Abstract
Abstract 4138
The addition of high-dose rituximab to conditioning regimens has been shown to improve outcomes of autologous stem cell transplantation (ASCT) for mantle cell lymphoma (MCL) patients (pts) in first partial (PR) or complete remission (CR)(Tam C et al. Blood 2009,113:4144). It has been suggested that absence of SOX11 expression can identify a subtype of indolent MCL with excellent outcomes that might be managed more conservatively than conventional MCL. We now report updated results of ASCT for 40 MCL pts treated at our center between 05/99 and 10/10. We also report on SOX-11 expression in a subset of these pts.
Pts had a median age of 54 years (range, 38–72), and 30% were older than 60 years. At diagnosis, 60% had IPI>1, 30% had intermediate/high MIPI, 88% had stage IV disease, and 78% had bone marrow involvement. 28% had blastic features and Ki-67 was ≥30% in 11/23 (52%) pts who were tested. Pts were treated with R-CHOP or R-hyper-CVAD × 4 cycles induction in 30% and 45% respectively (Group A). Since 2001, pts were referred to ASCT only if they failed to achieve CR with >4 cycles of R-hyper-CVAD induction (n= 10, 25%) (Group B). Prior to transplant, CR (or CR unconfirmed-CRu) was present in 62% pts; 38% were in PR, and 18% were PET+. Conditioning was R-BEAM and R-Cy-TBI conditioning in 77% and 23% respectively. Pts received R during stem cell collection with R administered at 375 mg/m2 on the day before initiating chemotherapy for stem cell mobilization, and again at 1000 mg/m2, 7 days later. Pts then received additional R at 1000 mg/m2 on days +1 and +8 after ASCT, as previously described. Pts were staged with CT, PET (whenever indicated) scans, bone marrow biopsy, and colonoscopy (if history of GI involvement) every 3 months for the first year, every 6 months for 5 years, then yearly thereafter.
SOX11: Formalin-fixed, paraffin-embedded tissue biopsy sections were assessed by immunohistochemistry (IHC) using anti-Sox-11 rabbit polyclonal antibody (Abcam, Cambridge, MA; 1:1500). For IHC controls we used a tissue microarray including 13 cases of MCL in addition to one complete section of MCL serving as positive controls, and sections from two cases of small lymphocytic lymphoma involving lymph node serving as negative controls. The 11 cases consisted of five GI biopsies, three lymph node biopsies, two bone marrows and one testis. 10/11 showed positive staining for SOX11. The case with negative staining, a GI biopsy, had scattered positive cells.
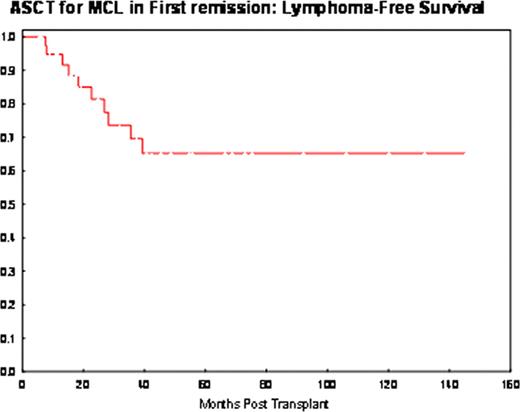
Clinical outcome : Following transplantation, CR/CRu was achieved in 100% pts. With a median follow-up of 37 months (range, 6–145), 10 pts experienced recurrent disease. All progressions occurred within 3 years, with a clear plateau emerging subsequently (Figure). The projected lymphoma-free-survival at 10-year, was 65% (95%CI, 44–80). A tendency for a higher risk of relapse was observed in R-hyper-CVAD resistant pts (Group B) pts [4/10 pts (40%) vs 6/30 (20%) in Group A; HR 2.5 (95%CI, 0.7–9.2), p=0.2)], and in pts with ki-67 ≥30% [HR 2.2 (95%CI, 0.4–11), p=0.3). MIPI, blastic histology, age (> 60 years), disease status at transplant (CR/PR) and conditioning were not found to be of prognostic value in our study. 2 pts (5%) developed myelodysplasia, one of which was concurrent with progression.
ASCT with high-dose rituximab has the potential to cure a proportion of pts with MCL after response to induction chemotherapy. Our results are favorable despite the inclusion of pts who were resistant to R-hyper-CVAD. Randomized studies comparing this strategy to conventional chemo-immunotherapy are warranted. The prognostic significance of Ki-67 level needs to be assessed in a larger cohort of patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.