefit from this approach. Elderly patients with GADD45A hyper-methylation may benefit from treatment with hypo-methylating agents which are associated with less toxicity (see Refs 7,8).
Wei:Celgene: Honoraria, Research Funding.
1.
2.
3.
4.
5.
6.
7.
8.
9.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.